Olive Oil/Pluronic Oleogels for Skin Delivery of Quercetin: In Vitro Characterization and Ex Vivo Skin Permeability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
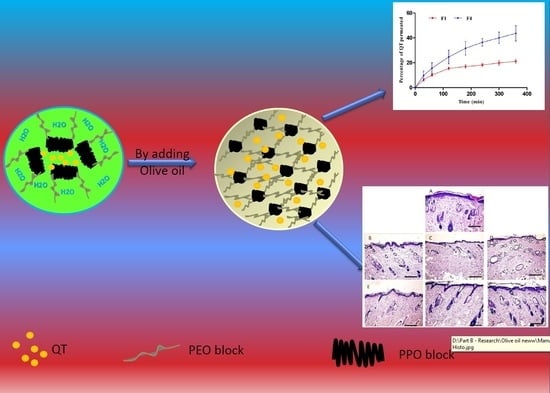
2.2. Preparation of QT-Loaded Pluronic F127/Olive Oil Oleogels
2.3. Particle Size and Zeta Potential
2.4. QT Content
2.5. Organoleptic Characteristics
2.6. pH Determination
2.7. Viscosity Measurement
2.8. In Vitro QT Release
2.9. Accelerated Stability Test
2.10. Differential Scanning Calorimetry (DSC)
2.11. Fourier Transform-Infrared Spectroscopy
2.12. Ex Vivo Skin Permeation
2.13. Permeation Kinetics
2.14. Histological Changes and Safety
2.15. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Formulation of QT Loaded Olive Oil/Pluronic Oleogels
3.2. Particle Size and Zeta Potential
3.3. QT Content and Organoleptic Characteristics
3.4. pH and Viscosity Measurements
3.5. In Vitro Release Studies
3.6. Accelerated Stability Test
3.7. Thermal Analysis
3.8. FT-IR
3.9. Ex Vivo Skin Permeation
3.10. Histopathology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- English, J.S.C.; Dawe, R.S.; Ferguson, J. Environmental effects and skin disease. Br. Med. Bull. 2003, 68, 129–142. [Google Scholar] [CrossRef] [Green Version]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef] [Green Version]
- Čáp, M.; Váchová, L.; Palková, Z. Reactive oxygen species in the signaling and adaptation of multicellular microbial communities. Oxid. Med. Cell Longev. 2012, 2012, 976753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Svobodová, A.; Psotová, J.; Walterová, D. Natural phenolics in the prevention of UV-induced skin damage. A review. Biomed. Pap. 2003, 147, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Pinnell, S.R. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. J. Am. Acad. Dermatol. 2003, 48, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.S.; Ramdath, D.D.; Marshall, J.R.; Isitor, G.N.; Eversley, M.; Xue, S.; Shi, J. Wound-healing activity of the skin of the common grape (Vitis Vinifera) variant, cabernet sauvignon. Phyther. Res. 2010, 24, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Morel, I.; Lescoat, G.; Cogrel, P.; Sergent, O.; Pasdeloup, N.; Brissot, P.; Cillard, P.; Cillard, J. Antioxidant and iron-chelating activities of the flavonoids catechin, quercetin and diosmetin on iron-loaded rat hepatocyte cultures. Biochem. Pharmacol. 1993, 45, 13–19. [Google Scholar] [CrossRef]
- Santos, A.C.; Uyemura, S.A.; Lopes, J.L.C.; Bazon, J.N.; Mingatto, F.E.; Curti, C. Effect of naturally occurring flavonoids on lipid peroxidation and membrane permeability transition in mitochondria. Free Radic. Biol. Med. 1998, 24, 1455–1461. [Google Scholar] [CrossRef]
- Bonina, F.; Lanza, M.; Montenegro, L.; Puglisi, C.; Tomaino, A.; Trombetta, D.; Francesco, C.; Saija, A. Flavonoids as potential protective agents against photo-oxidative skin damage. Int. J. Pharm. 1996, 145, 87–94. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Du, Y.; Takhistov, P.; Michniak-Kohn, B. Formulation optimization and topical delivery of quercetin from solid lipid based nanosystems. Int. J. Pharm. 2013, 441, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Paleco, R.; Vučen, S.R.; Crean, A.M.; Moore, A.; Scalia, S. Enhancement of the in vitro penetration of quercetin through pig skin by combined microneedles and lipid microparticles. Int. J. Pharm. 2014, 472, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Yoo, C.Y.; Park, S.N. Improved stability and skin permeability of sodium hyaluronate-chitosan multilayered liposomes by Layer-by-Layer electrostatic deposition for quercetin delivery. Colloids Surfaces B Biointerfaces 2015, 129, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Díez-Sales, O.; Pons, R.; Fernàndez-Busquets, X.; Fadda, A.M.; Manconi, M. Topical anti-inflammatory potential of quercetin in lipid-based nanosystems: In vivo and in vitro evaluation. Pharm. Res. 2014, 31, 959–968. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.; Liu, T.; Ma, H.; Tian, Y.; Li, L.; Li, Z.; Gao, M.; Zhang, J.; Tang, Z. Preparation of Essential Oil-Based Microemulsions for Improving the Solubility, pH Stability, Photostability, and Skin Permeation of Quercetin. AAPS PharmSciTech 2017, 18, 3097–3104. [Google Scholar] [CrossRef]
- Hughes, N.E.; Marangoni, A.G.; Wright, A.J.; Rogers, M.A.; Rush, J.W.E. Potential food applications of edible oil organogels. Trends Food Sci. Technol. 2009, 20, 470–480. [Google Scholar] [CrossRef]
- Ghosh, S.; Das Mahapatra, R.; Dey, J. Thermoreversible as well as thermoirreversible organogel formation by L-cysteine-based amphiphiles with poly (ethylene glycol) tail. Langmuir 2014, 30, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, V.; Gupta, V.; Ramteke, S.; Trivedi, P. Preparation and evaluation of tubular micelles of pluronic lecithin organogel for transdermal delivery of sumatriptan. Aaps PharmSciTech 2010, 11, 1718–1725. [Google Scholar] [CrossRef] [Green Version]
- Flo, A.; Calpena, A.C.; Halbaut, L.; Araya, E.I.; Fernández, F.; Clares, B. Melatonin delivery: Transdermal and transbuccal evaluation in different vehicles. Pharm. Res. 2016, 33, 1615–1627. [Google Scholar] [CrossRef]
- Mady, F.M.; Essa, H.; El-Ammawi, T.; Abdelkader, H.; Hussein, A.K. Formulation and clinical evaluation of silymarin pluronic-lecithin organogels for treatment of atopic dermatitis. Drug Des. Dev. Ther. 2016, 10, 1101. [Google Scholar]
- Jhawat, V.; Gupta, S.; Saini, V. Formulation and evaluation of novel controlled release of topical pluronic lecithin organogel of mefenamic acid. Drug Deliv. 2016, 23, 3573–3581. [Google Scholar] [CrossRef] [Green Version]
- Viljoen, J.M.; Cowley, A.; Du Preez, J.; Gerber, M.; Du Plessis, J. Penetration enhancing effects of selected natural oils utilized in topical dosage forms. Drug Dev. Ind. Pharm. 2015, 41, 2045–2054. [Google Scholar] [CrossRef] [PubMed]
- Baksi, R.; Singh, D.P.; Borse, S.P.; Rana, R.; Sharma, V.; Nivsarkar, M. In vitro and in vivo anticancer efficacy potential of Quercetin loaded polymeric nanoparticles. Biomed. Pharmacother. 2018, 106, 1513–1526. [Google Scholar] [CrossRef]
- Balata, G.; El Nahas, H.M.; Radwan, S. Propolis organogel as a novel topical delivery system for treating wounds. Drug Deliv. 2014, 21, 55–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanikkannan, N.; Singh, J.; Ramarao, P. In vitro transdermal iontophoretic transport of timolol maleate: Effect of age and species. J. Control. Release 2001, 71, 99–105. [Google Scholar] [CrossRef]
- Plamen, K.; Le, C.A.K.; Halima, R.; Rum, S.; Carla, V.; Haftek, M.; Pirot, F. Organogels for cosmetic and dermo-cosmetic applications. Househ. Pers. Care Today 2015, 10, 16–20. [Google Scholar]
- Kirilov, P.; Rum, S.; Gilbert, E.; Roussel, L.; Salmon, D.; Abdayem, R.; Serre, C.; Villa, C.; Haftek, M.; Falson, F.; et al. Aqueous dispersions of organogel nanoparticles–potential systems for cosmetic and dermo-cosmetic applications. Int. J. Cosmet. Sci. 2014, 36, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Pramanik, K.; Ray, S.S.; Pal, K. Development and characterization of sorbitan monostearate and sesame oil-based organogels for topical delivery of antimicrobials. Aaps PharmSciTech 2015, 16, 293–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, K.; Mahajan, A.; Sharma, G.; Singh, B.; Raza, K.; Chhibber, S.; Katare, O.P. Implementation of Quality by Design (QbD) approach in development of silver sulphadiazine loaded egg oil organogel: An improved dermatokinetic profile and therapeutic efficacy in burn wounds. Int. J. Pharm. 2020, 576, 118977. [Google Scholar] [CrossRef]
- Sharma, G.; Kamboj, S.; Thakur, K.; Negi, P.; Raza, K.; Katare, O.P. Delivery of thermoresponsive-tailored mixed micellar nanogel of lidocaine and prilocaine with improved dermatokinetic profile and therapeutic efficacy in topical anaesthesia. AAPS PharmSciTech 2017, 18, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, M.; Shalaby, K.; Ali, H.M.; Alruwaili, N.K.; Salama, A.; Ibrahim, M.F.; Akl, M.A.; Ahmed, T.A. Impact of nanostructured lipid carriers on dapsone delivery to the skin: In vitro and in vivo studies. Int. J. Pharm. 2019, 572, 118781. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.; Barba, F.; Prieto, M.; Simal-Gandara, J. Bioactive compounds and quality of extra virgin olive oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef]
- Behera, B.; Patil, V.; Sagiri, S.S.; Pal, K.; Ray, S.S. Span-60-based organogels as probable matrices for transdermal/topical delivery systems. J. Appl. Polym. Sci. 2012, 125, 852–863. [Google Scholar] [CrossRef]
- Sharma, P.K.; Matthew, J.E.; Bhatia, S.R. Structure and assembly of PEO-PPO-PEO co-polymers in mammalian cell-culture media. J. Biomater. Sci. Polym. Ed. 2005, 16, 1139–1151. [Google Scholar] [CrossRef]
- Liu, T.; Chu, B. Formation of homogeneous gel-like phases by mixed triblock copolymer micelles in aqueous solution: FCC to BCC phase transition. J. Appl. Crystallogr. 2000, 33, 727–730. [Google Scholar] [CrossRef] [Green Version]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, J.; Lenaerts, V.; Raymond, P.; Ong, H. Diffusion of rat atrial natriuretic factor in thermoreversible poloxamer gels. Biomaterials 1989, 10, 265–268. [Google Scholar] [CrossRef]
- Wright, A.J.; Marangoni, A.G. Formation, structure, and rheological properties of ricinelaidic acid-vegetable oil organogels. J. Am. Oil Chem. Soc. 2006, 83, 497–503. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Fang, C.-L.; Liu, C.-H.; Su, Y.-H. Lipid nanoparticles as vehicles for topical psoralen delivery: Solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC). Eur. J. Pharm. Biopharm. 2008, 70, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Baran, N.; Singh, V.K.; Pal, K.; Anis, A.; Pradhan, D.K.; Pramanik, K. Development and Characterization of Soy Lecithin and Palm Oil-based Organogels. Polym. Plast. Technol. Eng. 2014, 53, 865–879. [Google Scholar] [CrossRef]
- Li, Y.; Xu, R.; Couderc, S.; Bloor, M.; Wyn-Jones, E.; Holzwarth, J.F. Binding of sodium dodecyl sulfate (SDS) to the ABA block copolymer Pluronic F127 (EO97PO69EO97): F127 aggregation induced by SDS. Langmuir 2001, 17, 183–188. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Li, S.; Gao, W. Preparation, physicochemical characterization and in vitro digestibility on solid complex of maize starches with quercetin. LWT Food Sci. Technol. 2011, 44, 787–792. [Google Scholar] [CrossRef]
- Gurdeniz, G.; Tokatli, F.; Ozen, B. Differentiation of mixtures of monovarietal olive oils by mid-infrared spectroscopy and chemometrics. Eur. J. Lipid Sci. Technol. 2007, 109, 1194–1202. [Google Scholar] [CrossRef] [Green Version]
- Elmowafy, M.; Alruwaili, N.K.; Shalaby, K.; Alharbi, K.S.; Altowayan, W.M.; Ahmed, N.; Zafar, A.; Elkomy, M. Long-acting paliperidone parenteral formulations based on polycaprolactone nanoparticles; the influence of stabilizer and chitosan on in vitro release, protein adsorption, and cytotoxicity. Pharmaceutics 2020, 12, 160. [Google Scholar] [CrossRef] [Green Version]
- Satapathy, D.; Biswas, D.; Behera, B.; Sagiri, S.S.; Pal, K.; Pramanik, K. Sunflower-oil-based lecithin organogels as matrices for controlled drug delivery. J. Appl. Polym. Sci. 2013, 129, 585–594. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Ramsey, J.D.; Samadi, A.; Atoufi, Z.; Yazdi, M.K.; Ganjali, M.R.; Amirabad, L.M.; Zangene, E.; Farokhi, M.; Formela, K.; et al. Poloxamer: A versatile tri-block copolymer for biomedical applications. Acta Biomater. 2020, 110, 37–67. [Google Scholar] [CrossRef]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Lobo, J.M.S. Pluronic® F-127 and Pluronic Lecithin Organogel (PLO): Main features and their applications in topical and transdermal administration of drugs. J. Pharm. Pharm. Sci. 2012, 15, 592–605. [Google Scholar] [CrossRef] [Green Version]
- Dhawan, B.; Aggarwal, G.; Harikumar, S.L. Enhanced transdermal permeability of piroxicam through novel nanoemulgel formulation. Int. J. Pharm. Investig. 2014, 4, 65. [Google Scholar]
- Fang, J.-Y.; Hong, C.-T.; Chiu, W.-T.; Wang, Y.-Y. Effect of liposomes and niosomes on skin permeation of enoxacin. Int. J. Pharm. 2001, 219, 61–72. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Muller, R.H.; Shegokar, R.; Keck, C.M. 20 Years of Lipid Nanoparticles (SLN & NLC): Present State of Development & Industrial Applications. Curr. Drug Discov. Technol. 2011, 8, 207–227. [Google Scholar] [PubMed]






| Code | Olive Oil % | Pluronic F127 % | Particle Size (nm) | Zeta Potential (mV) | Drug Content % |
|---|---|---|---|---|---|
| F1 | - | 20 | 345.3 ± 5.3 | −15.1 ± 2.3 | 96.5 ± 2.8 |
| F2 | 5 | 20 | 375.9 ± 5.7 | −19.7 ± 3.7 | 95.1 ± 4.6 |
| F3 | 10 | 20 | 389.4 ± 3.8 | −19.2 ± 2.5 | 95.7 ± 7.3 |
| F4 | 15 | 20 | 372.8 ± 7.9 | −16.1 ± 3.3 | 96.0 ± 9.2 |
| F5 | 20 | 20 | 369.8 ± 8.5 | −17.3 ± 6.1 | 97.1 ± 2.8 |
| F6 | 25 | 20 | 401.5 ± 2.8 | −15.5 ± 1.2 | 96.1 ± 4.9 |
| F7 | 30 | 20 | 392.8 ± 9.7 | −17.2 ± 3.1 | 97.6 ± 8.5 |
| Code | Organoleptic Characteristics | pH | Viscosity (cp) | ||||
|---|---|---|---|---|---|---|---|
| Phase Separation | Greasiness | Grittiness | Consistency | Exudate | |||
| F1 | None | None | None | Viscous, spreadable gel | Nil | 6.6 ± 0.8 | 6367 ± 28 |
| F2 | None | None | None | Viscous, spreadable gel | Nil | 6.5 ± 0.2 | 6241 ± 34 |
| F3 | None | None | None | Less viscous, spreadable gel | Nil | 6.3 ± 0.7 | 5836 ± 27 |
| F4 | None | None | None | Less viscous, spreadable gel | Nil | 6.2 ± 0.4 | 5633 ± 49 |
| F5 | None | None | None | Thin, fluid | Nil | 6.0 ± 0.4 | 5289 ± 23 |
| F6 | None | None | None | Thin, fluid | Nil | 6.0 ± 1.1 | 5003 ± 36 |
| F7 | None | Less observable | None | More thin and fluid | Nil | 5.8 ± 0.6 | 4823 ± 29 |
| Code | Zero Order Kinetics | First Order Kinetics | Higuchi Model | KP Model | ||||
|---|---|---|---|---|---|---|---|---|
| r2 | K0 | r2 | K1 | r2 | KH | r2 | n | |
| F1 | 0.630 | 0.07 | 0.697 | 0.001 | 0.973 | 1.19 | 0.986 | 0.413 |
| F2 | 0.855 | 0.14 | 0.934 | 0.002 | 0.992 | 2.29 | 0.996 | 0.562 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmowafy, M.; Musa, A.; Alnusaire, T.S.; Shalaby, K.; Fouda, M.M.A.; Salama, A.; Al-Sanea, M.M.; Abdelgawad, M.A.; Gamal, M.; Fouad, S.A. Olive Oil/Pluronic Oleogels for Skin Delivery of Quercetin: In Vitro Characterization and Ex Vivo Skin Permeability. Polymers 2021, 13, 1808. https://doi.org/10.3390/polym13111808
Elmowafy M, Musa A, Alnusaire TS, Shalaby K, Fouda MMA, Salama A, Al-Sanea MM, Abdelgawad MA, Gamal M, Fouad SA. Olive Oil/Pluronic Oleogels for Skin Delivery of Quercetin: In Vitro Characterization and Ex Vivo Skin Permeability. Polymers. 2021; 13(11):1808. https://doi.org/10.3390/polym13111808
Chicago/Turabian StyleElmowafy, Mohammed, Arafa Musa, Taghreed S. Alnusaire, Khaled Shalaby, Maged M. A. Fouda, Ayman Salama, Mohammad M. Al-Sanea, Mohamed A. Abdelgawad, Mohammed Gamal, and Shahinaze A. Fouad. 2021. "Olive Oil/Pluronic Oleogels for Skin Delivery of Quercetin: In Vitro Characterization and Ex Vivo Skin Permeability" Polymers 13, no. 11: 1808. https://doi.org/10.3390/polym13111808
APA StyleElmowafy, M., Musa, A., Alnusaire, T. S., Shalaby, K., Fouda, M. M. A., Salama, A., Al-Sanea, M. M., Abdelgawad, M. A., Gamal, M., & Fouad, S. A. (2021). Olive Oil/Pluronic Oleogels for Skin Delivery of Quercetin: In Vitro Characterization and Ex Vivo Skin Permeability. Polymers, 13(11), 1808. https://doi.org/10.3390/polym13111808