Polymer Capsules with Hydrophobic Liquid Cores as Functional Nanocarriers
Abstract
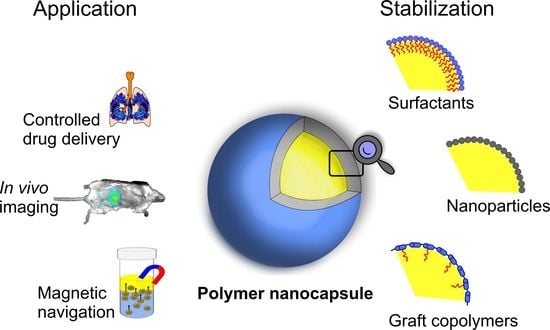
:1. Introduction
2. Fabrication of Polymer Core-Shell Nanocapsules
3. Stabilization of Oil Cores of Nanocapsules
4. Amphiphilic Polymers as Nanoemulsion Stabilizers: From Synthetic Graft Copolymers to Modified Polysaccharides of Natural Origin
5. Applications of Oil-Core Nanocapsules
5.1. Controlled Delivery and Release of Active Compounds
5.2. Multifunctional Polymer Nanocapsules
5.3. In Vivo Imaging
5.4. Magnetically Responsive Nanocapsules as Targeted Drug Delivery Systems and Chemical Reactors
6. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Sun, Q.; Du, Y.; Hall, E.A.H.; Luo, D.; Sukhorukov, G.B.; Routh, A.F. A fabrication method of gold coated colloidosomes and their application as targeted drug carriers. Soft Matter 2018, 14, 2594–2603. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, A.B.; Argin, S.; Ozilgen, M.; McClements, D.J. Formation and stabilization of nanoemulsion-based vitamin E delivery systems using natural biopolymers: Whey protein isolate and gum. Food Chem. 2015, 188, 256–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bratovčić, A.; Odobašić, A.; Ćatić, S.; Šestan, I. Application of polymer nanocomposite materials in food packaging. Croat. J. Food Sci. Technol. 2015, 7, 86–94. [Google Scholar] [CrossRef]
- Couch, L.M.; Wien, M.; Brown, J.L.; Davidson, P. Food nanotechnology: Proposed uses, safety concerns and regulations. Agro Food Ind. Hi Tech 2016, 27, 36–39. [Google Scholar]
- Singh, T.; Shukla, S.; Kumar, P.; Wahla, V.; Bajpai, V.K. Application of Nanotechnology in Food Science: Perception and Overview. Front. Microbiol. 2017, 8, 1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OH, J.-W.; Chun, S.C.; Chandrasekaran, M. Preparation and in Vitro Characterization of Chitosan Nanoparticles and Their Broad-Spectrum Antifungal Action Compared to Antibacterial Activities against Phytopathogens of Tomato. Agronomy 2019, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Esser-Kahn, A.P.; Odom, S.A.; Sottos, N.R.; White, S.R.; Moore, J.S. Triggered Release from Polymer Capsules. Macromolecules 2011, 44, 5539–5553. [Google Scholar] [CrossRef]
- Trojer, M.A.; Andersson, H.; Li, Y.; Borg, J.; Holmberg, K.; Nyden, M.; Nordstierna, L. Charged microcapsules for controlled release of hydrophobic actives. Part III: The effect of polyelectrolyte brush- and multilayers on sustained release. Phys. Chem. Chem. Phys. 2013, 15, 6456–6466. [Google Scholar] [CrossRef] [Green Version]
- Sari, P.; Mann, B.; Kumar, R.; Singh, R.R.B.; Sharma, R.; Bhardwaj, M.; Athira, M. Preparation and characterization of nanoemulsion encapsulating curcumin. Food Hydrocol. 2015, 43, 540–546. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, E.; Yang, J.; Cao, Z. Strategies to improve micelle stability for drug delivery. Nano Res. 2018, 11, 4985–4998. [Google Scholar] [CrossRef]
- Lu, Y.; Yue, Z.; Xie, J.; Wang, W.; Zhu, H.; Zhang, E.; Cao, Z. Micelles with ultralow critical micelle concentration as carriers for drug delivery. Nat. Biomed. Eng. 2018, 2, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Tücking, K.-S.; Grützner, V.; Unger, R.E.; Schönherr, H. Dual Enzyme-Responsive Capsules of Hyaluronic Acid-block-Poly(Lactic Acid) for Sensing Bacterial Enzymes. Macromol. Rapid Commun. 2015, 36, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Alwani, S.; Badea, I. Polymeric Nanoparticles in Gene Therapy: New Avenues of Design and Optimization for Delivery Applications. Polymers 2019, 11, 745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Ganbold, T.; Bao, Q.; Yoshida, T.; Baigude, H. Sugar Functionalized Synergistic Dendrimers for Biocompatible Delivery of Nucleic Acid Therapeutics. Polymers 2018, 10, 1034. [Google Scholar] [CrossRef] [Green Version]
- Taabache, S.; Bertin, A. Vesicles from Amphiphilic Dumbbells and Janus Dendrimers: Bioinspired Self-Assembled Structures for Biomedical Applications. Polymers 2017, 9, 280. [Google Scholar] [CrossRef] [Green Version]
- Kunzler, C.; Handschuh-Wang, S.; Roesener, M.; Schönherr, H. Giant Biodegradable Poly(ethylene glycol)-block-Poly(ε-caprolactone) Polymersomes by Electroformation. Macromol. Biosci. 2020, 20, 2000014. [Google Scholar] [CrossRef]
- Marturano, V.; Cerruti, P.; Giamberini, M.; Tylkowski, B.; Ambrogi, V. Light-Responsive Polymer Micro- and Nano-Capsules. Polymers 2017, 9, 8. [Google Scholar] [CrossRef]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-based Nanocapsules for Drug Delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef]
- Vecchione, R.; Ciotola, U.; Sagliano, A.; Bianchini, P.; Diaspro, A.; Netti, P.A. Tunable stability of monodisperse secondary O/W nano-emulsions. Nanoscale 2014, 6, 9300–9307. [Google Scholar] [CrossRef]
- Yang, S.; Ding, F.; Gao, Z.; Guo, J.; Cui, J.; Zhang, P. Fabrication of Poly(ethylene glycol) Capsules via Emulsion Templating Method for Targeted Drug Delivery. Polymers 2020, 12, 1124. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, J.-C. Oxidation–Responsive Emulsions Stabilized with Poly(Vinyl Pyrrolidone-co-allyl Phenyl Sulfide). Polymers 2020, 12, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, C.; Medronho, B.; Filipe, A.; Mira, I.; Lindman, B.; Edlund, H.; Norgren, M. Emulsion Formation and Stabilization by Biomolecules: The Leading Role of Cellulose. Polymers 2019, 11, 1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szczepanowicz, K.; Jantas, D.; Piotrowski, M.; Staroń, J.; Leśkiewicz, M.; Regulska, M.; Lasoń, W.; Warszyński, P. Encapsulation of curcumin in polyelectrolyte nanocapsules and their neuroprotective activity. Nanotechnology 2016, 27, 355101. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Li, Q.; Wang, G. Photodegradable polymer nanocapsles fabricated from dimethyldiethoxysilane emulsion templates for controlled release. Polym. Chem. 2017, 8, 6817–6823. [Google Scholar] [CrossRef]
- Szczepanowicz, K.; Podgórna, K.; Szyk-Warszyńska, L.; Warszyński, P. Formation of oil filled nanocapsules with silica shells modified by sequential adsorption of polyelectrolytes. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 885–889. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Szczepanowicz, K.; Podgórna, K.; Błasiak, E.; Majeed, N.; Ögren, S.-O.; Nowak, W.; Warszyński, P.; Dziedzicka-Wasylewska, M. Encapsulation of clozapine in polymeric nanocapsules and its biological effects. Colloids Surf. B Biointerfaces 2016, 140, 342–352. [Google Scholar] [CrossRef]
- Kopeć, M.; Szczepanowicz, K.; Warszyński, P.; Nowak, P. Liquid-core polyelectrolyte nanocapsules produced by membrane emulsification as carriers for corrosion inhibitors. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 510, 2–10. [Google Scholar] [CrossRef]
- Feng, J.; Chen, Q.; Wu, X.; Jafari, S.M.; McClements, D.J. Formulation of oil-in-water emulsions for pesticide applications: Impact of surfactant type and concentration on physical stability. Environ. Sci. Pollut. Res. 2018, 25, 21742–21751. [Google Scholar] [CrossRef]
- Syed, I.; Banerjee, P.; Sarkar, P. Oil-in-water emulsions of geraniol and carvacrol improve the antibacterial activity of these compounds on raw goat meat surface during extended storage at 4 °C. Food Control 2020, 107, 106757. [Google Scholar] [CrossRef]
- Kalepu, S.; Nekkanti, V. Insoluble drugdelivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Wang, Y.; Postma, A.; Hao, J.; Hosta-Rigau, L.; Caruso, F. Monodisperse Polymer Capsules: Tailoring Size, Shell Thickness, and Hydrophobic Cargo Loading via Emulsion Templating. Adv. Funct. Mater. 2010, 20, 1625–1631. [Google Scholar] [CrossRef]
- Donath, E.; Sukhorukov, G.B.; Caruso, F.; Davis, S.A.; Möhwald, H. Novel Hollow Polymer Shells by Colloid-Templated Assembly of Polyelectrolytes. Angew. Chem. Int. Ed. 1998, 37, 2201–2205. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy Nanoassemblies:Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Kopeć, M.; Rozpedzik, A.; Łapok, Ł.; Geue, T.; Laschewsky, A.; Zapotoczny, S. Stratified Micellar Multilayers—Toward Nanostructured Photoreactors. Chem. Mater. 2016, 28, 2219–2228. [Google Scholar] [CrossRef]
- Puciul-Malinowska, A.; Zapotoczny, S. Robust nanocoatings based on ionic silicones. Nanoscale 2018, 10, 12497–12504. [Google Scholar] [CrossRef]
- Zhao, S.; Caruso, F.; Dähne, L.; Decher, G.; De Geest, B.G.; Fan, J.; Feliu, N.; Gogotsi, Y.; Hammond, P.T.; Hersam, M.C.; et al. The Future of Layer-by-Layer Assembly: A Tribute to ACS Nano Associate Editor Helmuth Möhwald. ACS Nano 2019, 13, 6151–6169. [Google Scholar] [CrossRef] [Green Version]
- Richardson, J.J.; Cui, J.; Björnmalm, M.; Braunger, J.A.; Ejima, H.; Caruso, F. Innovation in layer-by-layer assembly. Chem. Rev. 2016, 116, 14828–14867. [Google Scholar] [CrossRef] [Green Version]
- Elbaz, N.M.; Owen, A.; Rannard, S.; McDonald, T.O. Controlled synthesis of calcium carbonate nanoparticles and stimuli-responsive multi-layered nanocapsules for oral drug delivery. Int. J. Pharm. 2019, 574, 118866. [Google Scholar] [CrossRef]
- Menard, M.; Meyer, F.; Parkhomenko, K.; Leuvrey, C.; Francius, G.; Begin-Colin, S.; Mertz, D. Mesoporous silica templated-albumin nanoparticles with high doxorubicin payload for drug delivery assessed with a 3-D tumor cell model. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 332–341. [Google Scholar] [CrossRef]
- Chojnacka-Górka, K.; Rozpędzik, A.; Zapotoczny, S. Robust Polyelectrolytes Microcapsules Reinforced with Carbon Nanotubes. RSC Adv. 2016, 6, 114639–114643. [Google Scholar] [CrossRef]
- Shang, B.; Zhang, X.; Ji, R.; Wang, Y.; Hu, H.; Peng, B.; Deng, Z. Preparation of colloidal polydopamine/Au hollow spheres for enhanced ultrasound contrast imaging and photothermal therapy. Mater. Sci. Eng. C 2020, 106, 110174. [Google Scholar] [CrossRef] [PubMed]
- Pucek, A.; Tokarek, B.; Waglewska, E.; Bazylińska, U. Recent Advances in the Structural Design of Photosensitive Agent Formulations Using “Soft” Colloidal Nanocarriers. Pharmaceutics 2020, 12, 587. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Gigliobianco, M.R.; Censi, R.; Di Martino, P. Polymeric Nanocapsules as Nanotechnological Alternative for Drug Delivery System: Current Status, Challenges and Opportunities. Nanometarials 2020, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, M.; Szczepanowicz, K.; Jantas, D.; Leśkiewicz, M.; Lasoń, W.; Warszyński, P. Emulsion-core and polyelectrolyte-shell nanocapsules: Biocompatibility and neuroprotection against SH-SY5Y cells. J. Nanopart. Res. 2013, 15, 2035. [Google Scholar] [CrossRef]
- Kim, C.S.; Mout, R.; Zhao, Y.; Yeh, Y.-C.; Tang, R.; Jeong, Y.; Duncan, B.; Hardy, J.A.; Rotello, V.M. Co-delivery of protein and small molecule therapeutics using nanoparticle-stabilized nanocapsules. Bioconjugate Chem. 2015, 26, 950–954. [Google Scholar] [CrossRef] [Green Version]
- Prasetyanto, E.A.; Bertucci, A.; Septiadi, D.; Corradini, R.; Castro-Hartmann, P.; De Cola, L. Breakable hybrid organosilica nanocapsules for protein delivery. Angew. Chem. Int. Ed. 2016, 55, 3323–3327. [Google Scholar] [CrossRef]
- Weber, D.M.; Voss, G.T.; De Oliveira, R.L.; Da Fonseca, C.A.R.; Paltian, J.; Rodrigues, K.C.; Ianiski, F.R.; Vaucher, R.A.; Luchese, C.; Wilhelm, E.A. Topic application of meloxicam-loaded polymeric nanocapsules as a technological alternative for treatment of the atopic dermatitis in mice. J. Appl. Biomed. 2018, 16, 337–343. [Google Scholar] [CrossRef]
- Dos Santos Chaves, P.; Frank, L.A.; Torge, A.; Schneider, M.; Pohlmann, A.R.; Guterres, S.S.; Beck, R.C.R. Spray-dried carvedilol-loaded nanocapsules for sublingual administration: Mucoadhesive properties and drug permeability. Powder Technol. 2019, 354, 348–357. [Google Scholar] [CrossRef]
- Ganassin, R.; Merker, C.; Corrêa Rodrigues, M.; Guimarães, N.F.; Sampaio Cerqueira Sodré, C.; da Silva Ferreira, Q.; da Silva, S.W.; Simalie Ombredane, A.; Anselmo Joanitti, G.; Rapp Py-Daniel, K.; et al. Nanocapsules for the co-delivery of selol and doxorubicin to breast adenocarcinoma 4T1 cells in vitro. Artif. Cells Nanomed. Biotechnol. 2018, 46, 2002–2012. [Google Scholar] [CrossRef]
- Tallian, C.; Herrero-Rollett, A.; Stadler, K.; Vielnascher, R.; Wieland, K.; Weihs, A.M.; Pellis, A.; Teuschl, A.H.; Lendl, B.; Amenitsch, H.; et al. Structural insights into pH-responsive drug release of self-assembling human serum albumin-silk fibroin nanocapsules. Eur. J. Pharm. Biopharm. 2018, 133, 176–187. [Google Scholar] [CrossRef]
- Drozdek, S.; Bazylińska, U. Biocompatible oil core nanocapsules as potential co-carriers of paclitaxel and fluorescent markers: Preparation, characterization, and bioimaging. Colloid Polym. Sci. 2016, 294, 225–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamczak, M.; Krok, M.; Pamuła, E.; Posadowska, U.; Szczepanowicz, K.; Barbasz, J.; Warszyński, P. Linseed Oil Based Nanocapsules as Delivery System for Hydrophobic Quantum Dots. Colloids Surf. B Biointerfaces 2013, 110, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; García-Gabilondo, M.; Rosell, A.; Roig, A. MRI/Photoluminescence Dual-Modal Imaging Magnetic PLGA Nanocapsules for Theranostics. Pharmaceutics 2020, 12, 16. [Google Scholar] [CrossRef] [Green Version]
- Nandwana, V.; Singh, A.; You, M.M.; Zhang, G.; Higham, J.; Zheng, T.S.; Li, Y.; Prasad, P.V.; Dravid, V.P. Magnetic lipid nanocapsules (MLNCs): Self-assembled lipid-based nanoconstruct for non-invasive theranostic applications. J. Mater. Chem. B 2018, 6, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.H.; Chen, S.Y.; Gao, X. Multifunctional nanocapsules for simultaneous encapsulation of hydrophilic and hydrophobic compounds and on-demand release. ACS Nano 2012, 6, 2558–2565. [Google Scholar] [CrossRef]
- Nishimura, T.; Akiyoshi, K. Biotransporting Biocatalytic Reactors toward Therapeutic Nanofactories. Adv. Sci. 2018, 5, 1800801. [Google Scholar] [CrossRef] [Green Version]
- Bazylińska, U.; Warszyński, P.; Wilk, K.A. Influence of pH upon in vitro sustained dye-release from oil-core nanocapsules with multilayer shells. Colloids Surfaces A Physicochem. Eng. Asp. 2012, 413, 266–272. [Google Scholar] [CrossRef]
- Gaber, M.; Hany, M.; Mokhtar, S.; Helmy, M.W.; Elkodairy, K.A.; Elzoghby, A.O. Boronic-targeted albumin-shell oily-core nanocapsules for synergistic aromatase inhibitor/herbal breast cancer therapy. Mater. Sci. Eng. C 2019, 105, 110099. [Google Scholar] [CrossRef]
- Bzowska, M.; Karabasz, A.; Szczepanowicz, K. Encapsulation of camptothecin into pegylated polyelectrolyte nanocarriers. Colloids Surf. A Physicochem. Eng. Asp. 2018, 557, 36–42. [Google Scholar] [CrossRef]
- Shi, C.; Zhong, S.; Sun, Y.; Xu, L.; He, S.; Dou, Y.; Zhao, S.; Gao, Y.; Cui, X. Sonochemical preparation of folic acid-decorated reductive-responsive epsilon-poly-l-lysine-based microcapsules for targeted drug delivery and reductive-triggered release. Mater. Sci. Eng. C 2020, 106, 110251. [Google Scholar] [CrossRef]
- Bazylińska, U.; Pietkiewicz, J.; Saczko, J.; Nattich-Rak, M.; Rossowska, J.; Garbiec, A.; Wilk, K.A. Nanoemulsion-templated multilayer nanocapsules for cyanine-type photosensitizer delivery to human breast carcinoma cells. Eur. J. Pharm. Sci. 2012, 47, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Bazylińska, U.; Kulbacka, J.; Wilk, K.A. Dicephalic ionic surfactants in fabrication of biocompatible nanoemulsions: Factors influencing droplet size and stability. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 312–320. [Google Scholar] [CrossRef]
- Bazylińska, U.; Skrzela, R.; Piotrowski, K.; Szczepanowicz, K.; Warszyński, P.; Wilk, K.A. Influence of dicephalic ionic surfactant interactions with oppositely charged polyelectrolyte upon the in vitro dye release from oil core nanocapsules. Bioelectrochemisty 2012, 87, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Szczepanowicz, K.; Bazylińska, U.; Pietkiewicz, J.; Szyk-Warszyńska, L.; Wilk, K.A.; Warszyński, P. Biocompatible long-sustained release oil-core polyelectrolyte nanocarriers: From controlling physical state and stability to biological impact. Adv. Colloid Interface Sci. 2015, 222, 678–691. [Google Scholar] [CrossRef] [PubMed]
- Sharipova, A.; Aidarova, S.; Cernoch, P.; Miller, R. Effect of surfactant hydrophobicity on the interfacial properties of polyallylamine hydrochloride/sodium alkylsulphate at water/hexane interface. Colloids Surf. A Physicochem. Eng. Asp. 2013, 438, 141–147. [Google Scholar] [CrossRef]
- Bain, C.D.; Claesson, P.M.; Langevin, D.; Meszaros, R.; Nylander, T.; Stubenrauch, C.; Titmuss, S.; von Klitzing, R. Complexes of surfactants with oppositely charged polymers at surfaces and in bulk. Adv. Colloid Interface Sci. 2010, 155, 32–49. [Google Scholar] [CrossRef]
- Kogej, K. Association and structure formation in oppositely charged polyelectrolyte–surfactant mixtures. Adv. Colloid Interface Sci. 2010, 158, 68–83. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.; Yuan, W. An Overview of Pickering Emulsions: Solid-Particle Materials, Classification, Morphology, and Applications. Front. Pharmacol. 2017, 8, 287. [Google Scholar] [CrossRef] [Green Version]
- Monegier du Sorbier, Q.; Aimable, A.; Pagnoux, C. Influence of the electrostatic interactions in a Pickering emulsion polymerization for the synthesis of silica-polystyrene hybrid nanoparticles. J. Colloid Interface Sci. 2015, 448, 306–314. [Google Scholar] [CrossRef]
- Albert, C.; Beladjine, M.; Tsapis, N.; Fattal, E.; Agnely, F.; Huang, N. Pickering emulsions: Preparation processes, key parameters governing their properties and potential for pharmaceutical applications. J. Control. Release 2019, 309, 302–332. [Google Scholar] [CrossRef]
- Gonzalez Ortiz, D.; Pochat-Bohatier, C.; Cambedouzou, J.; Bechelany, M.; Miele, P. Current Trends in Pickering Emulsions: Particle Morphology and Applications. Engineering 2020, 6, 468–482. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadros, T.; Levecke, B.; Booten, K. Polymeric surfactants and their applications: Steric, emulsions, and suspension stabilization. In Handbook of Detergents, Part E: Applications, 1st ed.; Zoller, U., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 345–348. [Google Scholar]
- Szafraniec, J.; Janik, M.; Odrobińska, J.; Zapotoczny, S. Nanocapsules templated on liquid cores stabilized by graft amphiphilic polyelectrolytes. Nanoscale 2015, 7, 5525–5536. [Google Scholar] [CrossRef] [PubMed]
- Szafraniec, J.; Odrobińska, J.; Lachowicz, D.; Kania, G.; Zapotoczny, S. Chitosan-based nanocapsules of core-shell architecture. Polimery 2017, 62, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Rymarczyk-Machał, M.; Szafraniec, J.; Zapotoczny, S.; Nowakowska, M. Photoactive graft amphiphilic polyelectrolyte: Facile synthesis, intramolecular aggregation and photosensitizing activity. Eur. Polym. J. 2014, 55, 76. [Google Scholar] [CrossRef]
- Szafraniec, J.; Odrobińska, J.; Zapotoczny, S. Polymeric nanocapsules templated on liquid cores as efficient photoreactors. RSC Adv. 2016, 6, 31290–31300. [Google Scholar] [CrossRef]
- Kumar, D.; Pandey, J.; Raj, V.; Kumar, P. A Review on the Modification of Polysaccharide through Graft Copolymerization for Various Potential Applications. Open Med Chem. J. 2017, 11, 109–126. [Google Scholar] [CrossRef]
- Podgórna, K.; Jankowska, K.; Szczepanowicz, K. Polysaccharide gel nanoparticles modified by the Layer-by-Layer technique for biomedical applications. Colloids Surf. A Physicochem. Eng. Asp. 2017, 519, 192–198. [Google Scholar] [CrossRef]
- Wang, H.; Yan, X.; Liang Li, G.; Pilz-Allen, C.; Möhwald, H.; Shchukin, D. Sono-Assembly of Highly Biocompatible Polysaccharide Capsules for Hydrophobic Drug Delivery. Adv. Healthc. Mater. 2014, 3, 825–831. [Google Scholar] [CrossRef]
- Tekie, F.S.M.; Kiani, M.; Zakerian, A.; Pilevarian, F.; Assali, A.; Soleimani, M.; Dinarvand, R.; Arefian, E.; Atashi, A.; Amini, M. Nano polyelectrolyte complexes of carboxymethyl dextran and chitosan to improve chitosan-mediated delivery of miR-145. Carbohydr. Polym. 2017, 159, 66–75. [Google Scholar] [CrossRef]
- Ismail, M.; Du, Y.; Ling, L.; Li, X. Artesunate-heparin conjugate based nanocapsules with improved pharmacokinetics to combat malaria. Int. J. Pharm. 2019, 562, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Belbekhouche, S.; Oniszczuk, J.; Pawlak, A.; El Joukhar, I.; Goffin, A.; Varrault, G.; Sahali, D.; Carbonnier, B. Cationic poly(cyclodextrin)/alginate nanocapsules: From design to application as effcient delivery vehicle of 4-hydroxy tamoxifen to podocyte in vitro. Colloids Surf. B Biointerfaces 2019, 179, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef]
- Du, X.; Liu, Y.; Yan, H.; Rafique, M.; Li, S.; Shan, X.; Wu, L.; Qiao, M.; Kong, D.; Wang, L. Anti-Infective and Pro-Coagulant Chitosan-Based Hydrogel Tissue Adhesive for Sutureless Wound Closure. Biomacromolecules 2020, 21, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Saharian, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef] [PubMed]
- Szafraniec, J.; Błażejczyk, A.; Kus, E.; Janik, M.; Zając, G.; Wietrzyk, J.; Chlopicki, S.; Zapotoczny, S. Robust oil-core nanocapsules with hyaluronate-based shells as promising nanovehicles for lipophilic compounds. Nanoscale 2017, 9, 18867–18880. [Google Scholar] [CrossRef]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M.; Ferrari, F. Hyaluronic acid and chitosan-based nanosystems: A new dressing generation for wound care. Expert Opin. Drug Deliv. 2019, 16, 715–740. [Google Scholar] [CrossRef]
- Graca, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic acid—Based wound dressings: A review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef]
- Wang, W.; Gaus, K.; Tilley, R.D.; Gooding, J.J. The impact of nanoparticle shape on cellular internalisation and transport: What do the different analysis methods tell us? Mater. Horiz. 2019, 6, 1538–1547. [Google Scholar] [CrossRef]
- Kuehl, C.; Zhang, Z.; Kaminskas, L.M.; Porter, C.J.H.; Davies, N.M.; Forrest, L.; Berkland, C. Hyaluronic Acid Molecular Weight Determines Lung Clearance and Biodistribution after Instillation. Mol. Pharm. 2016, 13, 1904–1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, J.D.; Horton, M.R. Threat matrix: Low-molecular-weight hyaluronan (HA) as a danger signal. Immunol. Res. 2005, 31, 207–218. [Google Scholar] [CrossRef]
- Stern, R.; Asari, A.A.; Sugahara, K.N. Hyaluronan fragments: An information-rich system. Eur. J. Cell Biol. 2006, 85, 699–715. [Google Scholar] [CrossRef] [PubMed]
- Greish, K. Enhanced permeability and retention effect for selective targeting of anticancer nanomedicine: Are we there yet? Drug Discov. Today Technol. 2012, 9, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Fadaka, A.; Ajiboye, B.; Ojo, O.; Adewale, O.; Olayide, I.; Emuowhochere, R. Biology of glucose metabolization in cancer cells. J. Oncol. Sci. 2017, 3, 45–51. [Google Scholar] [CrossRef]
- Srinivasarao, M.; Low, P.S. Ligand-Targeted Drug Delivery. Chem. Rev. 2017, 117, 12133–12164. [Google Scholar] [CrossRef]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef]
- Lopes, C.M.; Barata, P.; Oliveira, R. Stimuli-responsive nanosystems for drug-targeted delivery. In Drug Targeting and Stimuli Sensitive Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 155–209. ISBN 978012813689. [Google Scholar]
- Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Matsumura, Y.; Maruo, K.; Kimura, M.; Yamamoto, T.; Konno, T.; Maeda, H. Kinin-generating Cascade in Advanced Cancer Patients and in vitro Study. Jpn. J. Cancer Res. 1991, 82, 732–741. [Google Scholar] [CrossRef]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.G.; Palade, G.E. Neovasculature Induced by Vascular Endothelial Growth Factor Is Fenestrated. Cancer Res. 1997, 57, 765–772. [Google Scholar] [PubMed]
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Golombek, S.K.; May, J.N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Sawa, T.; Konno, T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J. Control. Release 2001, 74, 47–61. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The entry of nanoparticles into solid tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef]
- Szczęch, M.; Szczepanowicz, K. Polymeric core-shell nanoparticles prepared by spontaneous emulsification solvent evaporation and functionalized by the layer-by-layer method. Nanomaterials 2020, 10, 496. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.A.; Gadde, S.; Pfirschke, C.; Engblom, C.; Sprachman, M.M.; Kohler, R.H.; Yang, K.S.; Laughney, A.M.; Wojtkiewicz, G.; Kamaly, N.; et al. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Sci. Transl. Med. 2015, 7, 314ra183. [Google Scholar] [CrossRef] [Green Version]
- Zhan, Y.; Gonçalves, M.; Yi, P.; Capelo, D.; Zhang, Y.; Rodrigues, J.; Liu, C.; Tomás, H.; Li, Y.; He, P. Thermo/redox/pH-triple sensitive poly(N-isopropylacrylamide-co-acrylic acid) nanogels for anticancer drug delivery. J. Mater. Chem. B 2015, 3, 4221–4230. [Google Scholar] [CrossRef] [PubMed]
- Ang, C.Y.; Tan, S.Y.; Teh, C.; Lee, J.M.; Wong, M.F.E.; Qu, Q.; Poh, L.Q.; Li, M.; Zhang, Y.; Korzh, V.; et al. Redox and pH Dual Responsive Polymer Based Nanoparticles for in Vivo Drug Delivery. Small 2017, 13, 1602379. [Google Scholar] [CrossRef] [PubMed]
- Don, T.M.; Lu, K.Y.; Lin, L.J.; Hsu, C.H.; Wu, J.Y.; Mi, F.L. Temperature/pH/Enzyme Triple-Responsive Cationic Protein/PAA-b-PNIPAAm Nanogels for Controlled Anticancer Drug and Photosensitizer Delivery against Multidrug Resistant Breast Cancer Cells. Mol. Pharm. 2017, 14, 4648–4660. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bui, Q.N.; Duy, L.T.M.; Yang, H.Y.; Lee, D.S. One-Step Preparation of pH-Responsive Polymeric Nanogels as Intelligent Drug Delivery Systems for Tumor Therapy. Biomacromolecules 2018, 19, 2062–2071. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, H.Y.; Thambi, T.; Park, J.H.; Lee, D.S. Charge-convertible polymers for improved tumor targeting and enhanced therapy. Biomaterials 2019, 217, 119299. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Luo, L.; Wang, Y.; Wu, Q.; Dai, H.B.; Li, J.S.; Durkan, C.; Wang, N.; Wang, G.X. Endogenous pH-responsive nanoparticles with programmable size changes for targeted tumor therapy and imaging applications. Theranostics 2018, 8, 3038–3058. [Google Scholar] [CrossRef]
- Li, Z.; Wu, M.; Bai, H.; Liu, X.; Tang, G. Light-enhanced hypoxia-responsive nanoparticles for deep tumor penetration and combined chemo-photodynamic therapy. Chem. Commun. 2018, 54, 13127–13130. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef] [Green Version]
- Guan, X.; Li, Y.; Jiao, Z.; Lin, L.; Chen, J.; Guo, Z.; Tian, H.; Chen, X. Codelivery of antitumor drug and gene by a ph-sensitive charge-conversion system. ACS Appl. Mater. Interfaces 2015, 7, 3207–3215. [Google Scholar] [CrossRef]
- Yuan, Y.Y.; Mao, C.Q.; Du, X.J.; Du, J.Z.; Wang, F.; Wang, J. Surface charge switchable nanoparticles based on zwitterionic polymer for enhanced drug delivery to tumor. Adv. Mater. 2012, 24, 5476–5480. [Google Scholar] [CrossRef]
- Han, S.S.; Li, Z.Y.; Zhu, J.Y.; Han, K.; Zeng, Z.Y.; Hong, W.; Li, W.X.; Jia, H.Z.; Liu, Y.; Zhuo, R.X.; et al. Dual-pH sensitive charge-reversal polypeptide micelles for tumor-triggered targeting uptake and nuclear drug delivery. Small 2015, 11, 2543–2554. [Google Scholar] [CrossRef] [PubMed]
- Alkanawati, M.S.; da Costa Marques, R.; Mailänder, V.; Landfester, K.; Thérien-Aubin, H. Polysaccharide-Based pH-Responsive Nanocapsules Prepared with Bio-Orthogonal Chemistry and Their Use as Responsive Delivery Systems. Biomacromolecules 2020, 21, 2764–2771. [Google Scholar] [CrossRef] [PubMed]
- Benito, J.M.; Gómez-García, M.; Ortiz Mellet, C.; Baussanne, I.; Defaye, J.; García Fernández, J.M. Optimizing saccharide-directed molecular delivery to biological receptors: Design, synthesis, and biological evaluation of glycodendrimer-cyclodextrin conjugates. J. Am. Chem. Soc. 2004, 126, 10355–10363. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, O.; Ming, X.; Huang, L.; Juliano, R.L. Targeted intracellular delivery of antisense oligonucleotides via conjugation with small-molecule ligands. J. Am. Chem. Soc. 2010, 132, 8848–8849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Xia, J.; Liu, S.; Stein, S.; Ramon, C.; Xi, H.; Wang, L.; Xiong, X.; Zhang, L.; He, D.; et al. Endocytosis and membrane receptor internalization: Implication of F-BAR protein Carom. Front. Biosci. Landmark 2017, 22, 1439–1457. [Google Scholar] [CrossRef] [Green Version]
- Sapra, P.; Allen, T.M. Ligand-targeted liposomal anticancer drugs. Prog. Lipid Res. 2003, 42, 439–462. [Google Scholar] [CrossRef]
- Nateghian, N.; Goodarzi, N.; Amini, M.; Atyabi, F.; Khorramizadeh, M.R.; Dinarvand, R. Biotin/Folate-decorated Human Serum Albumin Nanoparticles of Docetaxel: Comparison of Chemically Conjugated Nanostructures and Physically Loaded Nanoparticles for Targeting of Breast Cancer. Chem. Biol. Drug Des. 2016, 87, 69–82. [Google Scholar] [CrossRef]
- Hatami, E.; Mu, Y.; Shields, D.N.; Chauhan, S.C.; Kumar, S.; Cory, T.J.; Yallapu, M.M. Mannose-decorated hybrid nanoparticles for enhanced macrophage targeting. Biochem. Biophys. Rep. 2019, 17, 197–207. [Google Scholar] [CrossRef]
- Guhagarkar, S.A.; Majee, S.B.; Samad, A.; Devarajan, P.V. Evaluation of pullulan-functionalized doxorubicin nanoparticles for asialoglycoprotein receptor-mediated uptake in Hep G2 cell line. Cancer Nanotechnol. 2011, 2, 49–55. [Google Scholar] [CrossRef]
- Shargh, V.H.; Hondermarck, H.; Liang, M. Antibody-targeted biodegradable nanoparticles for cancer therapy. Nanomedicine 2016, 11, 63–79. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D.; Ma, Y.; Zhao, Q.; Fallon, J.K.; Liu, D.; Xu, X.E.; Wang, Y.; He, Z.; Liu, F. Theranostic nanoemulsions: Codelivery of hydrophobic drug and hydrophilic imaging probe for cancer therapy and imaging. Nanomedicine 2014, 9, 2773–2785. [Google Scholar] [CrossRef] [PubMed]
- Parish, C.R.; Snowden, J.M. Lymphocytes express a diverse array of specific receptors for sulfated polysaccharides. Cell. Immunol. 1985, 91, 201–214. [Google Scholar] [CrossRef]
- van Kooyk, Y. C-type lectins on dendritic cells: Key modulators for the induction of immune responses. Biochem. Soc. Trans. 2008, 36, 1478–1481. [Google Scholar] [CrossRef] [PubMed]
- Janik-Hazuka, M.; Szafraniec-Szczęsny, J.; Kamiński, K.; Odrobińska, J.; Zapotoczny, S. Uptake and in vitro anticancer activity of oleic acid delivered in nanocapsules stabilized by amphiphilic derivatives of hyaluronic acid and chitosan. Int. J. Biol. Macromolecul. 2020, 164, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Bazylińska, U.; Saczko, J. Nanoemulsion-templated polylelectrolyte multifunctional nanocapsules for DNA entrapment and bioimaging. Colloids Surfaces B Biointerfaces 2016, 137, 191–202. [Google Scholar] [CrossRef]
- Bazylińska, U.; Pietkiewicz, J.; Rossowska, J.; Chodaczek, G.; Gamian, A.; Wilk, K.A. Polyelectrolyte Oil-Core Nanocarriers for Localized and Sustained Delivery of Daunorubicin to Colon Carcinoma MC38 Cells: The Case of Polysaccharide Multilayer Film in Relation to PEG-ylated Shell. Macromol. Biosci. 2017, 17, 54–66. [Google Scholar] [CrossRef]
- Bazylińska, U.; Wawrzyńczyk, D.; Kulbacka, J.; Fraçkowiak, R.; Cichy, B.; Bednarkiewicz, A.; Samoć, M.; Wilk, K.A. Polymeric nanocapsules with up-converting nanocrystals cargo make ideal fluorescent bioprobes. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Martins, J.G.; de Oliveira, A.C.; Garcia, P.S.; Kipper, M.J.; Martins, A.F. Durable pectin/chitosan membranes with self-assembling, water resistance and enhanced mechanical properties. Carbohydr. Polym. 2018, 188, 136–142. [Google Scholar] [CrossRef]
- Ventura, C.A.; Cannavà, C.; Stancanelli, R.; Paolino, D.; Cosco, D.; La Mantia, A.; Pignatello, R.; Tommasini, S. Gemcitabine-loaded chitosan microspheres. Characterization and biological in vitro evaluation. Biomed. Microdevices 2011, 13, 799–807. [Google Scholar] [CrossRef]
- Perrault, S.D.; Chan, W.C.W. In vivo assembly of nanoparticle components to improve targeted cancer imaging. Proc. Natl. Acad. Sci. USA 2010, 107, 11194–11199. [Google Scholar] [CrossRef] [Green Version]
- Lachowicz, D.; Szpak, A.; Malek-Zietek, K.E.; Kepczynski, M.; Muller, R.N.; Laurent, S.; Nowakowska, M.; Zapotoczny, S. Biocompatible and fluorescent superparamagnetic iron oxide nanoparticles with superior magnetic properties coated with charged polysaccharide derivatives. Colloids Surf. B Biointerfaces 2017, 150, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.K.H.; Ho, D. Cancer nanomedicine: From drug delivery to imaging. Sci. Transl. Med. 2013, 5, 216. [Google Scholar] [CrossRef] [PubMed]
- Bazylińska, U.; Drozdek, S.; Nyk, M.; Kulbacka, J.; Samoć, M.; Wilk, K.A. Core/shell quantum dots encapsulated in biocompatible oil-core nanocarriers as two-photon fluorescent markers for bioimaging. Langmuir 2014, 30, 14931–14943. [Google Scholar] [CrossRef] [PubMed]
- Kircher, M.F.; De La Zerda, A.; Jokerst, J.V.; Zavaleta, C.L.; Kempen, P.J.; Mittra, E.; Pitter, K.; Huang, R.; Campos, C.; Habte, F.; et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat. Med. 2012, 18, 829–834. [Google Scholar] [CrossRef]
- Tirotta, I.; Dichiarante, V.; Pigliacelli, C.; Cavallo, G.; Terraneo, G.; Bombelli, F.B.; Metrangolo, P.; Resnati, G. 19F magnetic resonance imaging (MRI): From design of materials to clinical applications. Chem. Rev. 2015, 115, 1106–1129. [Google Scholar] [CrossRef] [PubMed]
- Krafft, M.P. Large organized surface domains self-assembled from nonpolar amphiphiles. Acc. Chem. Res. 2012, 45, 514–524. [Google Scholar] [CrossRef]
- Cametti, M.; Crousse, B.; Metrangolo, P.; Milani, R.; Resnati, G. The fluorous effect in biomolecular applications. Chem. Soc. Rev. 2012, 41, 31–42. [Google Scholar] [CrossRef]
- Koshkina, O.; White, P.B.; Staal, A.H.J.; Schweins, R.; Swider, E.; Tirotta, I.; Tinnemans, P.; Fokkink, R.; Veltien, A.; Koen Van Riessen, N.; et al. Nanoparticles for “two color” 19 F magnetic resonance imaging: Towards combined imaging of biodistribution and degradation. J. Colloid Interface Sci. 2019, 565, 278–287. [Google Scholar] [CrossRef]
- Czyzynska-Cichon, I.; Janik-Hazuka, M.; Szafraniec-Szczęsny, J.; Jasinski, K.; Węglarz, W.P.; Zapotoczny, S.; Chlopicki, S. Low dose curcumin administered in hyaluronic acid–based nanocapsules induces hypotensive effect in hypertensive rats. in review.
- Koutsokeras, A.; Purkayastha, N.; Rigby, A.; Subang, M.C.; Sclanders, M.; Vessillier, S.; Mullen, L.; Chernajovsky, Y.; Gould, D. Generation of an efficiently secreted, cell penetrating NF-κB inhibitor. FASEB J. 2014, 28, 373–381. [Google Scholar] [CrossRef]
- Kong, S.D.; Lee, J.; Ramachandran, S.; Eliceiri, B.P.; Shubayev, V.I.; Lal, R.; Jin, S. Magnetic targeting of nanoparticles across the intact blood-brain barrier. J. Control. Release 2012, 164, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorin, D.A.; Portnov, S.A.; Inozemtseva, O.A.; Luklinska, Z.; Yashchenok, A.M.; Pavlov, A.M.; Skirtach, A.G.; Möhwald, H.; Sukhorukov, G.B. Magnetic/gold nanoparticle functionalized biocompatible microcapsules with sensitivity to laser irradiation. Phys. Chem. Chem. Phys. 2008, 10, 6899–6905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, M.; Katagiri, K.; Koumoto, K. Preparation of hybrid hollow capsules formed with Fe3O4 and polyelectrolytes via the layer-by-layer assembly and the aqueous solution process. J. Colloid Interface Sci. 2010, 341, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, A.M.; Gabriel, S.A.; Sukhorukov, G.B.; Gould, D.J. Improved and targeted delivery of bioactive molecules to cells with magnetic layer-by-layer assembled microcapsules. Nanoscale 2015, 7, 9686–9693. [Google Scholar] [CrossRef]
- Ai, H. Layer-by-layer capsules for magnetic resonance imaging and drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 772–788. [Google Scholar] [CrossRef]
- Kolesnikova, T.A.; Akchurin, G.G.; Portnov, S.A.; Khomutov, G.B.; Akchurin, G.G.; Naumova, O.G.; Sukhorukov, G.B.; Gorin, D.A. Visualization of magnetic microcapsules in liquid by optical coherent tomography and control of their arrangement via external magnetic field. Laser Phys. Lett. 2012, 9, 643–648. [Google Scholar] [CrossRef]
- Yi, Q.; Li, D.; Lin, B.; Pavlov, A.M.; Gong, Q.; Song, B.; Ai, H.; Sukhorukov, G.B. Magnetic Resonance Imaging for Monitoring of Magnetic Polyelectrolyte Capsule in Vivo Delivery. Bionanoscience 2014, 4, 59–70. [Google Scholar] [CrossRef]
- Cristofolini, L.; Szczepanowicz, K.; Orsi, D.; Rimoldi, T.; Albertini, F.; Warszyński, P. Hybrid Polyelectrolyte/Fe3O4 Nanocapsules for Hyperthermia Applications. ACS Appl. Mater. Interfaces 2016, 8, 25043–25050. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Sukhorukov, G.B.; Möhwald, H. Smart inorganic/organic nanocomposite hollow microcapsules. Angew. Chem. Int. Ed. 2003, 42, 4472–4475. [Google Scholar] [CrossRef]
- Hu, S.; Tsai, C.; Liao, C.; Liu, D.; Chen, S. Controlled Rupture of Magnetic Polyelectrolyte Microcapsules for Drug Delivery. Langmuir 2008, 24, 11811–11818. [Google Scholar] [CrossRef]
- Szczepanowicz, K.; Warszyński, P. Magnetically responsive liquid core polyelectrolyte nanocapsules. J. Microencapsul. 2015, 32, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Carregal-Romero, S.; Guardia, P.; Yu, X.; Hartmann, R.; Pellegrino, T.; Parak, W.J. Magnetically triggered release of molecular cargo from iron oxide nanoparticle loaded microcapsules. Nanoscale 2015, 7, 570–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szczepanowicz, K.; Piechota, P.; Weglarz, W.P.; Warszyński, P. Polyelectrolyte nanocapsules containing iron oxide nanoparticles as MRI detectable drug delivery system. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 532, 351–356. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Radtchenko, I.L.; Sukhorukov, G.B. Synthesis of nanosized magnetic ferrite particles inside hollow polyelectrolyte capsules. J. Phys. Chem. B 2003, 107, 86–90. [Google Scholar] [CrossRef]
- Guo, J.; Yang, W.; Deng, Y.; Wang, C.; Fu, S. Organic-dye-coupled magnetic nanoparticles encaged inside thermoresponsive PNIPAM microcapsules. Small 2005, 1, 737–743. [Google Scholar] [CrossRef]
- Du, P.; Mu, B.; Wang, Y.; Shi, H.; Xue, D.; Liu, P. Facile approach for temperature-responsive polymeric nanocapsules with movable magnetic cores. Mater. Lett. 2011, 65, 1579–1581. [Google Scholar] [CrossRef]
- Du, P.; Wang, T.; Liu, P. Double-walled hollow polymeric microspheres with independent pH and temperature dual-responsive and magnetic-targeting function from onion-shaped core-shell structures. Colloids Surf. B Biointerfaces 2013, 102, 1–8. [Google Scholar] [CrossRef]
- Li, A.; Ma, H.; Feng, S.; Liu, J. A copolymer capsule with a magnetic core for hydrophilic or hydrophobic drug delivery via thermo-responsive stimuli or carrier biodegradation. RSC Adv. 2016, 6, 33138–33147. [Google Scholar] [CrossRef]
- Veyret, R.; Delair, T.; Elaissari, A. Preparation and biomedical application of layer-by-layer encapsulated oil in water magnetic emulsion. J. Magn. Magn. Mater. 2005, 293, 171–176. [Google Scholar] [CrossRef]
- Mu, B.; Zhong, W.; Dong, Y.; Du, P.; Liu, P. Encapsulation of drug microparticles with self-assembled Fe3O4/alginate hybrid multilayers for targeted controlled release. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 825–831. [Google Scholar] [CrossRef]
- Pavlov, A.M.; De Geest, B.G.; Louage, B.; Lybaert, L.; De Koker, S.; Koudelka, Z.; Sapelkin, A.; Sukhorukov, G.B. Magnetically engineered microcapsules as intracellular anchors for remote control over cellular mobility. Adv. Mater. 2013, 25, 6945–6950. [Google Scholar] [CrossRef] [PubMed]
- Lepik, K.V.; Muslimov, A.R.; Timim, A.S.; Sergeev, V.S.; Romanyuk, D.S.; Moiseev, I.S.; Popova, E.V.; Radchenko, I.L.; Vilesov, A.D.; Galibin, O.V.; et al. Mesenchymal Stem Cell Magnetization: Magnetic Multilayer Microcapsule Uptake, Toxicity, Impact on Functional Properties, and Perspectives for Magnetic Delivery. Adv. Healthc. Mater. 2016, 5, 3182–3190. [Google Scholar] [CrossRef] [PubMed]
- Vidiasheva, I.V.; Abalymov, A.A.; Kurochkin, M.A.; Mayorova, O.A.; Lomova, M.V.; German, S.V.; Khalenkow, D.N.; Zharkov, M.N.; Gorin, D.A.; Skirtach, A.G.; et al. Transfer of cells with uptaken nanocomposite, magnetite-nanoparticle functionalized capsules with electromagnetic tweezers. Biomater. Sci. 2018, 6, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Podgórna, K.; Szczepanowicz, K. Synthesis of polyelectrolyte nanocapsules with iron oxide (Fe3O4) nanoparticles for magnetic targeting. Colloids Surf. A Physicochem. Eng. Asp. 2016, 505, 132–137. [Google Scholar] [CrossRef]
- Bae, K.H.; Ha, Y.J.; Kim, C.; Lee, K.-R.; Park, T.G. Pluronic/chitosan shell cross-linked nanocapsules encapsulating magnetic nanoparticles. J. Biomater. Sci. Polym. Ed. 2008, 19, 1571–1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, L.; Liu, F.; Lin, S.; Hu, J.; Liu, G.; Yang, Y.; Tu, Y.; Hou, C.; Li, F.; Hu, M.; et al. Superparamagnetic-Oil-Filled Nanocapsules of a Ternary Graft Copolymer. Langmuir 2014, 30, 3996–4004. [Google Scholar] [CrossRef]
- Gumieniczek-Chłopek, E.; Odrobińska, J.; Strączek, T.; Radziszewska, A.; Zapotoczny, S.; Kapusta, C. Hydrophobically coated superparamagnetic iron oxides nanoparticles incorporated into polymer-based nanocapsules dispersed in water. Materials 2020, 13, 1219. [Google Scholar] [CrossRef] [Green Version]
- Odrobińska, J.; Gumieniczek-Chłopek, E.; Szuwarzyński, M.; Radziszewska, A.; Fiejdasz, S.; Straczek, T.; Kapusta, C.; Zapotoczny, S. Magnetically Navigated Core-Shell Polymer Capsules as Nanoreactors Loadable at the Oil/Water Interface. ACS Appl. Mater. Interfaces 2019, 11, 10905–10913. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, H.B.; Koo, H.Y.; Choi, W.S. Remote-Controlled Magnetic Sponge Balls and Threads for Oil/Water Separation in a Confined Space and Anaerobic Reactions. ACS Appl. Mater. Interfaces 2019, 11, 40886–40897. [Google Scholar] [CrossRef]
- Whelehan, M.; von Stockar, U.; Marison, I.W. Removal of pharmaceuticals from water: Using liquid-core microcapsules as a novel approach. Water Res. 2010, 44, 2314–2324. [Google Scholar] [CrossRef]
- Ali, I.; Peng, C.; Naz, I.; Lin, D.; Saroj, D.P.; Ali, M. Development and application of novel bio-magnetic membrane capsules for the removal of the cationic dye malachite green in wastewater treatment. RSC Adv. 2019, 9, 3625–3646. [Google Scholar] [CrossRef] [Green Version]
- Lipshutz, B.H.; Ghorai, S.; Leong, W.W.Y.; Taft, B.R.; Krogstad, D.V. Manipulating micellar environments for enhancing transition metal-catalyzed cross-couplings in water at room temperature. J. Org. Chem. 2011, 76, 5061–5073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolb, G. Review: Microstructured reactors for distributed and renewable production of fuels and electrical energy. Chem. Eng. Process. Process Intensif. 2013, 65, 1–44. [Google Scholar] [CrossRef]
- Ghan, R.; Shutava, T.; Patel, A.; John, V.T.; Lvov, Y. Enzyme-Catalyzed Polymerization of Phenols within Polyelectrolyte Microcapsules. Macromolecules 2004, 37, 4519–4524. [Google Scholar] [CrossRef]
- Price, A.D.; Zelikin, A.N.; Wang, Y.; Caruso, F. Triggered Enzymatic Degradation of DNA within Selectively Permeable Polymer Capsule Microreactors. Angew. Chem. Int. Ed. 2009, 48, 329–332. [Google Scholar] [CrossRef]
- Alford, A.; Kozlovskaya, V.; Xue, B.; Gupta, N.; Higgins, W.; Pham-Hua, D.; He, L.; Urban, V.S.; Tse, H.M.; Kharlampieva, E. Manganoporphyrin-Polyphenol Multilayer Capsules as Radical and Reactive Oxygen Species (ROS) Scavengers. Chem. Mater. 2018, 30, 344–357. [Google Scholar] [CrossRef]
- Tiourina, O.P.; Antipov, A.A.; Sukhorukov, G.B.; Larionova, N.I.; Lvov, Y.; Möhwald, H. Entrapment of α-Chymotrypsin into Hollow Polyelectrolyte Microcapsules. Macromol. Biosci. 2001, 1, 209–214. [Google Scholar] [CrossRef]
- Fischer, A.; Lilienthal, S.; Vázquez-González, M.; Fadeev, M.; Sohn, Y.S.; Nechushtai, R.; Willner, I. Triggered Release of Loads from Microcapsule-in-Microcapsule Hydrogel Microcarriers: En-Route to an ‘artificial Pancreas’. J. Am. Chem. Soc. 2020, 142, 4223–4234. [Google Scholar] [CrossRef]
- Skirtach, A.G.; Antipov, A.A.; Shchukin, D.G.; Sukhorukov, G.B. Remote activation of capsules containing Ag nanoparticles and IR dye by laser light. Langmuir 2004, 20, 6988–6992. [Google Scholar] [CrossRef]
- Kreft, B.O.; Skirtach, A.G.; Sukhorukov, G.B.; Möhwald, H. Remote Control of Bioreactions in Multicompartment Capsules. Adv. Mater. 2007, 19, 3142–3145. [Google Scholar] [CrossRef]
- Skirtach, A.G.; Dejugnat, C.; Braun, D.; Susha, A.S.; Rogach, A.L.; Parak, W.J.; Möhwald, H.; Sukhorukov, G.B. The role of metal nanoparticles in remote release of encapsulated materials. Nano Lett. 2005, 5, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Skirtach, A.G.; Muñoz Javier, A.; Kreft, O.; Köhler, K.; Piera Alberola, A.; Möhwald, H.; Parak, W.J.; Sukhorukov, G.B. Laser-induced release of encapsulated materials inside living cells. Angew. Chem. Int. Ed. 2006, 45, 4612–4617. [Google Scholar] [CrossRef] [PubMed]
- Angelatos, A.S.; Radt, B.; Caruso, F. Light-responsive polyelectrolyte/gold nanoparticle microcapsules. J. Phys. Chem. B 2005, 109, 3071–3076. [Google Scholar] [CrossRef] [PubMed]
- Skirtach, A.G.; De Geest, B.G.; Mamedov, A.; Antipov, A.A.; Kotov, N.A.; Sukhorukov, G.B. Ultrasound stimulated release and catalysis using polyelectrolyte multilayer capsules. J. Mater. Chem. 2007, 17, 1050–1054. [Google Scholar] [CrossRef]
- De Geest, B.G.; Skirtach, A.G.; Mamedov, A.; Antipov, A.A.; Kotov, N.A.; De Smedt, S.C.; Sukhorukov, G.B. Ultrasound-Triggered Release from Multilayered Capsules. Small 2007, 3, 804–808. [Google Scholar] [CrossRef]
- Timin, A.S.; Muslimov, A.R.; Lepik, K.V.; Okilova, M.V.; Tcvetkov, N.Y.; Shakirova, A.I.; Afanasyev, B.V.; Gorin, D.A.; Sukhorukov, G.B. Intracellular Breakable and Ultrasound-Responsive Hybrid Microsized Containers for Selective Drug Release into Cancerous Cells. Part. Part. Syst. Charact. 2017, 34, 1600417. [Google Scholar] [CrossRef]
- Kubiak, T.; Banaszak, J.; Józefczak, A.; Rozynek, Z. Direction-Specific Release from Capsules with Homogeneous or Janus Shells Using an Ultrasound Approach. ACS Appl. Mater. Interfaces 2020, 12, 15810–15822. [Google Scholar] [CrossRef]
- Sukhorukov, G.B.; Antipov, A.A.; Voigt, A.; Donath, E.; Möhwald, H. pH-Controlled Macromolecule Encapsulation in and Release from Polyelectrolyte Multilayer Nanocapsules. Macromol. Rapid Commun. 2001, 22, 44–46. [Google Scholar] [CrossRef]
- Bolinger, P.; Stamou, D.; Vogel, H. Integrated Nanoreactor Systems: Triggering the Release and Mixing of Compounds Inside Single Vesicles. J. Am. Chem. Soc. 2004, 126, 8594–8595. [Google Scholar] [CrossRef]
- Lu, Z.; Prouty, M.D.; Guo, Z.; Golub, V.O.; Kumar, C.S.S.R.; Lvov, Y.M. Magnetic Switch of Permeability for Polyelectrolyte Microcapsules Embedded with Co@Au Nanoparticles. Langmuir 2005, 21, 2042–2050. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Sukhorukov, G.B.; Möhwald, H. Fabrication of Fluorescent Rare Earth Phosphates in Confined Media of Polyelectrolyte Microcapsules. J. Phys. Chem. B 2004, 108, 19109–19113. [Google Scholar] [CrossRef]
- Sharma, V.; Sundaramurthy, A. Reusable Hollow Polymer Microreactors Incorporated with Anisotropic Nanoparticles for Catalysis Application. ACS Omega 2019, 4, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Dong, S.; Zhou, H.; Yang, L.; Yuan, F.; Yang, Y.; Lei, J.; Bao, L.; Bian, L.; Wang, J. n-Dodecanol nanocapsules with supramolecular lock shell layer for thermal energy storage. Chem. Eng. J. 2020, 389, 124483. [Google Scholar] [CrossRef]




| Liquid Core | Shell | Additional Stabilizing Agent | Size | Application | Refs. |
|---|---|---|---|---|---|
| toluene | PAMPS-graft-PVN | - | 50–300 nm | nanocontainers for hydrophobic fluorescent probes | [74] |
| toluene | PAH-graft-PVN | - | 50–100 nm | nanoreactors for photosensitized reactions | [77] |
| oleic acid corn oil | Hyal-C12 | - | 100–350 nm | nanodelivery system (anticancer, anti-hypertensive), encapsulation of contrast agent (19F MRI) | [88,136,151] |
| oleic acid | Chit-C12 | - | 200–600 nm | reloadable, magnetically navigated nanoreactors | [75,179,180] |
| n-octadecane | Hyal-C12, Chit-C12 | - | 300–500 nm | imaging, morphology characterization | [75,88] |
| chloroform | silica | DTSACl a | 70 nm | encapsulation of a hydrophobic fluorescent probes | [25] |
| chloroform | PLL-PGA | AOT b | 100 nm | delivery of hydrophobic drugs, bioavailability improvement | [23,26] |
| linseed oil | PLL-PGA | lecithin | 100 nm | drug delivery system for poorly water-soluble compounds, thus eliminating their potential toxic effects | [52] |
| chloroform | PLL-PGA, Fe2O3 | AOT | 140 nm | platform for multifunctional biomedical applications (controlled release of pharmaceuticals, hyperthermia treatment) | [160,165] |
| oleic acid | PSS, PLL, PDADMAC | C12(TAPAMS)2, c oleic acid | 70–120 nm | pH-responsive and long sustained release nanocapsules | [57] |
| MT d, MBT e | PDADMAC | AOT | 3–4 µm | emulsions of corrosion inhibitors | [27] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szafraniec-Szczęsny, J.; Janik-Hazuka, M.; Odrobińska, J.; Zapotoczny, S. Polymer Capsules with Hydrophobic Liquid Cores as Functional Nanocarriers. Polymers 2020, 12, 1999. https://doi.org/10.3390/polym12091999
Szafraniec-Szczęsny J, Janik-Hazuka M, Odrobińska J, Zapotoczny S. Polymer Capsules with Hydrophobic Liquid Cores as Functional Nanocarriers. Polymers. 2020; 12(9):1999. https://doi.org/10.3390/polym12091999
Chicago/Turabian StyleSzafraniec-Szczęsny, Joanna, Małgorzata Janik-Hazuka, Joanna Odrobińska, and Szczepan Zapotoczny. 2020. "Polymer Capsules with Hydrophobic Liquid Cores as Functional Nanocarriers" Polymers 12, no. 9: 1999. https://doi.org/10.3390/polym12091999
APA StyleSzafraniec-Szczęsny, J., Janik-Hazuka, M., Odrobińska, J., & Zapotoczny, S. (2020). Polymer Capsules with Hydrophobic Liquid Cores as Functional Nanocarriers. Polymers, 12(9), 1999. https://doi.org/10.3390/polym12091999