Adsorption and Release of Rose Bengal on Layer-by-Layer Films of Poly(Vinyl Alcohol) and Poly(Amidoamine) Dendrimers Bearing 4-Carboxyphenylboronic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
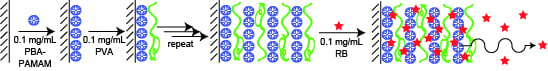
2.2. Preparation of LbL Films
2.3. Adsorption and Release of RB on LbL Films
3. Results and Discussion
3.1. Preparation of LbL Films
3.2. Adsorption of RB on LbL Films
3.3. Release of RB on LbL Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Chen, G.Q.; Yang, X.; Deng, H. Preparation of layer-by-layer nanofiltration membranes by dynamic deposition and crosslinking. Membranes 2019, 9, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Gao, C.; van der Bruggen, B. Technology-driven layer-by-layer assembly of a membrane for selective separation of monovalent anions and antifouling. Nanoscale 2019, 11, 2264–2274. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, M.; Guan, Y.; Chen, M.; Zhang, Y.A. CO2-responsive hydrogel film for optical sensing of dissolved CO2. Soft Matter. 2019, 15, 6107–6115. [Google Scholar] [CrossRef] [PubMed]
- Locke, A.K.; Means, A.K.; Dong, P.; Nichols, T.J.; Coté, G.L.; Grunlan, M.A. A layer-by-layer approach to retain a fluorescent glucose sensing assay within the cavity of a hydrogel membrane. ACS Appl. Bio Mater. 2018, 1, 1319–1327. [Google Scholar] [CrossRef] [Green Version]
- Mascagni, D.B.T.; Miyazaki, C.M.; Cruz, N.C.; Moraes, M.L.; Riul, A., Jr.; Ferreira, M. Layer-by-layer assembly of functionalized reduced graphene oxide for direct electrochemistry and glucose detection. Mater. Sci. Eng. C 2016, 68, 739–745. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Choi, D.; Heo, J.; Jung, S.Y.; Hong, J. Studies on the drug loading and release profiles of degradable chitosan-based multilayer films for anticancer treatment. Cancers 2020, 12, 593. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Ono, T.; Kashiwagi, K.; Takahashi, S.; Sato, K.; Anzai, J.I. PH-dependent release of insulin from layer-by-layer-deposited polyelectrolyte microcapsules. Polymers 2015, 7, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef]
- Sydow, S.; de Cassan, D.; Hänsch, R.; Gengenbach, T.R.; Easton, C.D.; Thissen, H.; Menzel, H. Layer-by-layer deposition of chitosan nanoparticles as drug-release coatings for PCL nanofibers. Biomater. Sci. 2018, 7, 233–246. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Yoshida, K.; Sato, K.; Ono, T.; Dairaku, T.; Kashiwagi, Y. Preparation of nafion/polycation layer-by-layer films for adsorption and release of insulin. Polymers 2018, 10, 812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, S.; Yoo, C.Y.; Park, S.N. Improved stability and skin permeability of sodium hyaluronate-chitosan multilayered liposomes by layer-by-layer electrostatic deposition for quercetin delivery. Colloids Surf. B Biointerfaces 2015, 129, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Rochín-Wong, S.; Rosas-Durazo, A.; Zavala-Rivera, P.; Maldonado, A.; Martínez-Barbosa, M.E.; Vélaz, I.; Tánori, J. Drug release properties of diflunisal from layer-by-layer self-assembled κ-carrageenan/chitosan nanocapsules: Effect of deposited layers. Polymers 2018, 10, 760. [Google Scholar] [CrossRef] [Green Version]
- Shutava, T.G.; Livanovich, K.S.; Sharamet, A.A. Layer-by-layer films of polysaccharides modified with polyethylene glycol and dextran. Colloids Surf. B Biointerfaces 2019, 173, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Vander Straeten, A.; Lefèvre, D.; Demoustier-Champagne, S.; Dupont-Gillain, C. Protein-based polyelectrolyte multilayers. Adv. Colloids Interface Sci. 2020, 280, 102161. [Google Scholar] [CrossRef] [PubMed]
- Hashide, R.; Yoshida, K.; Hasebe, Y.; Seno, M.; Takahashi, S.; Sato, K.; Anzai, J.I. Poly(lactic acid) microparticles coated with insulin-containing layer-by-layer films and their pH-dependent insulin release. J. Nanosci. Nanotechnol. 2014, 14, 3100–3105. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Kashimura, Y.; Kamijo, T.; Ono, T.; Dairaku, T.; Sato, T.; Kashiwagi, Y.; Sato, K. Decomposition of glucose-sensitive layer-by-layer films using hemin, DNA, and glucose oxidase. Polymers 2020, 12, 319. [Google Scholar] [CrossRef] [Green Version]
- Peng, N.; Yu, H.; Wang, Z.; Zhang, Y.; Deng, K.; Li, J.; Lu, L.; Zou, T.; Liu, Y.; Huang, S. Dendrimer-grafted bioreducible polycation/DNA multilayered films with low cytotoxicity and high transfection ability. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 737–745. [Google Scholar] [CrossRef]
- Guo, P.; Shi, Z.L.; Liu, A.; Lin, T.; Bi, F.G.; Shi, M.M.; Yan, S.G. Cartilage oligomeric matrix protein gene multilayers inhibit osteogenic differentiation and promote chondrogenic differentiation of mesenchymal stem cells. Int. J. Mol. Sci. 2014, 15, 20117–20133. [Google Scholar] [CrossRef]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Mustafa, R.; Luo, Y.; Wu, Y.; Guo, R.; Shi, X. Dendrimer-functionalized laponite nanodisks as a platform for anticancer drug delivery. Nanomaterials 2015, 5, 1716–1731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decher, G.; Hong, J.D. Buildup of ultrathin multilayer films by a self-assembly process, 1 consecutive adsorption of anionic and cationic bipolar amphiphiles on charged surfaces. Makromol. Chem. Macromol. Symp. 1991, 46, 321–327. [Google Scholar] [CrossRef]
- Volodkin, D.; von Klitzing, R.; Moehwald, H. Polyelectrolyte multilayers: Toward cell studies. Polymers 2014, 6, 1502–1527. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Guan, Y.; Tan, S.; Xu, J.; Cheng, S.; Zhang, X. Water uptake behavior of hydrogen-bonded PVPON-PAA LbL film. Soft Matter 2006, 2, 699–704. [Google Scholar] [CrossRef]
- Tomita, S.; Sato, K.; Anzai, J.I. Layer-by-layer assembled thin films composed of carboxylterminated poly(amidoamine) dendrimer as a pH-sensitibe nanodevice. J. Colloids Interface Sci. 2008, 326, 35–40. [Google Scholar] [CrossRef]
- Sato, K.; Imoto, Y.; Sugama, J.; Seki, S.; Inoue, H.; Odagiri, T.; Hoshi, T.; Anzai, J.I. Sugar-induced disintegration of layer-by-layer assemblies composed of concanavalin A and glycogen. Langmuir 2005, 21, 797–799. [Google Scholar] [CrossRef]
- Akiba, U.; Minaki, D.; Anzai, J.I. Host-guest chemistry in layer-by-layer assemblies containing calix[n]arenes and cucurbit[n]urils: A review. Polymers 2018, 10, 130. [Google Scholar] [CrossRef] [Green Version]
- Springsteen, G.; Wang, B. A detailed examination of boronic acid–diol complexation. Tetrahedron 2002, 58, 5291–5300. [Google Scholar] [CrossRef]
- Yan, J.; Springsteen, G.; Deeter, S.; Wang, B. The relationship among pKa, pH, and binding constants in the interactions between boronic acids and diols-it is not as simple as it appears. Tetrahedron 2004, 60, 11205–11209. [Google Scholar] [CrossRef]
- Suwa, K.; Nagasaka, M.; Niina, S.; Egawa, Y.; Seki, T.; Anzai, J.I. Sugar response of layer-by-layer films composed of poly(vinyl alcohol) and poly(amidoamine) dendrimer bearing 4-carboxyphenylboronic acid. Colloids Polym. Sci. 2015, 293, 1043–1048. [Google Scholar] [CrossRef]
- Seno, M.; Yoshida, K.; Sato, K.; Anzai, J.I. PH- and sugar-senstive multilayer films composed of phenylboronic acid (PBA)-modified poly(allyamine hydrochloride) (PBA-PAH) and poly(vinyl alcohol) (PVA): A signigicant effect of PBA content on the film stability. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Van den Brittner, G.C.; Dubikovskaya, E.A.; Bertozzi, C.R.; Chang, C.J. In vivo imaging of hydrogen peroxide production in amurine tumor model with chemoselective bioluminescent reporter. Proc. Natl. Acad. Sci. USA 2010, 107, 21316–21321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lux, C.G.; Joshi-Barr, S.; Lux, N.T.; Mahmoud, E.; Schopf, E.; Fomina, N.; Almutari, A. Biocompatible polymeric nanoparticles degrade and release cargo in response to biologically relevant levels of hydrogen peroxide. J. Am. Chem. Soc. 2012, 134, 15758–15764. [Google Scholar]
- Faria, R.A.D.; Iden, H.; Dias, H.; Matencio, T.; Messaddeq, Y. Non-enzymatic impedimetric sensor based on 3-aminophenylboronic acid functionalized screen-printed carbon electrode for highly sensitive glucose detection. Sensors 2019, 19, 1686. [Google Scholar] [CrossRef] [Green Version]
- Broaders, K.E.; Grandhe, S.; Fréchet, J.M.J. A biocompatible oxidation-triggered carrier polymer with potential in therapeutics. J. Am. Chem Soc. 2011, 133, 756–758. [Google Scholar] [CrossRef]
- Sato, K.; Takahashi, M.; Ito, M.; Abe, E.; Anzai, J. H2O2-induced decomposition of layer-By-layer films consisting of phenylboronic acid-bearing poly (allylamine) and poly (vinyl alcohol). Langmuir 2014, 30, 9247–9250. [Google Scholar] [CrossRef]
- Burke, S.E.; Barrett, C.J. PH-Responsive properties of multilayered poly (l-lysine)/hyaluronic acid surfaces. Biomacromolecules 2003, 4, 1773–1783. [Google Scholar] [CrossRef]
- Hübsch, E.; Fleith, G.; Fatisson, J.; Labbé, P.; Voegel, J.C.; Schaaf, P.; Ball, V. Multivalent ion/polyelectrolyte exchange processes in exponentially growing multilayers. Langmuir 2005, 21, 3664–3669. [Google Scholar] [CrossRef]
- Liu, Y.; Bryantsev, V.S.; Diallo, M.S.; Goddard, W.A., III. PAMAM dendrimers undergo pH responsive conformational changes without swelling. J. Am. Chem. Soc. 2009, 131, 2798–2799. [Google Scholar] [CrossRef] [Green Version]
- Karthikeyan, K.; Anish Babu, A.; Sang-Jae Kim, S.; Murugesan, R.; Jeyasubramanian, K. Enhanced photodynamic efficacy and efficient delivery of rose bengal using nanostructured poly(amidoamine) dendrimers: Potential application in photodynamic therapy of cancer. Cancer Nanotechnol. 2011, 2, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, N.; Othaman, R.; Abdullah, I.; Jon, N.; Baharum, A. Studies on the adsorption of phenol red dye Using silica-filledENR/PVC beads. J. Emerg. Trends Eng. Appl. Sci. 2012, 3, 845–850. [Google Scholar]
- Gerweck, L.E.; Seetharaman, K. Cellular pH gradient in tumor versus normal tissue: Potential exploitation for the treatment of cancer. Cancer Res. 1996, 56, 1194–1198. [Google Scholar] [PubMed]
- Izumi, H.; Torigoe, T.; Ishiguchi, H.; Uramoto, H.; Yoshida, Y.; Tanabe, M.; Ise, T.; Murakami, T.; Yoshida, T.; Nomoto, M.; et al. Cellular pH regulators: Potentially promising molecular targets for cancer chemotherapy. Cancer Treat. Rev. 2003, 29, 541–549. [Google Scholar] [CrossRef]











© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, K.; Yamaguchi, A.; Midorikawa, H.; Kamijo, T.; Ono, T.; Dairaku, T.; Sato, T.; Fujimura, T.; Kashiwagi, Y.; Sato, K. Adsorption and Release of Rose Bengal on Layer-by-Layer Films of Poly(Vinyl Alcohol) and Poly(Amidoamine) Dendrimers Bearing 4-Carboxyphenylboronic Acid. Polymers 2020, 12, 1854. https://doi.org/10.3390/polym12081854
Yoshida K, Yamaguchi A, Midorikawa H, Kamijo T, Ono T, Dairaku T, Sato T, Fujimura T, Kashiwagi Y, Sato K. Adsorption and Release of Rose Bengal on Layer-by-Layer Films of Poly(Vinyl Alcohol) and Poly(Amidoamine) Dendrimers Bearing 4-Carboxyphenylboronic Acid. Polymers. 2020; 12(8):1854. https://doi.org/10.3390/polym12081854
Chicago/Turabian StyleYoshida, Kentaro, Akane Yamaguchi, Hiroki Midorikawa, Toshio Kamijo, Tetsuya Ono, Takenori Dairaku, Takaya Sato, Tsutomu Fujimura, Yoshitomo Kashiwagi, and Katsuhiko Sato. 2020. "Adsorption and Release of Rose Bengal on Layer-by-Layer Films of Poly(Vinyl Alcohol) and Poly(Amidoamine) Dendrimers Bearing 4-Carboxyphenylboronic Acid" Polymers 12, no. 8: 1854. https://doi.org/10.3390/polym12081854
APA StyleYoshida, K., Yamaguchi, A., Midorikawa, H., Kamijo, T., Ono, T., Dairaku, T., Sato, T., Fujimura, T., Kashiwagi, Y., & Sato, K. (2020). Adsorption and Release of Rose Bengal on Layer-by-Layer Films of Poly(Vinyl Alcohol) and Poly(Amidoamine) Dendrimers Bearing 4-Carboxyphenylboronic Acid. Polymers, 12(8), 1854. https://doi.org/10.3390/polym12081854