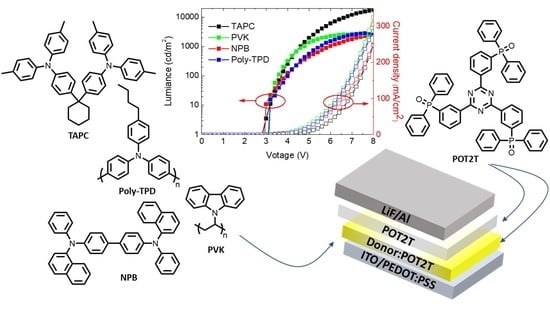
Thermally Activated Delayed Fluorescence in Commercially Available Materials for Solution-Process Exciplex OLEDs
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 2012, 492, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Nobuyasu, R.S.; Ren, Z.; Griffiths, G.C.; Batsanov, A.S.; Data, P.; Yan, S.; Monkman, A.P.; Bryce, M.R.; Dias, F.B. Rational design of TADF polymers using a donor–acceptor monomer with enhanced TADF efficiency induced by the energy alignment of charge transfer and local triplet excited states. Adv. Opt. Mater. 2016, 4, 597–607. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Tsang, D.; Kuwabara, H.; Hatae, Y.; Li, B.; Takahashi, T.; Lee, S.Y.; Yasuda, T.; Adachi, C. Nearly 100% internal quantum efficiency in undoped electroluminescent devices employing pure organic emitters. Adv. Mater. 2015, 27, 2096–2100. [Google Scholar] [CrossRef] [PubMed]
- Kaji, H.; Suzuki, H.; Fukushima, T.; Shizu, K.; Suzuki, K.; Kubo, S.; Komino, T.; Oiwa, H.; Suzuki, F.; Wakamiya, A. Purely organic electroluminescent material realizing 100% conversion from electricity to light. Nat. Commun. 2015, 6, 8476. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.A.; Chatterjee, T.; Tsai, W.L.; Lee, W.K.; Wu, M.J.; Jiao, M.; Pan, K.C.; Yi, C.L.; Chung, C.L.; Wong, K.T. Sky-blue organic light emitting diode with 37% external quantum efficiency using thermally activated delayed fluorescence from spiroacridine-triazine hybrid. Adv. Mater. 2016, 28, 6976–6983. [Google Scholar] [CrossRef]
- Rajamalli, P.; Senthilkumar, N.; Huang, P.-Y.; Ren-Wu, C.-C.; Lin, H.-W.; Cheng, C.-H. New molecular design concurrently providing superior pure blue, thermally activated delayed fluorescence and optical out-coupling efficiencies. J. Am. Chem. Soc. 2017, 139, 10948–10951. [Google Scholar] [CrossRef] [Green Version]
- Maeng, J.H.; Ahn, D.H.; Lee, H.; Jung, Y.H.; Karthik, D.; Lee, J.Y.; Kwon, J.H. Rigid indolocarbazole donor moiety for highly efficient thermally activated delayed fluorescent device. Dye. Pigment. 2020, 180, 108485. [Google Scholar] [CrossRef]
- Xie, F.-M.; Zhou, J.-X.; Li, Y.-Q.; Tang, J.-X. Effects of the relative position and number of donors and acceptors on the properties of TADF materials. J. Mater. Chem. C 2020, 8, 9476–9494. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, Y.; Ren, Z.; Yan, S. The design, synthesis and performance of thermally activated delayed fluorescence macromolecules. Polym. Chem. 2020, 11, 1555–1571. [Google Scholar] [CrossRef]
- Barman, D.; Gogoi, R.; Narang, K.; Iyer, P.K. Recent Developments on Multi-Functional Metal-Free Mechanochromic Luminescence and Thermally Activated Delayed Fluorescence Organic Materials. Front. Chem. 2020, 8, 483. [Google Scholar] [CrossRef]
- Goushi, K.; Yoshida, K.; Sato, K.; Adachi, C. Organic light-emitting diodes employing efficient reverse intersystem crossing for triplet-to-singlet state conversion. Nat. Photonics 2012, 6, 253–258. [Google Scholar] [CrossRef]
- Zhang, T.; Chu, B.; Li, W.; Su, Z.; Peng, Q.M.; Zhao, B.; Luo, Y.; Jin, F.; Yan, X.; Gao, Y. Efficient triplet application in exciplex delayed-fluorescence oleds using a reverse intersystem crossing mechanism based on a δ es–t of around zero. ACS Appl. Mater. Interfaces 2014, 6, 11907–11914. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.-Y.; Fang, G.-C.; Chang, Y.-C.; Kuo, T.-Y.; Chou, P.-T.; Lin, S.-W.; Wong, K.-T. Highly efficient bilayer interface exciplex for yellow organic light-emitting diode. ACS Appl. Mater. Interfaces 2013, 5, 6826–6831. [Google Scholar] [CrossRef] [PubMed]
- Data, P.; Pander, P.; Okazaki, M.; Takeda, Y.; Minakata, S.; Monkman, A.P. Dibenzo [a, j] phenazine-Cored Donor–Acceptor–Donor Compounds as Green-to-Red/NIR Thermally Activated Delayed Fluorescence Organic Light Emitters. Angew. Chem. Int. Ed. 2016, 55, 5739–5744. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, P.L.; Dias, F.B.; Monkman, A.P. Investigation of the mechanisms giving rise to TADF in exciplex states. J. Phys. Chem. C 2016, 120, 18259–18267. [Google Scholar] [CrossRef] [Green Version]
- Hung, W.-Y.; Fang, G.-C.; Lin, S.-W.; Cheng, S.-H.; Wong, K.-T.; Kuo, T.-Y.; Chou, P.-T. The first tandem, all-exciplex-based WOLED. Sci. Rep. 2014, 4, 5161. [Google Scholar] [CrossRef] [PubMed]
- Cherpak, V.; Stakhira, P.; Minaev, B.; Baryshnikov, G.; Stromylo, E.; Helzhynskyy, I.; Chapran, M.; Volyniuk, D.; Hotra, Z.; Dabuliene, A. Mixing of phosphorescent and exciplex emission in efficient organic electroluminescent devices. ACS Appl. Mater. Interfaces 2015, 7, 1219–1225. [Google Scholar] [CrossRef]
- Cherpak, V.; Gassmann, A.; Stakhira, P.; Volyniuk, D.; Grazulevicius, J.V.; Michaleviciute, A.; Tomkeviciene, A.; Barylo, G.; von Seggern, H. Three-terminal light-emitting device with adjustable emission color. Org. Electron. 2014, 15, 1396–1400. [Google Scholar] [CrossRef]
- Volyniuk, D.; Cherpak, V.; Stakhira, P.; Minaev, B.; Baryshnikov, G.; Chapran, M.; Tomkeviciene, A.; Keruckas, J.; Grazulevicius, J.V. Highly efficient blue organic light-emitting diodes based on intermolecular triplet–singlet energy transfer. J. Phys. Chem. C 2013, 117, 22538–22544. [Google Scholar] [CrossRef]
- Pander, P.; Gogoc, S.; Colella, M.; Data, P.; Dias, F.B. Thermally Activated Delayed Fluorescence in Polymer–Small-Molecule Exciplex Blends for Solution-Processed Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 28796–28802. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, C.; Zhou, Y.; Li, C.; Ye, J.; Du, X.; Li, W.; He, Z.; Zhang, M.; Lin, H. Novel small-molecule electron donor for solution-processed ternary exciplex with 24% external quantum efficiency in organic light-emitting diode. Mater. Horiz. 2019, 6, 1425–1432. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, Y.; Guo, J.; Zhang, N.; Zheng, C.; Du, X.; Lin, H.; Tao, S. Study of All Solution Processed Exciplex Organic Light-Emitting Diode. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2021; p. 022014. [Google Scholar]
- Zhong, P.-L.; Zheng, C.-J.; Zhang, M.; Zhao, J.-W.; Yang, H.-Y.; He, Z.-Y.; Lin, H.; Tao, S.-L.; Zhang, X.-H. Highly efficient ternary polymer-based solution-processable exciplex with over 20% external quantum efficiency in organic light-emitting diode. Org. Electron. 2020, 76, 105449. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, H.; Miao, Y.; Wang, Z.; Gao, L.; Wang, H.; Hao, Y.; Li, W. High color stability and CRI (> 80) fluorescent white organic light-emitting diode based pure emission of exciplexes by employing merely complementary colors. J. Mater. Chem. C 2018, 6, 304–311. [Google Scholar] [CrossRef]
- Wei, X.; Gao, L.; Miao, Y.; Zhao, Y.; Yin, M.; Wang, H.; Xu, B. A new strategy for structuring white organic light-emitting diodes by combining complementary emissions in the same interface. J. Mater. Chem. C 2020, 8, 2772–2779. [Google Scholar] [CrossRef]
- Gordon, M. The Exciplex; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Pander, P.; Kudelko, A.; Brzeczek, A.; Wroblowska, M.; Walczak, K. Analysis of exciplex emitters. Disp. Imaging 2017, 2, 265–277. [Google Scholar]
- Świst, A.; Cabaj, J.; Sołoducho, J.; Data, P.; Łapkowski, M. Novel acridone-based branched blocks as highly fluorescent materials. Synth. Met. 2013, 180, 1–8. [Google Scholar] [CrossRef]
- Vamvounis, G.; Aziz, H.; Hu, N.-X.; Popovic, Z.D. Temperature dependence of operational stability of organic light emitting diodes based on mixed emitter layers. Synth. Met. 2004, 143, 69–73. [Google Scholar] [CrossRef]
- Kondakov, D. Characterization of triplet-triplet annihilation in organic light-emitting diodes based on anthracene derivatives. J. Appl. Phys. 2007, 102, 114504. [Google Scholar] [CrossRef]






| Year | Von (V) | EML | CE (cd/A) | PE (lm/W) | EQE (%) | Method | Reference |
|---|---|---|---|---|---|---|---|
| 2018 | 4.9 | PVK:PO-T2T | 13.3 | - | 4.5 | Solution process | [20] |
| 2018 | 3 | TAPC:PO-T2T | 11.8 | 11.8 | 5.1 | Vacuum deposition | [24] |
| 2019 | 2.4 | TPA-3:PO-T2T | 44.8 | 41.5 | 14.4 | Solution process | [21] |
| 2019 | 2.3 | DTF:PO-T2T | 19.7 | 24.7 | 6 | Solution process | [21] |
| 2020 | 3.5 | PVK:PO-T2T | 15 | 7 | 4.75 | Solution process | [22] |
| 2020 | 3.5 | mCP:PO-T2T | 6.7 | 5.4 | 3 | Solution process | [23] |
| 2020 | 3 | PVK:PO-T2T | 14.8 | 9.3 | 4.6 | Solution process | [23] |
| 2020 | 3 | TAPC:PO-T2T | 4.49 | 4.21 | 1.67 | Vacuum deposition | [25] |
| 2021 | 2.8 | TAPC:PO-T2T | 17.2 | 16.9 | 7.1 | Solution process | This work |
| Exciplex | IPD − EAA, eV a | CT, eV b | LT, eV c | ΔEST, eV d |
|---|---|---|---|---|
| TAPC:POT2T | 2.36 | 2.272 | 2.248 | 0.024 |
| PVK:POT2T | 2.50 | 2.399 | 2.382 | 0.017 |
| NPB:POT2T | 2.17 | 2.215 | 2.112 | 0.103 |
| Poly-TPD:POT2T | 2.16 | 2.116 | 2.087 | 0.029 |
| Device | EL (nm) | Turn-On Voltage, at 1 cd/m2 | Maximum EQE/EQE at 10 mA/cm2 (%) | Maximum Power Efficiency/Power Efficiency at 10 mA/cm2 (lm/W) |
|---|---|---|---|---|
| Dev1 | 556 | 2.81 | 7.10/6.53 | 16.9/12.94 |
| Dev2 | 535 | 2.82 | 1.92/1.91 | 4.43/4.16 |
| Dev3 | 593 | 3.01 | 1.10/1.09 | 2.08/1.50 |
| Dev4 | 598 | 3.01 | 1.53/1.49 | 3.56/2.01 |
| Device | Thickness (nm) | Turn-On Voltage, at 1 cd/m2 | Maximum EQE/EQE at 10 mA/cm2 (%) | Maximum Power Efficiency/Power Efficiency at 10 mA/cm2 (lm/W) |
|---|---|---|---|---|
| Dev5 | 43 ± 2 | 3.41 | 6.48/5.68 | 13.51/8.92 |
| Dev6 | 36 ± 2 | 2.81 | 7.10/6.53 | 16.90/12.94 |
| Dev7 | 27 ± 3 | 2.62 | 5.75/5.22 | 16.06/11.49 |
| Dev8 | 14 ± 2 | 2.22 | 4.61/4.37 | 15.10/12.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, Z.-L.; Huang, W.-L.; Yeh, T.-H.; Xu, Y.-X.; Chiang, C.-H. Thermally Activated Delayed Fluorescence in Commercially Available Materials for Solution-Process Exciplex OLEDs. Polymers 2021, 13, 1668. https://doi.org/10.3390/polym13101668
Tseng Z-L, Huang W-L, Yeh T-H, Xu Y-X, Chiang C-H. Thermally Activated Delayed Fluorescence in Commercially Available Materials for Solution-Process Exciplex OLEDs. Polymers. 2021; 13(10):1668. https://doi.org/10.3390/polym13101668
Chicago/Turabian StyleTseng, Zong-Liang, Wei-Lun Huang, Tzu-Hung Yeh, You-Xun Xu, and Chih-Hsun Chiang. 2021. "Thermally Activated Delayed Fluorescence in Commercially Available Materials for Solution-Process Exciplex OLEDs" Polymers 13, no. 10: 1668. https://doi.org/10.3390/polym13101668
APA StyleTseng, Z. -L., Huang, W. -L., Yeh, T. -H., Xu, Y. -X., & Chiang, C. -H. (2021). Thermally Activated Delayed Fluorescence in Commercially Available Materials for Solution-Process Exciplex OLEDs. Polymers, 13(10), 1668. https://doi.org/10.3390/polym13101668