Green Nanocomposites from Rosin-Limonene Copolymer and Algerian Clay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Organoclay (Mag-CTA+)
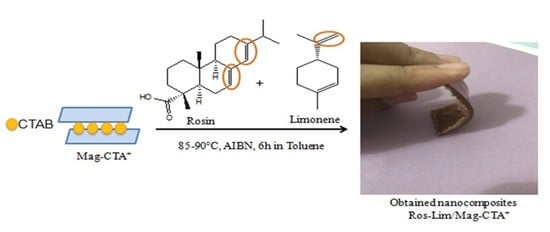
2.3. Preparation of Green Nanocomposites (Ros-lim/Mag-CTA+)
2.4. Characterization
3. Results
3.1. Modified Clays (Mag-Na+ and Mag-CTA+)
3.2. Obtained Nanocomposites (Ros-Lim/Mag-CTA+)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kojima, Y.; Usuki, A.; Kawasumi, M.; Okada, A.; Ukushima, Y.F.; Kamigaito, O. Mechanical properties of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1185–1189. [Google Scholar] [CrossRef]
- Harrane, A.; Meghabar, R.; Belbachir, M. In situ polymerization of ε-caprolactone catalyzed by Maghnite TOA to produce poly(ε-caprolactone)/montmorillonite nanocomposites. Des. Monomers Polym. 2006, 9, 181–191. [Google Scholar] [CrossRef]
- Zare, Y.; Fasihi, M.; Rhee, K.Y. Efficiency of stress transfer between polymer matrix and nanoplatelets in clay/polymer nanocomposites. Appl. Clay Sci. 2017, 143, 265–272. [Google Scholar] [CrossRef]
- Kotal, M.; Bhowmick, A.K. Polymer nanocomposites from modified clays: Recent advances and challenges. Prog. Polym. Sci. 2015, 51, 127–187. [Google Scholar] [CrossRef] [Green Version]
- Mykola, S.; Olga, N.; Dmitry, M. The influence of alkylammonium modified clays on the fungal resistance and biodeterioration of epoxy-clay nanocomposites. Int. Biodeterior. Biodegrad. 2016, 110, 136–140. [Google Scholar] [CrossRef]
- Wypych, F.; Satyanarayana, K.G. Functionalization of single layers and nanofibers: A new strategy to produce polymer nanocomposites with optimized properties. Coll. J. Interf. Sci. 2005, 285, 532–543. [Google Scholar] [CrossRef]
- Mitchell, G.R.; Biscaia, S.; Mahendra, V.S.; Mateus, A. High Value Materials from the Forests. Adv. Mater. Phys. Chem. 2016, 6, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Mahendra, V. Rosin Product Review. Appl. Mech. Mater. 2019, 890, 77–91. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Alvaro, G.H.; Frank, W.; Pietro, L. Influence of cement on rheology and stability of rosin emulsified anionic bitumen emulsion. J. Mater. Civ. Eng. 2016, 28. [Google Scholar] [CrossRef]
- Hui Dai, D.; Sun, C.Y.; Li, F.Y.; Yang, Y.J.; Ren, X.G.; Wang, Z.M. Study on the waterproofing agent for the mineral woolboard. Appl. Mech. Mater. 2012, 204, 3672–3676. [Google Scholar] [CrossRef]
- Karlberg, A.T. Colophony: Rosin in unmodified and modified form BT. In Kanerva’s Occupational Dermatology; John, S.M., Johansen, J.D., Rustemeyer, T., Elsner, P., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 607–664. [Google Scholar]
- Xie, Y.; Fu, Q.; Wang, Q.; Xiao, Z.; Militz, H. Effects of chemical modification on the mechanical properties of wood. Eur. J. Wood Prod. 2013, 71, 401–416. [Google Scholar] [CrossRef]
- Rukel, E.; Wojcik, R.; Arlt, H. Cationic Polymerization of α-Pinene Oxide and β-Pinene Oxide by a Unique OxoniumlonCarbenium Ion Sequence. J. Macromol. Sci. Part A. 1976, 10, 1371–1390. [Google Scholar] [CrossRef]
- Sharma, S.; Srivastava, A.K. Synthesis and characterization of copolymers of limonene with styrene initiated by azobisisobutyronitrile. Eur. Polym. J. 2004, 40, 2235–2240. [Google Scholar] [CrossRef]
- Derdar, H.; Belbachir, M.; Hennaoui, F.; Akeb, M.; Harrane, A. Green copolymerization of limonene with β-pinene catalyzed by an eco-catalyst Maghnite-H+. Polym. Sci. Ser. B. 2018, 60, 555–562. [Google Scholar] [CrossRef]
- Roberts, W.; Day, A. A Study of the Polymerization of α- and β-Pinene with Friedel- Crafts Type Catalysts. J. Am. Chem. Soc. 1950, 72, 1226–1230. [Google Scholar] [CrossRef]
- Derdar, H.; Belbachir, M.; Harrane, A. A green synthesis of polylimonene using Maghnite-H+, an exchanged montmorillonite clay, as eco-catalyst. BCREC 2019, 14, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Modena, M.; Bates, R.B.; Marvel, S.C. Some low molecular weight polymers of d-limonene and related terpenes obtained by Ziegler-type catalysts. J. Polym. Sci. Part A Gen. Pap. 1965, 3, 949–960. [Google Scholar] [CrossRef]
- Barros, M.T.; Petrova, K.T.; Ramos, A.M. Potentially Biodegradable Polymers based on α- or β-Pinene and Sugar Derivatives or Styrene, Obtained under Normal Conditions and Microwave Irradiation. Eur. J. Org. Chem. 2007, 8, 1357–1363. [Google Scholar] [CrossRef]
- Cherifi, Z.; Boukoussa, B.; Zaoui, A.; Belbachir, M.; Meghabar, R. Structural, morphological and thermal properties of nanocomposites poly(GMA)/clay prepared by ultrasound and in-situ polymerization. Ultrason. Sonochem. 2018, 48, 188–198. [Google Scholar] [CrossRef]
- Embarek, N.; Sahli, N.; Belbachir, M. Preparation and characterization of poly(3-glycidoxypropyltrimethoxysilane) nanocomposite using organophilic montmorillonite clay (Mag-cetyltrimethylammonium). J. Compos. Mater. 2019, 53, 4313–4322. [Google Scholar] [CrossRef]
- Mrah, L.; Meghabar, R. Influence of clay modification process in polypyrrole-layered silicate nanocomposite. Appl. Sci. 2020, 2, 659. [Google Scholar] [CrossRef] [Green Version]
- Mrah, L.; Meghabar, R. Dispersion and improvement of organoclays in nanocomposites based on poly(propylene oxide). J. Thermoplastic. Comp. Mater. 2020, 1–14. [Google Scholar] [CrossRef]
- Dike, A.S.; Yilmazer, U. Improvement of organoclay dispersion into polystyrene based nanocomposites by incorporation of SBS and maleic anhydride-grafted SBS. J. Thermoplastic. Comp. Mater. 2019, 33, 554–574. [Google Scholar] [CrossRef]
- Khenifi, A.; Zohra, B.; Kahina, B.; Houari, H.; Zoubir, D. Removal of 2,4-DCP from wastewater by CTAB/bentonite using one-step and two-step methods: A comparative study. Chem. Eng. J. 2009, 146, 345–354. [Google Scholar] [CrossRef]
- Belbachir, M.; Bensaoula, A. Composition and Method for Catalysis Using Bentonites. U.S. Patent 7094823 B2, 22 August 2006. [Google Scholar]
- Cicel, B. Mineralogical composition and distribution of Si, Al, Fe, Mg and Ca in the fine fractions of some Czech and Slovak bentonites. Geol. Carpath. Ser. Clays 1992, 43, 3–7. [Google Scholar]
- Grenier, A.; Wendorff, H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Salmi-Mani, H.; Ait-Touchente, Z.; Lamouri, A.; Carbonnier, B.; Caron, J.F.; Benzarti, K.; Chehimi, M.M. Diazonium salt-based photoiniferter as a new efficient pathway to clay–polymer nanocomposites. RSC Adv. 2016, 6, 88126. [Google Scholar] [CrossRef]
- Bureau, M.N.; Denault, J.; Cole, K.C.; Enright, G.D. The role of crystallinity and reinforcement in the mechanical behavior of polyamide-6/clay nanocomposites. Polym. Eng. Sci. 2002, 42, 1897–1906. [Google Scholar] [CrossRef]
- Kherroub, D.E.; Belbachir, M.; Lamouri, S. Nylon 6/clay nanocomposites prepared with Algerian modified clay (12-maghnite). Res. Chem. Int. 2014, 41, 5217–5228. [Google Scholar] [CrossRef]
- Vaia, R.A.; Price, G.; Ruth, P.N.; Nguyen, H.T.; Lichtenhan, J. Polymer/layered silicate nanocomposite as high performance ablative materials. Appl. Clay Sci. 1999, 15, 67–92. [Google Scholar] [CrossRef]
- Zhu, Z.K.; Yang, Y.; Yin, J.; Wang, X.Y.; Ke, Y.C.; Qi, Z.N. Preparation and properties of organosolublemontmorillonite/polyimide hybrid materials. J. Appl. Polym. Sci. 1999, 73, 2063–2068. [Google Scholar] [CrossRef]
- Wang, S.; Long, C.; Wang, X.; Li, Q.; Qi, Z. Synthesis and properties of silicone rubber/organomontmorillonite hybrid nanocomposites. J. Appl. Polym. Sci. 1998, 69, 1557–1561. [Google Scholar] [CrossRef]









| Samples | Ros | Lim | Mag-CTA+ | Time | Yield |
|---|---|---|---|---|---|
| Ros-Lim/Mag-CTA+ 1% | 9 g | 5 g | 1% (wt) | 6 h | 82% |
| Ros-Lim/Mag-CTA+ 5% | 9 g | 5 g | 5% (wt) | 6 h | 77% |
| Ros-Lim/Mag-CTA+ 10% | 9 g | 5 g | 10% (wt) | 6 h | 72% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derdar, H.; Mitchell, G.R.; Mahendra, V.S.; Benachour, M.; Haoue, S.; Cherifi, Z.; Bachari, K.; Harrane, A.; Meghabar, R. Green Nanocomposites from Rosin-Limonene Copolymer and Algerian Clay. Polymers 2020, 12, 1971. https://doi.org/10.3390/polym12091971
Derdar H, Mitchell GR, Mahendra VS, Benachour M, Haoue S, Cherifi Z, Bachari K, Harrane A, Meghabar R. Green Nanocomposites from Rosin-Limonene Copolymer and Algerian Clay. Polymers. 2020; 12(9):1971. https://doi.org/10.3390/polym12091971
Chicago/Turabian StyleDerdar, Hodhaifa, Geoffrey Robert Mitchell, Vidhura Subash Mahendra, Mohamed Benachour, Sara Haoue, Zakaria Cherifi, Khaldoun Bachari, Amine Harrane, and Rachid Meghabar. 2020. "Green Nanocomposites from Rosin-Limonene Copolymer and Algerian Clay" Polymers 12, no. 9: 1971. https://doi.org/10.3390/polym12091971
APA StyleDerdar, H., Mitchell, G. R., Mahendra, V. S., Benachour, M., Haoue, S., Cherifi, Z., Bachari, K., Harrane, A., & Meghabar, R. (2020). Green Nanocomposites from Rosin-Limonene Copolymer and Algerian Clay. Polymers, 12(9), 1971. https://doi.org/10.3390/polym12091971