Thiophene-Based Trimers and Their Bioapplications: An Overview
Abstract
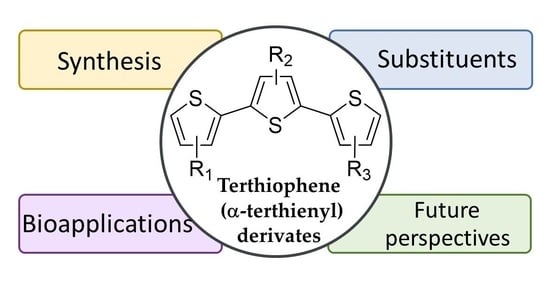
:1. Introduction
2. Synthetic Methodologies
2.1. Carbon–Carbon (C–C) Cross-Coupling Methods
2.1.1. Kumada Reaction
2.1.2. Stille Reaction
2.1.3. Suzuki Reaction
2.2. Ring Closure Reactions
2.2.1. Cyclization of 1,3-diynes
2.2.2. Cyclization of 1,4-diketone
2.3. Other Synthetic Strategies
3. Trimer Structures
3.1. Unsubstituted EDOT-Containing Thiophene-Based Trimers
3.2. Saturated Aliphatic Substituents
3.3. Unsaturated Aliphatic Substituents
3.4. Nitro Groups
3.5. Amines
3.6. Nitriles
3.7. Bromo Groups
3.8. Fluoro, Chloro, and Iodo Groups
3.9. Alcohols
3.10. Ethers
3.11. Thioethers
3.12. Ketones
3.13. Aldehydes
3.14. Carboxylic Acids
3.15. Esters
3.16. Amides
3.17. Fused Aromatics
3.18. Aryl and Heteroaryl Groups
3.19. Thiophene S,S-dioxide
3.20. Metal Complexes
3.21. Charged Trimers
3.22. Crosslinked Trimers
4. Bioapplications
4.1. Terthiophene and Terthiophene Derivatives
4.1.1. Pesticides
4.1.2. Antifungal and Antibacterial Activity
4.1.3. Sensing
4.1.4. Pharmacological Activity
4.2. Polimerized Trimers
4.2.1. Sensing
Poly(amino-terthiophene)s
Poly(acid-terthiophene)s
4.2.2. Antibacterial Activity
4.2.3. Tissue Engineering
4.2.4. Pharmacological Activity
5. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- MacDiarmid, A.G.; Epstein, A.J. Conducting Polymers: Past, Present and Future…. MRS Proc. 1993, 328, 133. [Google Scholar] [CrossRef]
- Mantione, D.; del Agua, I.; Sanchez-Sanchez, A.; Mecerreyes, D. Poly(3,4-ethylenedioxythiophene) (PEDOT) Derivatives: Innovative Conductive Polymers for Bioelectronics. Polymers 2017, 9, 354. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting polymers: a comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar] [CrossRef]
- Malliaras, G.; Abidian, M.R. Organic Bioelectronic Materials and Devices. Adv. Mater. 2015, 27, 7492. [Google Scholar] [CrossRef] [Green Version]
- Gómez, I.J.; Vázquez Sulleiro, M.; Mantione, D.; Alegret, N. Carbon Nanomaterials Embedded in Conductive Polymers: A State of the Art. Polymers 2021, 13, 745. [Google Scholar] [CrossRef]
- Döbbelin, M.; Marcilla, R.; Pozo-Gonzalo, C.; Mecerreyes, D. Innovative materials and applications based on poly(3,4-ethylenedioxythiophene) and ionic liquids. J. Mater. Chem. 2010, 20, 7613–7622. [Google Scholar] [CrossRef]
- Elschner, A.; Kirchmeyer, S.; Lovenich, W.; Merker, U.; Reuter, K. PEDOT; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9780429137389. [Google Scholar]
- Kaloni, T.P.; Giesbrecht, P.K.; Schreckenbach, G.; Freund, M.S. Polythiophene: From Fundamental Perspectives to Applications. Chem. Mater. 2017, 29, 10248–10283. [Google Scholar] [CrossRef]
- Steinkopf, W.; Leitsmann, R.; Hofmann, K.H. Studien in der Thiophenreihe. LVII. Über α-Polythienyle. Justus Liebig’s Ann. Chem. 1941, 546, 180–199. [Google Scholar] [CrossRef]
- Buerle, P.; Becher, J.; Lau, J.; Mark, P. Sulfur-Containing Oligomers. In Electronic Materials: The Oligomer Approach; Wiley-VCH Verlag GmbH: Weinheim, Germany, 1998; pp. 105–233. [Google Scholar]
- Zechmeister, L.; Sease, J.W. A Blue-fluorescing Compound, Terthienyl, Isolated from Marigolds. J. Am. Chem. Soc. 1947, 69, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.K.; Fincher, C.R.; Park, Y.W.; Heeger, A.J.; Shirakawa, H.; Louis, E.J.; Gau, S.C.; MacDiarmid, A.G. Electrical Conductivity in Doped Polyacetylene. Phys. Rev. Lett. 1977, 39, 1098–1101. [Google Scholar] [CrossRef]
- Tamao, K.; Sumitani, K.; Kumada, M. Selective carbon-carbon bond formation by cross-coupling of Grignard reagents with organic halides. Catalysis by nickel-phosphine complexes. J. Am. Chem. Soc. 1972, 94, 4374–4376. [Google Scholar] [CrossRef]
- Yamamoto, T.; Sanechika, K.; Yamamoto, A. Preparation of thermostable and electric-conducting poly(2,5-thienylene). J. Polym. Sci. Polym. Lett. Ed. 1980, 18, 9–12. [Google Scholar] [CrossRef]
- Lin, J.W.-P.; Dudek, L.P. Synthesis and properties of poly(2,5-thienylene). J. Polym. Sci. Polym. Chem. Ed. 1980, 18, 2869–2873. [Google Scholar] [CrossRef]
- Cunningham, D.D.; Laguren-Davidson, L.; Mark, H.B.; Van Pham, C.; Zimmer, H. Synthesis of oligomeric 2,5-thienylenes; their U.V. spectra and oxidation potentials. J. Chem. Soc. Chem. Commun. 1987, 1021–1023. [Google Scholar] [CrossRef]
- Roncali, J.; Giffard, M.; Frère, P.; Jubault, M.; Gorgues, A. Extensively conjugated tetrathiafulvalene (TTF) π-electron donors with oligothiophenes spacer groups. J. Chem. Soc. Chem. Commun. 1993, 689–691. [Google Scholar] [CrossRef]
- Li, Z.H.; Wong, M.S.; Fukutani, H.; Tao, Y. Full Emission Color Tuning in Bis-Dipolar Diphenylamino-Endcapped Oligoarylfluorenes. Chem. Mater. 2005, 17, 5032–5040. [Google Scholar] [CrossRef]
- Yin, B.; Jiang, C.; Wang, Y.; La, M.; Liu, P.; Deng, W. Synthesis and electrochromic properties of oligothiophene derivatives. Synth. Met. 2010, 160, 432–435. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Wang, C.; Li, H.; Lu, H.; Xu, B.; Tian, W. Novel low-bandgap oligothiophene-based donor-acceptor alternating conjugated copolymers: Synthesis, properties, and photovoltaic applications. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2765–2776. [Google Scholar] [CrossRef]
- Guan, L.; Wang, J.; Huang, J.; Jiang, C.; La, M.; Liu, P.; Deng, W. Synthesis and Photovoltaic Properties of Donor–Acceptor Oligothiophene Derivatives Possessing Mesogenic Properties. Synth. Commun. 2011, 41, 3662–3670. [Google Scholar] [CrossRef]
- Yu, J.; Shen, T.-L.; Weng, W.-H.; Huang, Y.-C.; Huang, C.-I.; Su, W.-F.; Rwei, S.-P.; Ho, K.-C.; Wang, L. Molecular Design of Interfacial Modifiers for Polymer-Inorganic Hybrid Solar Cells. Adv. Energy Mater. 2012, 2, 245–252. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Zhang, P.; Li, Y.; Yang, X.; Chen, L.; Tu, Y. Solution-processable tetrazine and oligothiophene based linear A–D–A small molecules: Synthesis, hierarchical structure and photovoltaic properties. Org. Electron. 2013, 14, 1424–1434. [Google Scholar] [CrossRef]
- Grübel, M.; Meister, S.; Schulze, U.; Raftopoulos, K.N.; Baumer, F.; Papadakis, C.M.; Nilges, T.; Rieger, B. Synthesis of Diisocyanate-Containing Thiophenes and Their Use in PDMS-Based Segmented Polymers. Macromol. Chem. Phys. 2016, 217, 59–71. [Google Scholar] [CrossRef]
- Crisp, G.T. Palladium Mediated Formation of Bithiophenes. Synth. Commun. 1989, 19, 307–316. [Google Scholar] [CrossRef]
- Chantarak, S.; Liu, F.; Emrick, T.; Russell, T.P. Solvent-Assisted Orientation of Poly(3-hexylthiophene)-Functionalized CdSe Nanorods Under an Electric Field. Macromol. Chem. Phys. 2014, 215, 1647–1653. [Google Scholar] [CrossRef]
- Kamal, M.R.; Al-taweel, S.A.; El-abadelah, M.M.; Abu Ajaj, K.M. SYNTHESIS OF α-THIOPHENE OLIGOMERS VIA ORGANOTIN COMPOUNDS. Phosphorus. Sulfur. Silicon Relat. Elem. 1997, 126, 65–74. [Google Scholar] [CrossRef]
- Ahn, S.; Yabumoto, K.; Jeong, Y.; Akagi, K. Low bandgap poly(thienylenemethine) derivatives bearing terarylene moieties in the side chains. Polym. Chem. 2014, 5, 6977–6989. [Google Scholar] [CrossRef]
- Amna, B.; Siddiqi, H.M.; Hassan, A.; Ozturk, T. Recent developments in the synthesis of regioregular thiophene-based conjugated polymers for electronic and optoelectronic applications using nickel and palladium-based catalytic systems. RSC Adv. 2020, 10, 4322–4396. [Google Scholar] [CrossRef] [Green Version]
- Lightowler, S.; Hird, M. Monodisperse Aromatic Oligomers of Defined Structure and Large Size through Selective and Sequential Suzuki Palladium-Catalyzed Cross-Coupling Reactions. Chem. Mater. 2005, 17, 5538–5549. [Google Scholar] [CrossRef]
- Yu, M.; Lynch, V.; Pagenkopf, B.L. Intramolecular Cyclopropanation of Glycals: Studies toward the Synthesis of Canadensolide, Sporothriolide, and Xylobovide. Org. Lett. 2001, 3, 2563–2566. [Google Scholar] [CrossRef]
- Hassan Omar, O.; Babudri, F.; Farinola, G.M.; Naso, F.; Operamolla, A.; Pedone, A. Synthesis of d-glucose and l-phenylalanine substituted phenylene–thiophene oligomers. Tetrahedron 2011, 67, 486–494. [Google Scholar] [CrossRef]
- Gronowitz, S.; Peters, D. Convenient synthesis of various terheterocyclic compounds by Pd(0)-catalyzed coupling reactions. Heterocycles 1990, 30, 645–658. [Google Scholar] [CrossRef]
- Melucci, M.; Barbarella, G.; Sotgiu, G. Solvent-Free, Microwave-Assisted Synthesis of Thiophene Oligomers via Suzuki Coupling. J. Org. Chem. 2002, 67, 8877–8884. [Google Scholar] [CrossRef] [PubMed]
- Alesi, S.; Di Maria, F.; Melucci, M.; Macquarrie, D.J.; Luque, R.; Barbarella, G. Microwave-assisted synthesis of oligothiophene semiconductors in aqueous media using silica and chitosan supported Pd catalysts. Green Chem. 2008, 10, 517. [Google Scholar] [CrossRef]
- DiMaria, F.; Barbarella, G. Facilitated synthesis of functional oligothiophenes for application in thin film devices and live cell imaging. J. Sulfur Chem. 2013, 34, 627–637. [Google Scholar] [CrossRef]
- Beny, J.P.; Dhawan, S.N.; Kagan, J.; Sundlass, S. Synthesis of 3,2′:5′,3″-terthiophene and other terthiophenes by the thiophenecarboxaldehyde.fwdarw. ethynylthiophene.fwdarw. dithienylbutadiyne route. J. Org. Chem. 1982, 47, 2201–2204. [Google Scholar] [CrossRef]
- Kagan, J.; Perrine, M.D. A Side Reaction in the Synthesis of 2-Ethynylthiophene from 2-Thiophenecarboxaldehyde by the Corey Procedure and an Inproved Synthesis of 2,2′:5′,2″-Terthiophene. Heterocycles 1986, 24, 365. [Google Scholar] [CrossRef]
- Carpita, A.; Rossi, R.; Veracini, C.A. Synthesis and 13C nmr characterization of some π-excessive heteropolyaromatic compounds. Tetrahedron 1985, 41, 1919–1929. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, P.; Cong, R.; Xu, N.; Tan, Y.; Tan, C.; Jiang, Y. Sensitive Conjugated-Polymer-Based Fluorescent ATP Probes and Their Application in Cell Imaging. ACS Appl. Mater. Interfaces 2016, 8, 3567–3574. [Google Scholar] [CrossRef]
- Press, D.J.; Gendy, C.; Pasalkar, S.; Schechtel, S.; Heyne, B.; Sutherland, T.C. Synthesis of Tetrathia–Oligothiophene Macrocycles. ACS Omega 2019, 4, 3405–3408. [Google Scholar] [CrossRef]
- Zheng, Q.; Hua, R.; Jiang, J.; Zhang, L. A general approach to arylated furans, pyrroles, and thiophenes. Tetrahedron 2014, 70, 8252–8256. [Google Scholar] [CrossRef]
- Zhang, G.; Yi, H.; Chen, H.; Bian, C.; Liu, C.; Lei, A. Trisulfur Radical Anion as the Key Intermediate for the Synthesis of Thiophene via the Interaction between Elemental Sulfur and NaO t Bu. Org. Lett. 2014, 16, 6156–6159. [Google Scholar] [CrossRef]
- Urselmann, D.; Antovic, D.; Müller, T.J.J. Pseudo five-component synthesis of 2,5-di(hetero)arylthiophenes via a one-pot Sonogashira–Glaser cyclization sequence. Beilstein J. Org. Chem. 2011, 7, 1499–1503. [Google Scholar] [CrossRef]
- Wynberg, H.; Metselaar, J. A Convenient Route To Polythiophenes. Synth. Commun. 1984, 14, 1–9. [Google Scholar] [CrossRef]
- Merz, A.; Ellinger, F. Convenient Synthesis of α-Terthienyl and α-Quinquethienyl via a Friedel-Crafts Route. Synthesis (Stuttgart) 1991, 1991, 462–464. [Google Scholar] [CrossRef]
- Asano, T.; Ito, S.; Saito, N.; Hatakeda, K. A Simple Synthesis of 2,2′,5′,2″-Terthienyl. Heterocycles 1977, 6, 317. [Google Scholar] [CrossRef]
- Nakayama, J.; Nakamura, Y.; Murabayashi, S.; Hoshino, M. Preparation of a-Quinque- and a-Septithiophenes and Their Positional Isomers. Heterocycles 1987, 26, 939. [Google Scholar] [CrossRef]
- Sørensen, A.R.; Overgaard, L.; Johannsen, I. Reactivity of 2,5-dithienyl-pyrroles and thiophenes. Synth. Met. 1993, 55, 1626–1631. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Lam, K.; Poleshchuk, O.K.; Kuz’mina, L.G.; Churakov, A.V. Oligothienyl catenated germanes and silanes: synthesis, structure, and properties. Dalt. Trans. 2018, 47, 5431–5444. [Google Scholar] [CrossRef]
- Shridhar, D.R.; Jogibhukta, M.; Rao, P.S.; Handa, V.K. An Improved Method for the Preparation of 2,5-Disubstituted Thiophenes. Synthesis 1982, 1982, 1061–1062. [Google Scholar] [CrossRef]
- Ben-Haida, A.; Hodge, P. Polymer-supported syntheses of thiophene-containing compounds using a new type of traceless linker. Org. Biomol. Chem. 2012, 10, 1754. [Google Scholar] [CrossRef]
- Leriche, P.; Aillerie, D.; Roquet, S.; Allain, M.; Cravino, A.; Frère, P.; Roncali, J. 3D-conjugated systems based on oligothiophenes and phosphorus nodes. Org. Biomol. Chem. 2008, 6, 3202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.S.; Al-Suti, M.K.; Shah, H.H.; Al-Humaimi, S.; Al-Battashi, F.R.; Bjernemose, J.K.; Male, L.; Raithby, P.R.; Zhang, N.; Köhler, A.; et al. Synthesis and characterization of platinum(ii) di-ynes and poly-ynes incorporating ethylenedioxythiophene (EDOT) spacers in the backbone. Dalt. Trans. 2011, 40, 10174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, H. Electrochemical Polymerization in crystal-preparation of polybithiophene with crystal order. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 622–628. [Google Scholar] [CrossRef]
- Li, Y.; Shu, Q.; Du, Q.; Dai, Y.; Zhao, S.; Zhang, J.; Li, L.; Chen, K. Surface Modification for Improving the Photocatalytic Polymerization of 3,4-Ethylenedioxythiophene over Inorganic Lead Halide Perovskite Quantum Dots. ACS Appl. Mater. Interfaces 2020, 12, 451–460. [Google Scholar] [CrossRef]
- Chen, K.; Deng, X.; Dodekatos, G.; Tüysüz, H. Photocatalytic Polymerization of 3,4-Ethylenedioxythiophene over Cesium Lead Iodide Perovskite Quantum Dots. J. Am. Chem. Soc. 2017, 139, 12267–12273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, H. Circular Dichroism of Bipolarons in a Chiroptically Active Conjugated Polymer. J. Macromol. Sci. Part B 2016, 55, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Goto, H. Vortex fibril structure and chiroptical electrochromic effect of optically active poly(3,4-ethylenedioxythiophene) (PEDOT*) prepared by chiral transcription electrochemical polymerisation in cholesteric liquid crystal. J. Mater. Chem. 2009, 19, 4914. [Google Scholar] [CrossRef] [Green Version]
- Jaafari, A.; Ouzeau, V.; Ely, M.; Rodriguez, F.; Chane-ching, K.; Yassar, A.; Aaron, J.J. Synthesis and optical properties of novel 1,3-propanedione bearing oligothiophene substituents. Synth. Met. 2004, 147, 183–189. [Google Scholar] [CrossRef]
- Zanardi, C.; Zanfrognini, B.; Morandi, S.; Terzi, F.; Pigani, L.; Pasquali, L.; Seeber, R. Synthesis, spectroscopic and electrochemical characterization of Co(II)-terpyridine based metallopolymer. Electrochim. Acta 2018, 260, 314–323. [Google Scholar] [CrossRef]
- Invernale, M.A.; Pendergraph, S.A.; Yavuz, M.S.; Ombaba, M.; Sotzing, G.A. Conjugated polymers atypically prepared in water. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2024–2031. [Google Scholar] [CrossRef] [Green Version]
- Pardieu, E.; Saad, A.; Dallery, L.; Garnier, F.; Vedrine, C.; Hauquier, F.; Dalko, P.; Pernelle, C. Synthesis and characterization of β-substituted 3,4-ethylenedioxy terthiophene monomers for conducting polymer applications. Synth. Met. 2013, 171, 23–31. [Google Scholar] [CrossRef]
- Ji, L.; Edkins, R.M.; Sewell, L.J.; Beeby, A.; Batsanov, A.S.; Fucke, K.; Drafz, M.; Howard, J.A.K.; Moutounet, O.; Ibersiene, F.; et al. Experimental and Theoretical Studies of Quadrupolar Oligothiophene-Cored Chromophores Containing Dimesitylboryl Moieties as π-Accepting End-Groups: Syntheses, Structures, Fluorescence, and One- and Two-Photon Absorption. Chem. Eur. J. 2014, 20, 13618–13635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imae, I.; Imabayashi, S.; Komaguchi, K.; Tan, Z.; Ooyama, Y.; Harima, Y. Synthesis and electrical properties of novel oligothiophenes partially containing 3,4-ethylenedioxythiophenes. RSC Adv. 2014, 4, 2501–2508. [Google Scholar] [CrossRef]
- Imae, I.; Korai, K.; Ooyama, Y.; Komaguchi, K.; Harima, Y. Synthesis of novel dyes having EDOT-containing oligothiophenes as π-linker for panchromatic dye-sensitized solar cells. Synth. Met. 2015, 207, 65–71. [Google Scholar] [CrossRef]
- Shen, L.; Liu, P.; Liu, C.; Jiang, Q.; Xu, J.; Duan, X.; Du, Y.; Jiang, F. Advances in Efficient Polymerization of Solid-State Trithiophenes for Organic Thermoelectric Thin-Film. ACS Appl. Polym. Mater. 2020, 2, 376–384. [Google Scholar] [CrossRef]
- Imae, I.; Sagawa, H.; Mashima, T.; Komaguchi, K.; Ooyama, Y.; Harima, Y.; Imae, I.; Sagawa, H.; Mashima, T.; Komaguchi, K.; et al. Synthesis of Soluble Polythiophene Partially Containing 3,4-Ethylenedioxythiophene and 3-Hexylthiophene by Polycondensation. Open J. Polym. Chem. 2014, 04, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Turbiez, M.; Frère, P.; Allain, M.; Videlot, C.; Ackermann, J.; Roncali, J. Design of Organic Semiconductors: Tuning the Electronic Properties of π-Conjugated Oligothiophenes with the 3,4-Ethylenedioxythiophene (EDOT) Building Block. Chem. Eur. J. 2005, 11, 3742–3752. [Google Scholar] [CrossRef]
- Abdiryim, T.; Jamal, R.; Zhao, C.; Awut, T.; Nurulla, I. Structure and properties of solid-state synthesized poly(3′,4′-ethylenedioxy-2,2′:5′,2″-terthiophene). Synth. Met. 2010, 160, 325–332. [Google Scholar] [CrossRef]
- Borghese, A.; Geldhof, G.; Antoine, L. Direct C–H arylation of 3-methoxythiophene catalyzed by Pd. Application to a more efficient synthesis of π-alkoxy-oligothiophene derivatives. Tetrahedron Lett. 2006, 47, 9249–9252. [Google Scholar] [CrossRef]
- Sease, J.W.; Zechmeister, L. Chromatographic and Spectral Characteristics of Some Polythienyls. J. Am. Chem. Soc. 1947, 69, 270–273. [Google Scholar] [CrossRef]
- Uhlenbroek, J.H.; Bijloo, J.D. Investigations on nematicides: III. Polythienyls and related compounds. Recl. Trav. Chim. Pays-Bas 1960, 79, 1181–1196. [Google Scholar] [CrossRef]
- Luo, T.-M.H.; Legoff, E. Facile Synthesis of α-Polythienyls via 1,4-Diketones. J. Chin. Chem. Soc. 1992, 39, 325–332. [Google Scholar] [CrossRef]
- Tao, T.; Qian, H.F.; Zhang, K.; Geng, J.; Huang, W. Functionalized oligothiophene-based heterocyclic aromatic fluorescent compounds with various donor-acceptor spacers and adjustable electronic properties: A theoretical and experimental perspective. Tetrahedron 2013, 69, 7290–7299. [Google Scholar] [CrossRef]
- Tamao, K.; Kodama, S.; Nakajima, I.; Kumada, M.; Minato, A.; Suzuki, K. Nickel-phosphine complex-catalyzed Grignard coupling-II. Grignard coupling of heterocyclic compounds. Tetrahedron 1982, 38, 3347–3354. [Google Scholar] [CrossRef]
- Roncali, J.; Gorgues, A.; Jubault, M. Effects of Substitution of the Median Thiophene Ring on the Electrodeposition and Structure of Poly(terthienyls). Chem. Mater. 1993, 5, 1456–1464. [Google Scholar] [CrossRef]
- Van Pham, C.; Burkhardt, A.; Shabana, R.; Cunningham, D.D.; Mark, H.B.; Zimmer, H. A convenient synthesis of 2, 5-thienylene oligomers; some of their spectroscopic and electrochemical properties. Phosphorus. Sulfur. Silicon Relat. Elem. 1989, 46, 153–168. [Google Scholar] [CrossRef]
- Gronowitz, S.; Hörnfeldt, A.B.; Galal, A.; Mark, H.B. Synthesis of mixed oligomeric heteroarylenes containing furan, thiophene, and selenophene rings; their uv spectra and oxidation potentials. Phosphorus. Sulfur. Silicon Relat. Elem. 1989, 42, 171–176. [Google Scholar] [CrossRef]
- Delabouglise, D.; Hmyene, M.; Horowitz, G.; Yassar, A.; Garnier, F. Electrochemical coupling of dialkylated sexithiophene. Adv. Mater. 1992, 4, 107–110. [Google Scholar] [CrossRef]
- Bäuerle, P.; Pfau, F.; Schlupp, H.; Würthner, F.; Gaudl, K.-U.; Caro, M.B.; Fischer, P. Synthesis and structural characterization of alkyl oligothiophenes—The first isomerically pure dialkylsexithiophene. J. Chem. Soc. Perkin Trans. 1993, 3, 489–494. [Google Scholar] [CrossRef]
- Ten Hoeve, W.; Wynberg, H. Substituted 2 2′:5′,2″:5″,2‴5‴,2″″:5″″,2″‴5″‴2″″″:5″″″,2″″‴:5‴‴′:5‴‴″,2″″″″′,2′′′′′′′′′′-Undecithiophenes: The Longest Characterized Oligothiophenes. J. Am. Chem. Soc. 1991, 113, 5887–5889. [Google Scholar] [CrossRef] [Green Version]
- Ferraris, J.P.; Newton, M.D. Electrochemical and optical properties of thiophene-alkylheteroaromatic copolymers. Polymer 1992, 33, 391–397. [Google Scholar] [CrossRef]
- Andersson, M.R.; Pei, Q.; Hjertberg, T.; Inganäs, O.; Wennerström, O.; Österholm, J.E. Synthesis of soluble poly(alkylthiophenes) which are thermally stable in the doped state. Synth. Met. 1993, 55, 1227–1231. [Google Scholar] [CrossRef]
- Li, W.; Han, Y.; Li, B.; Liu, C.; Bo, Z. Tris[tri(2-thienyl)phosphine]palladium as the catalyst precursor for thiophene-based Suzuki-Miyaura crosscoupling and polycondensation. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 4556–4563. [Google Scholar] [CrossRef]
- Gohier, F.; Frère, P.; Roncali, J. 3-Fluoro-4-hexylthiophene as a Building Block for Tuning the Electronic Properties of Conjugated Polythiophenes. J. Org. Chem. 2013, 78, 1497–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Y.; Mohin, J.; Tsai, C.-H.; Tristram-Nagle, S.; Gil, R.R.; Kowalewski, T.; Noonan, K.J.T. Stille Catalyst-Transfer Polycondensation Using Pd-PEPPSI-IPr for High-Molecular-Weight Regioregular Poly(3-hexylthiophene). Macromol. Rapid Commun. 2015, 36, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Gallazzi, M.C.; Castellani, L.; Marin, R.A.; Zerbi, G. Regiodefined substituted poly(2,5-thienylene)s. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 3339–3349. [Google Scholar] [CrossRef]
- Olinga, T.; Destri, S.; Porzio, W.; Selva, A. Synthesis and characterization of 3-hexyl multi-substituted α,ω-diformyl-α-oligothiophenes (n = 3, 6, 8). Macromol. Chem. Phys. 1997, 198, 1091–1107. [Google Scholar] [CrossRef]
- Jones, C.L.; Higgins, S.J. Symmetrical alkyl-substituted oligothiophenes as ligands: Complexation of the [(η-C5H5)Ru]+ moiety by hexyl-substituted ter-, quater- and quinque-thiophenes. J. Mater. Chem. 1999, 9, 865–874. [Google Scholar] [CrossRef]
- Yang, C.; Abley, M.; Holdcroft, S. Regioregular di(2′-(thienyl))furan- and di(2′-thienyl)benzene-based polymers: Steric and heavy-atom effects on the luminescence of conjugated systems. Macromolecules 1999, 32, 6889–6891. [Google Scholar] [CrossRef]
- Kokubo, H.; Yamamoto, T. Organometallic Syntheses of Head-to-Head Poly(3-hexylthiophene) and a Related Polymer With a Spacing Non-Substituted Thiophene Unit. Colloidal Solutions of the Polymers. Macromol. Chem. Phys. 2001, 202, 1031–1034. [Google Scholar] [CrossRef]
- Diaz-Quijada, G.A.; Weinberg, N.; Holdcroft, S.; Mario Pinto, B. Conformational analysis of oligothiophenes and oligo(thienyl)furans by use of a combined molecular dynamics/NMR spectroscopic protocol. J. Phys. Chem. A 2002, 106, 1277–1285. [Google Scholar] [CrossRef]
- Saravanan, C.; Liu, C.L.; Chang, Y.M.; Lu, J.D.; Hsieh, Y.J.; Rwei, S.P.; Wang, L. Fulleropyrrolidines bearing π-conjugated moiety for polymer solar cells: Contribution of the chromophoric substituent on C60 to the photocurrent. ACS Appl. Mater. Interfaces 2012, 4, 6133–6141. [Google Scholar] [CrossRef]
- XIA, P.F.; Lu, J.; Kwok, C.H.; Fukutani, H.; Wong, M.S.; Tao, Y. Synthesis and properties of monodisperse multi-triarylamine-substituted oligothiophenes and 4,7-bis(2′-oligothienyl)-2,1,3-benzothiadiazoles for organic solar cell applications. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 137–148. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Z.E.; Hu, J.; Hong, Y. Novel metal-free organic dyes containing linear planar 11,12-dihydroindolo[2,3-a]carbazole donor for dye-sensitized solar cells: Effects of π spacer and alkyl chain. Dyes Pigments 2019, 164, 213–221. [Google Scholar] [CrossRef]
- Ghosh, T.; Gopal, A.; Saeki, A.; Seki, S.; Nair, V.C. P/n-Polarity of thiophene oligomers in photovoltaic cells: Role of molecular vs. supramolecular properties. Phys. Chem. Chem. Phys. 2015, 17, 10630–10639. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, Y.; Fang, C.; Cai, X.; Hu, B.; Tu, G. Efficient deep-red electroluminescent donor-acceptor copolymers based on 6,7-dichloroquinoxaline. Org. Electron. 2017, 46, 276–282. [Google Scholar] [CrossRef]
- Diaz-Quijada, G.A.; Weinberg, N.; Holdcroft, S.; Pinto, B.M. Investigation of barriers to conformational interchange in oligothiophenes and oligo(thienyl)furans. J. Phys. Chem. A 2002, 106, 1266–1276. [Google Scholar] [CrossRef]
- Luo, T.-M.H.; Chen, L.-H. Synthesis of 2,5 -Bis(4-methyl-2-thienyl)thiophene and 2,5-Bis(4-methyl-2-thienyl)pyrrole. J. Chin. Chem. Soc. 1995, 42, 589–591. [Google Scholar] [CrossRef]
- Reddinger, J.L.; Reynolds, J.R. Site Specific Electropolymerization To Form Transition-Metal-Containing, Electroactive Polythiophenes. Chem. Mater. 1998, 10, 1236–1243. [Google Scholar] [CrossRef]
- Belkessam, F.; Mohand, A.; Soulé, J.-F.; Elias, A.; Doucet, H. Palladium-catalyzed 2,5-diheteroarylation of 2,5-dibromothiophene derivatives. Beilstein J. Org. Chem. 2014, 10, 2912–2919. [Google Scholar] [CrossRef] [Green Version]
- Araki, K.; Endo, H.; Masuda, G.; Ogawa, T. Bridging Nanogap Electrodes by In Situ Electropolymerization of a Bis(terthiophenylphenanthroline)ruthenium Complex. Chem. Eur. J. 2004, 10, 3331–3340. [Google Scholar] [CrossRef] [PubMed]
- Krömer, J.; Bäuerle, P. Homologous series of regioregular alkylsubstituted oligothiophenes up to an 11-mer. Tetrahedron 2001, 57, 3785–3794. [Google Scholar] [CrossRef]
- Wang, C.; Benz, M.E.; LeGoff, E.; Schindler, J.L.; Allbritton-Thomas, J.; Kannewurf, C.R.; Kanatzidis, M.G. Studies on Conjugated Polymers: Preparation, Spectroscopic, and Charge-Transport Properties of a New Soluble Polythiophene Derivative: Poly(3′,4′dibutyl-2,2′:5′2″-terthiophene). Chem. Mater. 1994, 6, 401–411. [Google Scholar] [CrossRef]
- Horne, J.C.; Blanchard, G.J.; LeGoff, E. Rotational Isomerization Barriers of Thiophene Oligomers in the Ground and First Excited Electronic States. A 1H NMR and Fluorescence Lifetime Investigation. J. Am. Chem. Soc. 1995, 117, 9551–9558. [Google Scholar] [CrossRef]
- Nakayama, J.; Ting, Y.; Sugihara, Y.; Ishii, A. Synthesis of highly congested bi- and terthiophenes; 3,4,3′,′-tetra-tert-butylbithiophene and 3′,4′-di-tert-butyl-2,2′:5′,2″-terthiophene. Heterocycles 1997, 44, 75–80. [Google Scholar] [CrossRef]
- Henderson, P.T.; Collard, D.M. Thiophene: Alkylthiophene Copolymers from Substituted Dialkyloligothiophenes. Chem. Mater. 1995, 7, 1879–1889. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Du, S.; Tong, J.; Zhang, P.; Guo, P.; Yang, C.; Xia, Y. A two-dimension medium band gap conjugated polymer based on 5,10-bis(alkylthien-2-yl)dithieno[3,2-d:3′,2′-d′]benzo[1,2-b:4,5-b′]dithiophene: Synthesis and photovoltaic application. J. Macromol. Sci. Part A Pure Appl. Chem. 2016, 53, 538–545. [Google Scholar] [CrossRef]
- Huang, W.; Meng, H.; Yu, W.L.; Pei, J.; Chen, Z.K.; Lai, Y.H. A novel series of p-N diblock light-emitting copolymers based on oligothiophenes and 1,4-Bis(oxadiazolyl)-2,5-dialkyloxybenzene. Macromolecules 1999, 32, 118–126. [Google Scholar] [CrossRef]
- Meng, H.; Huang, W. Novel photoluminescent polymers containing oligothiophene and m-phenylene-1,3,4-oxadiazole moieties: Synthesis and spectroscopic and electrochemical studies. J. Org. Chem. 2000, 65, 3894–3901. [Google Scholar] [CrossRef]
- Liu, P.; Shirota, Y.; Osada, Y. A novel class of low-molecular-weight organic gels based on terthiophene. Polym. Adv. Technol. 2000, 11, 512–517. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, Q.; Qin, Q.; Ye, S.; Lin, Y.; Liu, Z.; Bian, Z.; Chen, Y.; Huang, C. Design, synthesis and photophysical properties of A-D-A-D-A small molecules for photovoltaic application. Dyes Pigments 2015, 121, 99–108. [Google Scholar] [CrossRef]
- Pandolfi, F.; Rocco, D.; Mattiello, L. Synthesis and characterization of new D-π-A and A-π-D-π-A type oligothiophene derivatives. Org. Biomol. Chem. 2019, 17, 3018–3025. [Google Scholar] [CrossRef]
- Jeon, C.W.; Kang, S.H.; Yun, H.J.; An, T.K.; Cha, H.; Park, C.E.; Kim, Y.H. Synthesis and characterization of poly(dialkylterthiophene-bithiophene) and poly(dialkylterthiophene-thienothiophene) for organic thin film transistors and organic photovoltaic cells. Synth. Met. 2013, 185–186, 159–166. [Google Scholar] [CrossRef]
- Song, H.G.; Kim, Y.J.; Lee, J.S.; Kim, Y.H.; Park, C.E.; Kwon, S.K. Dithienobenzodithiophene-Based Small Molecule Organic Solar Cells with over 7% Efficiency via Additive- and Thermal-Annealing-Free Processing. ACS Appl. Mater. Interfaces 2016, 8, 34353–34359. [Google Scholar] [CrossRef] [PubMed]
- Pokrop, R.; Verilhac, J.M.; Gasior, A.; Wielgus, I.; Zagorska, M.; Travers, J.P.; Pron, A. Effect of molecular weight on electronic, electrochemical and spectroelectrochemical properties of poly(3,3″-dioctyl-2,2′5′,2″-terthiophene). J. Mater. Chem. 2006, 16, 3099–3106. [Google Scholar] [CrossRef]
- Tang, A.; Zhan, C.; Yao, J. Series of Quinoidal Methyl-Dioxocyano-Pyridine Based π-Extended Narrow-Bandgap Oligomers for Solution-Processed Small-Molecule Organic Solar Cells. Chem. Mater. 2015, 27, 4719–4730. [Google Scholar] [CrossRef]
- Ie, Y.; Hirose, T.; Aso, Y. Synthesis, properties, and FET performance of rectangular oligothiophene. J. Mater. Chem. 2009, 19, 8169–8175. [Google Scholar] [CrossRef]
- Effenberger, F.; Grube, G. Synthesis of Oligothienylfullerenes. Synthesis 1998, 1998, 1372–1379. [Google Scholar] [CrossRef]
- Andreani, F.; Salatelli, E.; Lanzi, M. Novel poly(3,3″- and 3′,4′-dialkyl-2,2′:5′,2″-terthiophene)s by chemical oxidative synthesis: Evidence for a new step towards the optimization of this process. Polymer 1996, 37, 661–665. [Google Scholar] [CrossRef]
- Amir, E.; Sivanandan, K.; Cochran, J.E.; Cowart, J.J.; Ku, S.-Y.; Seo, J.H.; Chabinyc, M.L.; Hawker, C.J. Synthesis and characterization of soluble low-bandgap oligothiophene-[all]- S,S -dioxides-based conjugated oligomers and polymers. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 1933–1941. [Google Scholar] [CrossRef]
- Speros, J.C.; Martinez, H.; Paulsen, B.D.; White, S.P.; Bonifas, A.D.; Goff, P.C.; Frisbie, C.D.; Hillmyer, M.A. Effects of olefin content and alkyl chain placement on optoelectronic and morphological properties in poly(thienylene vinylenes). Macromolecules 2013, 46, 5184–5194. [Google Scholar] [CrossRef]
- Ciofalo, M.; Ponterini, G. Generation of singlet oxygen by 2,2′:5′,2″-terthiophene and some of its derivatives. J. Photochem. Photobiol. A Chem. 1994, 83, 1–6. [Google Scholar] [CrossRef]
- Bricaud, Q.; Cravino, A.; Leriche, P.; Roncali, J. Terthiophene-cyanovinylene π-conjugated polymers as donor material for organic solar cells. Synth. Met. 2009, 159, 2534–2538. [Google Scholar] [CrossRef] [Green Version]
- Lundin, P.M.; Giri, G.; Bao, Z. A comparison of the properties of two structurally equivalent but regiochemically different mono-alkylated polybithiophenes prepared through AABB-type stille polycondensation. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 908–915. [Google Scholar] [CrossRef]
- Somanathan, N.; Radhakrishnan, S.; Mukundan, T.; Schmidt, H.W. Studies on 3-(2-Ethylhexyl)thiophene Polymers. Macromol. Mater. Eng. 2002, 287, 236–242. [Google Scholar] [CrossRef]
- Wang, H.J.; Tzeng, J.Y.; Chou, C.W.; Huang, C.Y.; Lee, R.H.; Jeng, R.J. Novel polythiophene derivatives functionalized with conjugated side-chain pendants comprising triphenylamine/carbazole moieties for photovoltaic cell applications. Polym. Chem. 2013, 4, 506–519. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.H.; Son, S.K.; Kim, K.; Lee, S.K.; Shin, W.S.; Moon, S.J.; Kang, I.N. Synthesis and characterization of new selenophene-based donor-acceptor low-bandgap polymers for organic photovoltaic cells. Macromolecules 2012, 45, 1303–1312. [Google Scholar] [CrossRef]
- Andreani, F.; Angiolini, L.; Caretta, D.; Salatelli, E. Synthesis and polymerization of 3,3″-di[(S)-(+)-2-methylbutyl]-2,2′:5′,2″-terthiophene: A new monomer precursor to chiral regioregular poly(thiophene). J. Mater. Chem. 1998, 8, 1109–1111. [Google Scholar] [CrossRef]
- Barbarell, G.; Zambianchi, M.; Bongini, A.; Antolini, L. Conformational chirality of oligothiophenes in the solid state. X-Ray structure of 3,4?,4?-trimethyl-2,2?:5?,2?-terthiophene. Adv. Mater. 1994, 6, 561–564. [Google Scholar] [CrossRef]
- Rossi, R.; Carpita, A.; Ciofalo, M.; Houben, J.L. ChemInform Abstract: Synthesis and Characterization of 2,2′:5′,2′′-Terthiophene Derivatives of Possible Therapeutic Use. ChemInform 2010, 22. [Google Scholar] [CrossRef]
- Martinez, F.; Neculqueo, G. Synthesis and polymerization of 3-octylsubstituted thiophene, bithiophene and terthiophene. Int. J. Polym. Mater. Polym. Biomater. 1999, 44, 265–274. [Google Scholar] [CrossRef]
- Wu, C.G.; Lai, C.Y.; Hsiao, N.L. Molecular engineering leading to better processability of conjugated chromophores: The optical properties of new soluble copolymers containing alternative oligo-octylthiophene and oligo-methylene blocks. Eur. Polym. J. 2009, 45, 879–887. [Google Scholar] [CrossRef]
- Bidan, G.; De Nicola, A.; Enée, V.; Guillerez, S. Synthesis and UV-Visible Properties of Soluble Regioregular Oligo(3-octylthiophenes), Monomer to Hexamer. Chem. Mater. 1998, 10, 1052–1058. [Google Scholar] [CrossRef]
- Leone, A.K.; Souther, K.D.; Vitek, A.K.; LaPointe, A.M.; Coates, G.W.; Zimmerman, P.M.; McNeil, A.J. Mechanistic Insight into Thiophene Catalyst-Transfer Polymerization Mediated by Nickel Diimine Catalysts. Macromolecules 2017, 50, 9121–9127. [Google Scholar] [CrossRef]
- Gondo, S.; Goto, Y.; Era, M. Preparation of Regioregular Alkylthiophene Oligomers and Their Optical Properties. Mol. Cryst. Liq. Cryst. 2007, 470, 353–358. [Google Scholar] [CrossRef]
- Beryozkina, T.; Senkovskyy, V.; Kaul, E.; Kiriy, A. Kumada catalyst-transfer poly condensation of thiophene-based oligomers: Robustness of a chain-growth mechanism. Macromolecules 2008, 41, 7817–7823. [Google Scholar] [CrossRef]
- Barbarella, G.; Bongini, A.; Zambianchi, M. Regiochemistry and Conformation of Poly(3-hexylthiophene) via the Synthesis and the Spectroscopic Characterization of the Model Configurational Triads. Macromolecules 1994, 27, 3039–3045. [Google Scholar] [CrossRef]
- Li, J.C.; Lee, S.H.; Hahn, Y.B.; Kim, K.J.; Zong, K.; Lee, Y.S. Synthesis and characterization of triphenylamine-3-hexylthiophene oligomer hybrids: A triphenylamine core carrying three terthiophene branches and triphenylamine end-capped quaterthiophene. Synth. Met. 2008, 158, 150–156. [Google Scholar] [CrossRef]
- Corriu, R.J.P.; Masse, J.P. Activation of Grignard reagents by transition-metal complexes. A new and simple synthesis of trans-stilbenes and polyphenyls. J. Chem. Soc. Chem. Commun. 1972, 144a. [Google Scholar] [CrossRef]
- Higuchi, H.; Nakayama, T.; Koyama, H.; Ojima, J.; Wada, T.; Sasabe, H. Synthesis and Properties of α,ω-Disubstituted Oligo(3-hexylthiophene)s and Oligothienoquinonoids in Head-to-head Orientation. Bull. Chem. Soc. Jpn. 1995, 68, 2363–2377. [Google Scholar] [CrossRef]
- Tanaka, S.; Tamba, S.; Tanaka, D.; Sugie, A.; Mori, A. Synthesis of well-defined head-to-tail-type oligothiophenes by regioselective deprotonation of 3-substituted thiophenes and nickel-catalyzed cross-coupling reaction. J. Am. Chem. Soc. 2011, 133, 16734–16737. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, H.; Abdilla, A.; Yun, H.; Han, J.; Stein, G.E.; Hawker, C.J.; Kim, B.J. Chain-Length-Dependent Self-Assembly Behaviors of Discrete Conjugated Oligo(3-hexylthiophene). Chem. Mater. 2020. [Google Scholar] [CrossRef]
- Tanaka, S.; Tanaka, D.; Tatsuta, G.; Murakami, K.; Tamba, S.; Sugie, A.; Mori, A. Concise Synthesis of Well-Defined Linear and Branched Oligothiophenes with Nickel-Catalyzed Regiocontrolled Cross-Coupling of 3-Substituted Thiophenes by Catalytically Generated Magnesium Amide. Chem. Eur. J. 2013, 19, 1658–1665. [Google Scholar] [CrossRef] [Green Version]
- Yagai, S.; Suzuki, M.; Lin, X.; Gushiken, M.; Noguchi, T.; Karatsu, T.; Kitamura, A.; Saeki, A.; Seki, S.; Kikkawa, Y.; et al. Supramolecular Engineering of Oligothiophene Nanorods without Insulators: Hierarchical Association of Rosettes and Photovoltaic Properties. Chem. Eur. J. 2014, 20, 16128–16137. [Google Scholar] [CrossRef]
- Liu, J.T.; Hase, H.; Taylor, S.; Salzmann, I.; Forgione, P. Approaching the Integer-Charge Transfer Regime in Molecularly Doped Oligothiophenes by Efficient Decarboxylative Cross-Coupling. Angew. Chem. Int. Ed. 2020, 59, 7146–7153. [Google Scholar] [CrossRef]
- Collis, G.E.; Burrell, A.K.; Blandford, E.J.; Officer, D.L. A modular procedure for the synthesis of functionalised β-substituted terthiophene monomers for conducting polymer applications. Tetrahedron 2007, 63, 11141–11152. [Google Scholar] [CrossRef]
- Kagan, J.; Liu, H. 3′-Vinyl-2,2′:5′,2″-terthiophene: Synthesis, polymerization and copolymerization with styrene. Synth. Met. 1996, 82, 75–81. [Google Scholar] [CrossRef]
- Inaoka, S.; Collard, D.M. Chemical and electrochemical polymerization of 3-alkylthiophenes on self-assembled monolayers of oligothiophene-substituted alkylsilanes. Langmuir 1999, 15, 3752–3758. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, J.-H.; Kim, T.H.; Kang, D.M.; Kim, Y.H.; Shim, Y.-B.; Shin, S.C. Polyterthiophene Appended by Organomolybdenum Sulfide Cluster: Electrochemical Synthesis and Electrochemical Properties of Poly[Mo2(μ-C5H5)2{μ-η2:η2-SC(R)C S[C4HS(C4H3S-2)2-2,5]}2]s. Chem. Mater. 2003, 15, 825–827. [Google Scholar] [CrossRef]
- Yamazaki, T.; Murata, Y.; Komatsu, K.; Furukawa, K.; Morita, M.; Maruyama, N.; Yamao, T.; Fujita, S. Synthesis and electrolytic polymerization of the ethylenedioxy-substituted terthiophene-fullerene dyad. Org. Lett. 2004, 6, 4865–4868. [Google Scholar] [CrossRef]
- Volz, W.; Vob, J. A mild and simple synthesis of benzo[c]thiophenes and 4,7-di-hydrobenzo[c]thiophenes. Synth. 1990, 1990, 670–674. [Google Scholar] [CrossRef]
- Manca, P.; Pilo, M.I.; Casu, G.; Gladiali, S.; Sanna, G.; Scanu, R.; Spano, N.; Zucca, A.; Zanardi, C.; Bagnis, D.; et al. A new terpyridine tethered polythiophene: Electrosynthesis and characterization. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3513–3523. [Google Scholar] [CrossRef]
- Han, A.; Bai, J.; Murata, Y.; Komatsu, K. Synthesis and characterization of the fullerene-terthiophene dyads. Heteroat. Chem. 2011, 22, 72–78. [Google Scholar] [CrossRef]
- Mitsudo, K.; Sato, H.; Yamasaki, A.; Kamimoto, N.; Goto, J.; Mandai, H.; Suga, S. Synthesis and Properties of Ethene-Bridged Terthiophenes. Org. Lett. 2015, 17, 4858–4861. [Google Scholar] [CrossRef]
- Sordello, F.; Minero, C.; Viscardi, G.; Quagliotto, P. Highly Photoactive Polythiophenes Obtained by Electrochemical Synthesis from Bipyridine-Containing Terthiophenes. Energies 2019, 12, 341. [Google Scholar] [CrossRef] [Green Version]
- Quagliotto, P.; Prosperini, S.; Viscardi, G. Improved Synthesis of a Terthiophene-Based Monomeric Ligand That Forms a Highly Active Polymer for the Carbon Dioxide Reduction. Lett. Org. Chem. 2017, 14. [Google Scholar] [CrossRef]
- Kim, D.H.; Kang, B.S.; Lim, S.M.; Bark, K.M.; Kim, B.G.; Shiro, M.; Shim, Y.B.; Shin, S.C. Polyterthiophene-bearing pendant organomolybdenum complexes: Electropolymerization of erythro-[Mo2(μ-C5H5)2(CO) 4{μ-η2:η2-C(R)≡C[C 4HS(C4H3S-2)2-2,5]}]. J. Chem. Soc. Dalt. Trans. 1998, 1893–1898. [Google Scholar] [CrossRef]
- Cao, Y.; Wolf, M.O.; Patrick, B.O. A terthiophene-containing alkynylplatinum terpyridine pacman complex: Controllable folding/unfolding modulated by weak intermolecular interactions. Inorg. Chem. 2013, 52, 5636–5638. [Google Scholar] [CrossRef] [PubMed]
- Kuchison, A.M.; Wolf, M.O.; Patrick, B.O. Photophysical properties and electropolymerization of gold complexes of 3,3″-diethynyl-2,2′:5′,2″-terthiophene. Inorg. Chem. 2010, 49, 8802–8812. [Google Scholar] [CrossRef] [PubMed]
- Wagner, P.; Partridge, A.C.; Jolley, K.W.; Officer, D.L. Facile synthesis of acetylene-substituted terthiophenes. Tetrahedron Lett. 2007, 48, 6245–6248. [Google Scholar] [CrossRef]
- Cheng, H.; Djukic, B.; Jenkins, H.A.; Gorelsky, S.I.; Lemaire, M.T. Iron(II) complexes containing thiophene-substituted “bispicen” ligands—Spin-crossover, ligand rearrangements, and ferromagnetic interactions. Can. J. Chem. 2010, 88, 954–963. [Google Scholar] [CrossRef]
- Xiao, Z.; Ye, G.; Liu, Y.; Chen, S.; Peng, Q.; Zuo, Q.; Ding, L. Pushing Fullerene Absorption into the Near-IR Region by Conjugately Fusing Oligothiophenes. Angew. Chem. Int. Ed. 2012, 51, 9038–9041. [Google Scholar] [CrossRef]
- Sharma, G.D.; Mikroyannidis, J.A.; Roy, M.S.; Thomas, K.R.J.; Ball, R.J.; Kurchania, R. Dithienylthienothiadiazole-based organic dye containing two cyanoacrylic acid anchoring units for dye-sensitized solar cells. RSC Adv. 2012, 2, 11457. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, Z.; Feng, Y.; Liu, Y.; Yang, B.; Liu, D.; Lv, Y.; Lu, P.; Ma, Y. Highly π-extended polymers based on phenanthro-pyrazine: Synthesis, characterization, theoretical calculation and photovoltaic properties. Polymer 2013, 54, 6191–6199. [Google Scholar] [CrossRef]
- Schwiderski, R.L.; Rasmussen, S.C. Synthesis and Characterization of Thieno[3,4- b ]pyrazine-Based Terthienyls: Tunable Precursors for Low Band Gap Conjugated Materials. J. Org. Chem. 2013, 78, 5453–5462. [Google Scholar] [CrossRef]
- Sen, C.P.; Shrestha, R.G.; Shrestha, L.K.; Ariga, K.; Valiyaveettil, S. Low-Band-Gap BODIPY Conjugated Copolymers for Sensing Volatile Organic Compounds. Chem. Eur. J. 2015, 21, 17344–17354. [Google Scholar] [CrossRef]
- Xu, X.; Wang, C.; Bäcke, O.; James, D.I.; Bini, K.; Olsson, E.; Andersson, M.R.; Fahlman, M.; Wang, E. Pyrrolo[3,4-g]quinoxaline-6,8-dione-based conjugated copolymers for bulk heterojunction solar cells with high photovoltages. Polym. Chem. 2015, 6, 4624–4633. [Google Scholar] [CrossRef] [Green Version]
- Abdulahi, B.A.; Li, X.; Mone, M.; Kiros, B.; Genene, Z.; Qiao, S.; Yang, R.; Wang, E.; Mammo, W. Structural engineering of pyrrolo[3,4- f ]benzotriazole-5,7(2 H,6 H)-dione-based polymers for non-fullerene organic solar cells with an efficiency over 12%. J. Mater. Chem. A 2019, 7, 19522–19530. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Guo, H.; Jones, R.A. Synthesis and electropolymerization of N-heterocyclic carbene complexes of Pd(ii) and Pt(ii) from an emissive imidazolium salt with a terthiophene backbone. Dalt. Trans. 2019, 48, 14440–14449. [Google Scholar] [CrossRef]
- Freese, T.; Lücke, A.-L.; Schmidt, C.A.S.; Polamo, M.; Nieger, M.; Namyslo, J.C.; Schmidt, A. Anionic N-heterocyclic carbenes derived from sydnone imines such as molsidomine. Trapping reactions with selenium, palladium, and gold. Tetrahedron 2017, 73, 5350–5357. [Google Scholar] [CrossRef] [Green Version]
- Mikroyannidis, J.A.A.; Tsagkournos, D.V.V.; Balraju, P.; Sharma, G.D.D. Efficient bulk heterojunction solar cells using an alternating phenylenevinylene copolymer with dithenyl(thienothiadiazole) segments as donor and PCBM or modified PCBM as acceptor. Sol. Energy Mater. Sol. Cells 2011, 95, 3025–3035. [Google Scholar] [CrossRef]
- Yen, W.-C.; Pal, B.; Yang, J.-S.; Hung, Y.-C.; Lin, S.-T.; Chao, C.-Y.; Su, W.-F. Synthesis and characterization of low bandgap copolymers based on indenofluorene and thiophene derivative. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 5044–5056. [Google Scholar] [CrossRef]
- Lepeltier, M.; Lukoyanova, O.; Jacobson, A.; Jeeva, S.; Perepichka, D.F. New azaborine-thiophene heteroacenes. Chem. Commun. 2010, 46, 7007. [Google Scholar] [CrossRef]
- Li, P.; Fenwick, O.; Yilmaz, S.; Breusov, D.; Caruana, D.J.; Allard, S.; Scherf, U.; Cacialli, F. Dual functions of a novel low-gap polymer for near infra-red photovoltaics and light-emitting diodes. Chem. Commun. 2011, 47, 8820. [Google Scholar] [CrossRef] [PubMed]
- Zoombelt, A.P.; Fonrodona, M.; Turbiez, M.G.R.; Wienk, M.M.; Janssen, R.A.J.R.A.J. Synthesis and photovoltaic performance of a series of small band gap polymers. J. Mater. Chem. 2009, 19, 5336. [Google Scholar] [CrossRef]
- Xia, Y.; Luo, J.; Deng, X.; Li, X.; Li, D.; Zhu, X.; Yang, W.; Cao, Y. Novel Random Low-Band-Gap Fluorene-Based Copolymers for Deep Red/Near Infrared Light-Emitting Diodes and Bulk Heterojunction Photovoltaic Cells. Macromol. Chem. Phys. 2006, 207, 511–520. [Google Scholar] [CrossRef]
- Huber, J.; Jung, C.; Mecking, S. Nanoparticles of Low Optical Band Gap Conjugated Polymers. Macromolecules 2012, 45, 7799–7805. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.; Kumar, R.S.; Naveen, M.; Son, Y.-A. Synthesis and characterization of triphenylamine-based polymers and their application towards solid-state electrochromic cells. RSC Adv. 2016, 6, 78984–78993. [Google Scholar] [CrossRef]
- Zoombelt, A.P.; Leenen, M.A.M.; Fonrodona, M.; Nicolas, Y.; Wienk, M.M.; Janssen, R.A.J. The influence of side chains on solubility and photovoltaic performance of dithiophene–thienopyrazine small band gap copolymers. Polymer 2009, 50, 4564–4570. [Google Scholar] [CrossRef]
- Beaupré, S.; Breton, A.-C.; Dumas, J.; Leclerc, M. Multicolored Electrochromic Cells Based On Poly(2,7-Carbazole) Derivatives For Adaptive Camouflage. Chem. Mater. 2009, 21, 1504–1513. [Google Scholar] [CrossRef]
- Lai, Y.-Y.; Cheng, Y.-J.; Chen, C.-H.; Cheng, S.-W.; Cao, F.-Y.; Hsu, C.-S. Synthesis, photophysical and photovoltaic properties of a new class of two-dimensional conjugated polymers containing donor–acceptor chromophores as pendant groups. Polym. Chem. 2013, 4, 3333. [Google Scholar] [CrossRef]
- Cimrová, V.; Kmínek, I.; Pavlačková, P.; Výprachtický, D.; Cimrova, V.; Kminek, I.; Pavlackova, P.; Vyprachticky, D. Low-bandgap donor-acceptor copolymers with 4,6-bis(3′-(2-ethylhexyl)thien-2′-yl)thieno[3,4-c][1,2,5]thiadiazole: synthesis, optical, electrochemical, and photovoltaic properties. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3426–3436. [Google Scholar] [CrossRef]
- Arrechea-Marcos, I.; de Echegaray, P.; Mancheño, M.J.; Ruiz Delgado, M.C.; Ramos, M.M.; Quintana, J.A.; Villalvilla, J.M.; Díaz-García, M.A.; López Navarrete, J.T.; Ponce Ortiz, R.; et al. Molecular aggregation of naphthalimide organic semiconductors assisted by amphiphilic and lipophilic interactions: a joint theoretical and experimental study. Phys. Chem. Chem. Phys. 2017, 19, 6206–6215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, Y.-J.; Kim, F.S.; Xin, H.; Jenekhe, S.A. New Thienothiadiazole-Based Conjugated Copolymers for Electronics and Optoelectronics. Macromolecules 2012, 45, 3732–3739. [Google Scholar] [CrossRef]
- Mondal, R.; Becerril, H.A.; Verploegen, E.; Kim, D.; Norton, J.E.; Ko, S.; Miyaki, N.; Lee, S.; Toney, M.F.; Brédas, J.-L.; et al. Thiophene-rich fused-aromatic thienopyrazine acceptor for donor–acceptor low band-gap polymers for OTFT and polymer solar cell applications. J. Mater. Chem. 2010, 20, 5823. [Google Scholar] [CrossRef]
- Verolet, Q.; Soleimanpour, S.; Fujisawa, K.; Dal Molin, M.; Sakai, N.; Matile, S. Design and Synthesis of Mixed Oligomers with Thiophenes, Dithienothiophene S, S -Dioxides, Thieno[3,4]pyrazines and 2,1,3-Benzothiadiazoles: Flipper Screening for Mechanosensitive Systems. ChemistryOpen 2015, 4, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Steinberger, S.; Mishra, A.; Reinold, E.; Mena-Osteritz, E.; Mueller, H.; Uhrich, C.; Pfeiffer, M.; Baeuerle, P.; Müller, H.; Uhrich, C.; et al. Synthesis and characterizations of red/near-IR absorbing A–D–A–D–A-type oligothiophenes containing thienothiadiazole and thienopyrazine central units. J. Mater. Chem. 2012, 22, 2701–2712. [Google Scholar] [CrossRef]
- Kmínek, I.; Výprachtický, D.; Kříž, J.; Dybal, J.J.; Cimrová, V.; Kminek, I.; Vyprachticky, D.; Kriz, J.; Dybal, J.J.; Cimrova, V. Low-band gap copolymers containing thienothiadiazole units: Synthesis, optical, and electrochemical properties. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2743–2756. [Google Scholar] [CrossRef]
- Zotti, G.; Zecchin, S.; Schiavon, G.; Vercelli, B.; Berlin, A. Novel polythiophene regular copolymers from 3′,4′-diamino- and 3′,4′-dinitro-terthiophenes. Electrochim. Acta 2005, 50, 1469–1474. [Google Scholar] [CrossRef]
- Soucy-Breau, C.; Eachern, A.M.; Leitch, L.C.; Arnason, T.; Morand, P. Synthesis and characterization of alkyl-, alkenyl-, acyl- and nitrogen-substituted derivatives of the potent phototoxin α-terthiophene. J. Heterocycl. Chem. 1991, 28, 411–416. [Google Scholar] [CrossRef]
- Smith, Z.C.; Meyer, D.M.; Simon, M.G.; Staii, C.; Shukla, D.; Thomas, S.W. Thiophene-Based Conjugated Polymers with Photolabile Solubilizing Side Chains. Macromolecules 2015, 48, 959–966. [Google Scholar] [CrossRef]
- Clarke, T.M.; Gordon, K.C.; Chan, W.S.; Phillips, D.L.; Wagner, P.; Officer, D.L. Raman Spectroscopy of Short-Lived Terthiophene Radical Cations Generated by Photochemical and Chemical Oxidation. ChemPhysChem 2006, 7, 1276–1285. [Google Scholar] [CrossRef]
- Mehenni, H.; Dao, L.H.L.H.; Mehenni, H.; Dao, L.H.L.H. Synthesis and characterization of novel conducting homopolymers based on amino β-styryl terthiophene. Can. J. Chem. 2008, 86, 1010–1018. [Google Scholar] [CrossRef]
- Tarkuc, S.; Unver, E.K.; Udum, Y.A.; Tanyeli, C.; Toppare, L. The effect of changes in π-conjugated terthienyl systems using thienyl and ethylenedioxybenzene functionalized thieno[3,4-b]pyrazine precursors: Multicolored low band gap polymers. Electrochim. Acta 2010, 55, 7254–7258. [Google Scholar] [CrossRef]
- Zhang, Q.T.; Tour, J.M. Alternating Donor/Acceptor Repeat Units in Polythiophenes. Intramolecular Charge Transfer for Reducing Band Gaps in Fully Substituted Conjugated Polymers. J. Am. Chem. Soc. 1998, 120, 5355–5362. [Google Scholar] [CrossRef]
- Gotz, G.; Scheib, S.; Klose, R.; Heinze, J.; Bauerle, P. Synthesis and properties of a series of regioregularly amino-substituted oligo- and polythiophenes. Adv. Funct. Mater. 2002, 12, 723–728. [Google Scholar] [CrossRef]
- Peng, Q.; Liu, X.; Qin, Y.; Zhou, D.; Xu, J. Thieno[3,4-b]pyrazine-based low bandgap photovoltaic copolymers: Turning the properties by different aza-heteroaromatic donors. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 4458–4467. [Google Scholar] [CrossRef]
- Herland, A.; Nilsson, K.P.R.; Olsson, J.D.M.; Hammarstroem, P.; Konradsson, P.; Inganaes, O. Synthesis of a Regioregular Zwitterionic Conjugated Oligoelectrolyte, Usable as an Optical Probe for Detection of Amyloid Fibril Formation at Acidic pH. J. Am. Chem. Soc. 2005, 127, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Kong, L.; Zhao, J.; Bai, G. Synthesis and electrochemical capacitive performance of thieno[3,4-b]pyrazine-based Donor-Acceptor type copolymers used as supercapacitor electrode material. Electrochim. Acta 2017, 238, 36–48. [Google Scholar] [CrossRef]
- Zhang, Q.; Tour, J.M. Low Optical Bandgap Polythiophenes by an Alternating Donor/Acceptor Repeat Unit Strategy. J. Am. Chem. Soc. 1997, 119, 5065–5066. [Google Scholar] [CrossRef]
- Schwiderski, R.L.; Rasmussen, S.C. Side chain tuning of frontier orbitals in polymers of thieno[3,4-b]-pyrazine-based terthienyls. Synth. Met. 2014, 193, 58–63. [Google Scholar] [CrossRef]
- Vyprachticky, D.; Kminek, I.; Pavlackova, P.; Cimrova, V. Syntheses of fluorene/carbazole-thienothiadiazole copolymers for organic photovoltaics. ECS Trans. 2011, 33, 111–118. [Google Scholar] [CrossRef]
- Karthik, D.; Kumar, V.; Justin Thomas, K.R.; Li, C.-T.; Ho, K.-C. Synthesis and characterization of thieno[3,4-d]imidazole-based organic sensitizers for photoelectrochemical cells. Dyes Pigments 2016, 129, 60–70. [Google Scholar] [CrossRef]
- Shen, X.; Chen, S.; Xiao, Z.; Zuo, Q.; Chen, Y.; Ding, L. Synthesis of thienoselenadiazole-containing conjugated copolymers and their application in polymer solar cells. Polym. J. 2012, 44, 978–981. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-L.; Lin, T.-P.; Chen, Y.-S.; Sun, S.-S. Probing receptor-anion interactions by ratiometric chemosensors containing pyrrolecarboxamide interacting sites. Eur. J. Org. Chem. 2007, 3999–4010. [Google Scholar] [CrossRef]
- Mangeney, C.; Lacroix, J.-C.; Chane-Ching, K.I.; Jouini, M.; Aeiyach, S.; Lacaze, P.-C. Poly(3′,4′-[bis(N,N’-ethyloxamyl)]terthiophene): A new functionalized conductive polymer with tunable pendent ethyloxamyl substituents. Phys. Chem. Chem. Phys. 1999, 1, 2755–2760. [Google Scholar] [CrossRef]
- Mangeney, C.; Lacroix, J.-C.; Chane-Ching, K.I.; Jouini, M.; Villain, F.; Ammar, S.; Jouini, N.; Lacaze, P.-C. Conducting-polymer electrochemical switching as an easy means for control of the molecular properties of grafted transition metal complexes. Chem. Eur. J. 2001, 7, 5029–5040. [Google Scholar] [CrossRef]
- Mehenni, H.; Dao, L.H. Towards the development of a direct electrochemical biodetector of avidin based on the poly(chloro amino-β-styryl terthiophene)-coated glassy carbon electrode. Aust. J. Chem. 2012, 65, 395–401. [Google Scholar] [CrossRef]
- Mehenni, H. Development of an avidin sensor based on the poly(methoxy amino-β-styryl terthiophene)-coated glassy carbon electrode. Can. J. Chem. 2012, 90, 271–277. [Google Scholar] [CrossRef]
- Abdelwahab, A.A.; Kim, D.-M.; Halappa, N.M.; Shim, Y.-B. A Selective Catalytic Oxidation of Ascorbic Acid at the Aminopyrimidyl Functionalized-Conductive Polymer Electrode. Electroanalysis 2013, 25, 1178–1184. [Google Scholar] [CrossRef]
- Park, M.-O.; Noh, H.-B.; Park, D.-S.; Yoon, J.-H.; Shim, Y.-B. Long-life Heavy Metal Ions Sensor Based on Graphene Oxide-anchored Conducting Polymer. Electroanalysis 2017, 29, 514–520. [Google Scholar] [CrossRef]
- Singh, R.P. A catechol biosensor based on a gold nanoparticles encapsulated-dendrimer. Analyst 2011, 136, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-M.; Shim, K.-B.; Son, J.I.; Reddy, S.S.; Shim, Y.-B. Spectroelectrochemical and electrochromic behaviors of newly synthesized poly[3′-(2-aminopyrimidyl)-2,2′:5′,2″-terthiophene]. Electrochim. Acta 2013, 104, 322–329. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.; Hwang, B.-Y.; Naveen, M.H.; Shim, Y.-B. Detection of Rocuronium in Whole Blood Using a Lipid-Bonded Conducting Polymer and Porous Carbon Composite Electrode. Electroanalysis 2018, 30, 1425–1431. [Google Scholar] [CrossRef]
- Park, M.-O.; Seo, K.-D.; Shim, Y.-B.; Yoon, J.-H.; Park, D.-S. Chromium(VI) sensor based on catalytic reduction using the nanoporous layer of poly(aminopyrimidyl- terthiophene) and AuNi composite. Sens. Actuators B Chem. 2019, 301, 127151. [Google Scholar] [CrossRef]
- Naveen, M.H.; Noh, H.-B.; Al Hossain, M.S.; Kim, J.H.; Shim, Y.-B. Facile potentiostatic preparation of functionalized polyterthiophene-anchored graphene oxide as a metal-free electrocatalyst for the oxygen reduction reaction. J. Mater. Chem. A Mater. Energy Sustain. 2015, 3, 5426–5433. [Google Scholar] [CrossRef]
- Herland, A.; Bjoerk, P.; Nilsson, K.P.R.; Olsson, J.D.M.; Asberg, P.; Konradsson, P.; Hammarstroem, P.; Inganaes, O. Electroactive luminescent self-assembled bio-organic nanowires: Integration of semiconducting oligoelectrolytes within amyloidogenic proteins. Adv. Mater. 2005, 17, 1466–1471. [Google Scholar] [CrossRef]
- Demanze, F.; Cornil, J.; Garnier, F.; Horowitz, G.; Valat, P.; Yassar, A.; Lazzaroni, R.; Brédas, J.-L. Tuning of the Electronic and Optical Properties of Oligothiophenes via Cyano Substitution: A Joint Experimental and Theoretical Study. J. Phys. Chem. B 1997, 101, 4553–4558. [Google Scholar] [CrossRef]
- Yassar, A.; Demanze, F.; Fichou, D. Synthesis and electrical properties of cyano-substituted oligothiophenes towards n-type organic semiconductors. Opt. Mater. 1999, 12, 379–382. [Google Scholar] [CrossRef]
- Hapiot, P.; Demanze, F.; Yassar, A.; Garnier, F. Molecular Engineering of Band Level Energies in Oligothiophenes, through Cyano-Substitutions. J. Phys. Chem. 1996, 100, 8397–8401. [Google Scholar] [CrossRef]
- Yassar, A.; Demanze, F.; Jaafari, A.; El Idrissi, M.; Coupry, C. Cyano-Substituted Oligothiophenes: A New Approach to n-Type Organic Semiconductors. Adv. Funct. Mater. 2002, 12, 699–708. [Google Scholar] [CrossRef]
- Hsu, D.-T.; Lin, C.-H. Synthesis of Benzo[c] and Naphtho[c]heterocycle Diesters and Dinitriles via Homoelongation. J. Org. Chem. 2009, 74, 9180–9187. [Google Scholar] [CrossRef]
- Demeter, D.; Allain, M.; Leriche, P.; Grosu, I.; Roncali, J. Synthesis and electronic properties of terthienyls β-substituted by (thienyl)cyanovinylene groups. Tetrahedron Lett. 2010, 51, 4117–4120. [Google Scholar] [CrossRef] [Green Version]
- Schweiger, L.F.; Ryder, K.S.; Morris, D.G.; Glidle, A.; Cooper, J.M. Strategies towards functionalised electronically conducting organic copolymers. J. Mater. Chem. 2000, 10, 107–114. [Google Scholar] [CrossRef]
- Pozo-Gonzalo, C.; Khan, T.; McDouall, J.J.W.; Skabara, P.J.; Roberts, D.M.; Light, M.E.; Coles, S.J.; Hursthouse, M.B.; Neugebauer, H.; Cravino, A.; et al. Synthesis and electropolymerisation of 3′,4′-bis(alkylsulfanyl)terthiophenes and the significance of the fused dithiin ring in 2,5-dithienyl-3,4-ethylenedithiothiophene (DT-EDTT). J. Mater. Chem. 2002, 12, 500–510. [Google Scholar] [CrossRef]
- Pozo-Gonzalo, C.; Roberts, D.M.M.; Skabara, P.J.J. 3,4-Disubstituted terthiophene systems: Synthesis and electropolymerization. Synth. Met. 2001, 119, 115–116. [Google Scholar] [CrossRef]
- Amb, C.M.; Rasmussen, S.C. Sterics versus Electronics: Regioselective Cross-Coupling of Polybrominated Thiophenes. Eur. J. Org. Chem. 2008, 2008, 801–804. [Google Scholar] [CrossRef]
- Wang, F.; Gu, H.; Swager, T.M. Carbon nanotube/polythiophene chemiresistive sensors for chemical warfare agents. J. Am. Chem. Soc. 2008, 130, 5392–5393. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Li, B.; Cao, Y.; Meng, X.; Yan, H.; Ge, S.; Lu, Y. Conjugated oligomers with thiophene and indole moieties: Synthesis, photoluminescence and electrochromic performances. Tetrahedron Lett. 2017, 58, 35–42. [Google Scholar] [CrossRef]
- Yamaguchi, I.; Nakahara, T. Reactive polythiophenes with zincke salt structure: Synthesis, polymer reactions, and chemical properties. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 3340–3349. [Google Scholar] [CrossRef]
- Schäferling, M.; Bäuerle, P. Porphyrin-functionalized oligo- and polythiophenes. J. Mater. Chem. 2004, 14, 1132–1141. [Google Scholar] [CrossRef]
- Bäuerle, P.; Scheib, S. Molecular recognition of alkali-ions by crown-ether-functionalized poly(alkylthiophenes). Adv. Mater. 1993, 5, 848–853. [Google Scholar] [CrossRef]
- Quagliotto, P.; Barbero, N.; Barolo, C.; Buscaino, R.; Carfora, P.; Prosperini, S.; Viscardi, G. Water based surfactant-assisted synthesis of thienylpyridines and thienylbipyridine intermediates. Dyes Pigments 2017, 137, 468–479. [Google Scholar] [CrossRef]
- Sun, C.; Prosperini, S.; Quagliotto, P.; Viscardi, G.; Yoon, S.S.; Gobetto, R.; Nervi, C. Electrocatalytic reduction of CO2 by thiophene-substituted rhenium(i) complexes and by their polymerized films. Dalt. Trans. 2016, 45, 14678–14688. [Google Scholar] [CrossRef] [PubMed]
- Vélez, J.H.; Díaz, F.R.; del Valle, M.A.; Bernède, J.C.; East, G.A. Synthesis of 3′,4′-disubstituted terthiophenes. Characterization and electropolymerization. I. 3′,4′-dibromo-2,2′:5′,2″-terthiophene in photovoltaic display. J. Appl. Polym. Sci. 2006, 102, 5314–5321. [Google Scholar] [CrossRef]
- Nagura, K.; Saito, S.; Yusa, H.; Yamawaki, H.; Fujihisa, H.; Sato, H.; Shimoikeda, Y.; Yamaguchi, S. Distinct responses to mechanical grinding and hydrostatic pressure in luminescent chromism of tetrathiazolylthiophene. J. Am. Chem. Soc. 2013, 135, 10322–10325. [Google Scholar] [CrossRef]
- Rahimi, A.; Namyslo, J.C.; Drafz, M.H.H.; Halm, J.; Hübner, E.; Nieger, M.; Rautzenberg, N.; Schmidt, A. Selective mono- to perarylations of tetrabromothiophene by a cyclobutene-1,2-diylbisimidazolium preligand. J. Org. Chem. 2011, 76, 7316–7325. [Google Scholar] [CrossRef]
- Tùng, Đ.T.; Tuân, Đ.T.; Rasool, N.; Villinger, A.; Reinke, H.; Fischer, C.; Langer, P. Regioselective Palladium(0)-Catalyzed Cross-Coupling Reactions and Metal-Halide Exchange Reactions of Tetrabromothiophene: Optimization, Scope and Limitations. Adv. Synth. Catal. 2009, 351, 1595–1609. [Google Scholar] [CrossRef]
- Dang, T.T.; Rasool, N.; Dang, T.T.; Reinke, H.; Langer, P. Synthesis of tetraarylthiophenes by regioselective Suzuki cross-coupling reactions of tetrabromothiophene. Tetrahedron Lett. 2007, 48, 845–847. [Google Scholar] [CrossRef]
- Lu, K.-M.; Li, W.-M.; Lin, P.-Y.; Liu, K.-T.; Liu, C.-Y. Direct C-H Arylation as a Chemoselective Single-Step Access to π-Acceptor-π Type Building Blocks. Adv. Synth. Catal. 2017, 359, 3805–3817. [Google Scholar] [CrossRef]
- Bilik, P.; Tanious, F.; Kumar, A.; Wilson, W.D.; Boykin, D.W.; Colson, P.; Houssier, C.; Facompré, M.; Tardy, C.; Bailly, C. Novel Dications with Unfused Aromatic Systems: Trithiophene and Trifuran Derivatives of Furimidazoline. ChemBioChem 2001, 2, 559–569. [Google Scholar] [CrossRef]
- Mitsudo, K.; Shimohara, S.; Mizoguchi, J.; Mandai, H.; Suga, S. Synthesis of nitrogen-bridged terthiophenes by tandem Buchwald-Hartwig coupling and their properties. Org. Lett. 2012, 14, 2702–2705. [Google Scholar] [CrossRef]
- Leitner, T.D.; Vogt, A.; Popović, D.; Mena-Osteritz, E.; Walzer, K.; Pfeiffer, M.; Bäuerle, P. Influence of alkyl chain length in S,N-heteropentacenes on the performance of organic solar cells. Mater. Chem. Front. 2018, 2, 959–968. [Google Scholar] [CrossRef]
- Lee, H.; Jo, H.; Kim, D.; Biswas, S.; Sharma, G.D.; Ko, J. The effect of acceptor end groups on the physical and photovoltaic properties of A-π-D-π-A type oligomers with same S, N-heteropentacene central electron donor unit for solution processed organic solar cells. Dyes Pigments 2016, 129, 209–219. [Google Scholar] [CrossRef]
- Schroeder, B.C.; Kirkus, M.; Nielsen, C.B.; Ashraf, R.S.; McCulloch, I. Dithienosilolothiophene: A New Polyfused Donor for Organic Electronics. Macromolecules 2015, 48, 5557–5562. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.; Popovic, D.; Vogt, A.; Kast, H.; Leitner, T.; Walzer, K.; Pfeiffer, M.; Mena-Osteritz, E.; Bäuerle, P. A-D-A-type S, N-Heteropentacenes: Next-Generation Molecular Donor Materials for Efficient Vacuum-Processed Organic Solar Cells. Adv. Mater. 2014, 26, 7217–7223. [Google Scholar] [CrossRef]
- Kimura, M.; Sakai, R.; Sato, S.; Fukawa, T.; Ikehara, T.; Maeda, R.; Mihara, T. Sensing of Vaporous Organic Compounds by TiO2 Porous Films Covered with Polythiophene Layers. Adv. Funct. Mater. 2012, 22, 469–476. [Google Scholar] [CrossRef]
- Bandini, M.; Pietrangelo, A.; Sinisi, R.; Umani-Ronchi, A.; Wolf, M.O. New Electrochemically Generated Polymeric Pd Complexes as Heterogeneous Catalysts for Suzuki Cross-Coupling Reactions. Eur. J. Org. Chem. 2009, 2009, 3554–3561. [Google Scholar] [CrossRef]
- SATAKE, Y.; ITO, S.; FUJIHARA, H. Synthesis and Electropolymerization of Terthiophene-modified Gold and Palladium Nanoparticles: Metal Nanoparticle-Polythiophene Composites. J. Japan Soc. Colour Mater. 2005, 78, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Michalitsch, R.; Elkassmi, A.; Yassar, A.; Gamier, F. A practical synthesis of functionalized alkyl-oligothiophenes for molecular self-assembly. J. Heterocycl. Chem. 2001, 38, 649–653. [Google Scholar] [CrossRef]
- Strover, L.; Roux, C.; Malmström, J.; Pei, Y.; Williams, D.E.; Travas-Sejdic, J. Switchable surfaces of electroactive polymer brushes grafted from polythiophene ATRP-macroinitiator. Synth. Met. 2012, 162, 381–390. [Google Scholar] [CrossRef]
- Foster, E.L.; De Leon, A.C.C.; Mangadlao, J.; Advincula, R. Electropolymerized and polymer grafted superhydrophobic, superoleophilic, and hemi-wicking coatings. J. Mater. Chem. 2012, 22, 11025–11031. [Google Scholar] [CrossRef]
- Robitaille, L.; Leclerc, M. Synthesis, Characterization, and Langmuir-Blodgett Films of Fluorinated Polythiophenes. Macromolecules 1994, 27, 1847–1851. [Google Scholar] [CrossRef]
- Crouch, D.J.; Sparrowe, D.; Heeney, M.; McCulloch, I.; Skabara, P.J. Polyterthiophenes Incorporating 3,4-Difluorothiophene Units: Application in Organic Field-Effect Transistors. Macromol. Chem. Phys. 2010, 211, 2642–2648. [Google Scholar] [CrossRef] [Green Version]
- Büchner, W.; Garreau, R.; Lemaire, M.; Roncali, J.; Garnier, F. Poly(fhiorinated 3-alkyl thiophene). J. Electroanal. Chem. 1990, 277, 355–358. [Google Scholar] [CrossRef]
- Facchetti, A.; Yoon, M.H.; Stern, C.L.; Hutchison, G.R.; Ratner, M.A.; Marks, T.J. Building blocks for N-type molecular and polymeric electronics. Perfluoroalkyl- versus alkyl-functionalized oligothiophenes (nTs; n = 2–6). Systematic synthesis, spectroscopy, electrochemistry, and solid-state organization. J. Am. Chem. Soc. 2004, 126, 13480–13501. [Google Scholar] [CrossRef]
- Le, Y.; Umemoto, Y.; Okabe, M.; Kusunoki, T.; Nakayama, K.I.; Pu, Y.J.; Kido, J.; Tada, H.; Aso, Y. Electronegative oligothiophenes based on difluorodioxocyclopentene- annelated thiophenes: Synthesis, properties, and n-Type FET performances. Org. Lett. 2008, 10, 833–836. [Google Scholar] [CrossRef]
- Ie, Y.; Umemoto, Y.; Kaneda, T.; Aso, Y. Electronegative oligothiophenes based on a hexafluorocyclopentene-annelated thiophene unit. Org. Lett. 2006, 8, 5381–5384. [Google Scholar] [CrossRef]
- Ie, Y.; Umemoto, Y.; Nitani, M.; Aso, Y. Perfluoroalkyl-annelated conjugated systems toward n-type organic semiconductors. Pure Appl. Chem. 2008, 80, 589–597. [Google Scholar] [CrossRef]
- Wu, T.; Boyer, J.-C.; Barker, M.; Wilson, D.; Branda, N.R. A “Plug-and-Play” Method to Prepare Water-Soluble Photoresponsive Encapsulated Upconverting Nanoparticles Containing Hydrophobic Molecular Switches. Chem. Mater. 2013, 25, 2495–2502. [Google Scholar] [CrossRef]
- Gronowitz, S.; Svensson, A. On the Ring-Opening of Some 3-Lithiobithienyls and 3′-Lithio-α-terthienyls. Isr. J. Chem. 1986, 27, 25–28. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.; Li, J.; Tu, G. Highly selective palladium-catalyzed Stille coupling reaction toward chlorine-containing NIR electroluminescent polymers. J. Mater. Chem. C 2015, 3, 7463–7468. [Google Scholar] [CrossRef]
- Imamura, K.; Hirayama, D.; Yoshimura, H.; Takimiya, K.; Aso, Y.; Otsubo, T. Application of flash vacuum pyrolysis to the synthesis of sulfur-containing heteroaromatic systems. Tetrahedron Lett. 1999, 40, 2789–2792. [Google Scholar] [CrossRef]
- Higgins, T.B.; Mirkin, C.A. Model Coordination Complexes for Designing Poly(terthiophene)/Rh(I) Hybrid Materials with Electrochemically Tunable Reactivities. Chem. Mater. 1998, 10, 1589–1595. [Google Scholar] [CrossRef]
- Awaji, H.; Nakahodo, T.; Fujihara, H. Synthesis of heterosegment-functioned hybrid nanotubes of polythiophene and heterometallic nanoparticles by sequential template-based electropolymerization. Chem. Commun. 2011, 47, 3547–3549. [Google Scholar] [CrossRef] [PubMed]
- Ponnapati, R.; Felipe, M.J.; Advincula, R. Electropolymerizable Terthiophene-Terminated Poly(aryl ether) Dendrimers with Naphthalene and Perylene Cores. Macromolecules 2011, 44, 7530–7537. [Google Scholar] [CrossRef]
- Tajima, T.; Nishihama, T.; Miyake, S.; Takahashi, N.; Takaguchi, Y. Synthesis and Properties of (Terthiophene) 4 –Poly(amidoamine)–C 60 Pentad. Bull. Chem. Soc. Jpn. 2015, 88, 736–745. [Google Scholar] [CrossRef]
- Taniguchi, N.; Nakabayashi, K.; Harada, T.; Tajima, N.; Shizuma, M.; Fujiki, M.; Imai, Y. Circularly Polarized Luminescence of Chiral Binaphthyl with Achiral Terthiophene Fluorophores. Chem. Lett. 2015, 44, 598–600. [Google Scholar] [CrossRef]
- Mantione, D.; Istif, E.; Dufil, G.; Vallan, L.; Parker, D.; Brochon, C.; Cloutet, E.; Hadziioannou, G.; Berggren, M.; Stavrinidou, E.; et al. Thiophene-Based Trimers for In Vivo Electronic Functionalization of Tissues. ACS Appl. Electron. Mater. 2020, 2, acsaelm.0c00861. [Google Scholar] [CrossRef]
- Ponnapati, R.; Felipe, M.J.; Park, J.Y.; Vargas, J.; Advincula, R. Terthiophene-Jacketed Poly(benzyl ether) Dendrimers: Sonication Synthesis, Electropolymerization, and Polythiophene Film Formation. Macromolecules 2010, 43, 10414–10421. [Google Scholar] [CrossRef]
- Melucci, M.; Dionigi, C.; Lanzani, G.; Viola, I.; Gigli, G.; Barbarella, G. Shaping Thiophene Oligomers into Fluorescent Nanobeads Forming Two-Dimensionally Patterned Assemblies by the Capillary Effect. Macromolecules 2005, 38, 10050–10054. [Google Scholar] [CrossRef]
- Mouffouk, F.; Brown, S.J.; Demetriou, A.M.; Higgins, S.J.; Nichols, R.J.; Rajapakse, R.M.G.; Reeman, S. Electrosynthesis and characterization of biotin-functionalized poly(terthiophene) copolymers, and their response to avidin. J. Mater. Chem. 2005, 15, 1186–1196. [Google Scholar] [CrossRef]
- Grande, C.D.; Tria, M.C.; Jiang, G.; Ponnapati, R.; Advincula, R. Surface-Grafted Polymers from Electropolymerized Polythiophene RAFT Agent. Macromolecules 2011, 44, 966–975. [Google Scholar] [CrossRef]
- Zanardi, C.; Scanu, R.; Pigani, L.; Pilo, M.I.; Sanna, G.; Seeber, R.; Spano, N.; Terzi, F.; Zucca, A. Synthesis and electrochemical polymerisation of 3′-functionalised terthiophenes. Electrochemical and spectroelectrochemical characterisation. Electrochim. Acta 2006, 51, 4859–4864. [Google Scholar] [CrossRef]
- Saha, S.; Baker, G.L. Surface-tethered conjugated polymers created via the grafting-from approach. J. Appl. Polym. Sci. 2015, 132, 41363/1–41363/9. [Google Scholar] [CrossRef]
- Xu, W.-C.; Zhou, Q.; Ashendel, C.L.; Chang, C.-T.; Chang, C.-J. Novel protein kinase C inhibitors: synthesis and PKC inhibition of β-substituted polythiophene derivatives. Bioorg. Med. Chem. Lett. 1999, 9, 2279–2282. [Google Scholar] [CrossRef]
- Spires, J.B.; Peng, H.; Williams, D.; Travas-Sejdic, J. An improved terthiophene conducting polymer for DNA-sensing. Electrochim. Acta 2011, 58, 134–141. [Google Scholar] [CrossRef]
- Janeliunas, D.; Eelkema, R.; Nieto-Ortega, B.; Ramirez Aguilar, F.J.; Lopez Navarrete, J.T.; van der Mee, L.; Stuart, M.C.A.; Casado, J.; van Esch, J.H. Designing new symmetrical facial oligothiophene amphiphiles. Org. Biomol. Chem. 2013, 11, 8435–8442. [Google Scholar] [CrossRef] [PubMed]
- van Rijn, P.; Savenije, T.J.; Stuart, M.C.A.; van Esch, J.H. Amphiphilic conjugated thiophenes for self-assembling antenna systems in water. Chem. Commun. 2009, 2163–2165. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Chong, H.; Lin, H.-A.; Yamashita, Y.; Zhang, B.; Huang, K.; Hashizume, D.; Yu, H. Palladium-catalyzed direct C–H arylations of dioxythiophenes bearing reactive functional groups: a step-economical approach for functional π-conjugated oligoarenes. Org. Biomol. Chem. 2015, 13, 8505–8511. [Google Scholar] [CrossRef] [Green Version]
- Miller, L.L.; Yu, Y. Synthesis of β-Methoxy, Methyl-Capped α-Oligothiophenes. J. Org. Chem. 1995, 60, 6813–6819. [Google Scholar] [CrossRef]
- Torsi, L.; Tafuri, A.; Cioffi, N.; Gallazzi, M.C.; Sassella, A.; Sabbatini, L.; Zambonin, P.G. Regioregular polythiophene field-effect transistors employed as chemical sensors. Sens. Actuators B Chem. 2003, 93, 257–262. [Google Scholar] [CrossRef]
- Rodriguez-Alba, E.; Ortiz-Palacios, J.; Morales-Espinoza, E.G.; Vonlanthen, M.; Valderrama, B.X.; Rivera, E. Synthesis, characterization and optical properties of novel oligothiophenes bearing pyrene units attached via well defined oligo(ethylene glycol) spacers. Synth. Met. 2015, 206, 92–105. [Google Scholar] [CrossRef]
- Rodriguez-Alba, E.; Ortiz-Palacios, J.; Vonlanthen, M.; Rojas-Montoya, S.M.; Porcu, P.; Ruiu, A.; Rivera, E. Design of novel well-defined oligothiophenes bearing donor-acceptor groups (pyrene-porphyrin): Synthesis, characterization, optical properties and energy transfer. J. Mol. Struct. 2019, 1183, 28–36. [Google Scholar] [CrossRef]
- Zotti, G.; Marin, R.A.; Gallazzi, M.C. Electrochemical Polymerization of Mixed Alkyl-AlkoxyBithiophenes and -terthiophenes. Substitution-Driven Polymerization from Thiophene Hexamers to Long-Chain Polymers. Chem. Mater. 1997, 9, 2945–2950. [Google Scholar] [CrossRef]
- Girotto, E.M.; Casalbore-Miceli, G.; Camaioni, N.; de Paoli, M.A.; Fichera, A.M.; Belobrzeckaja, L.; Gallazzi, M.C. Effect of the synthesis temperature and the length of alkyl substituents on photoelectrical properties of polyterthiophenes. J. Mater. Chem. 2001, 11, 1072–1076. [Google Scholar] [CrossRef] [Green Version]
- Arbizzani, C.; Gallazzi, M.C.; Mastragostino, M.; Rossi, M.; Soavi, F. Capacitance and cycling stability of poly(alkoxythiophene) derivative electrodes. Electrochem. Commun. 2001, 3, 16–19. [Google Scholar] [CrossRef]
- Gambhir, S.; Wagner, K.; Officer, D.L. Towards functionalized terthiophene-based polymers. Synth. Met. 2005, 154, 117–120. [Google Scholar] [CrossRef]
- Santos, M.J.L.; Girotto, E.M.; Nogueira, A.F. Photoelectrochemical properties of poly(terthiophene) films modified with a fullerene derivative. Thin Solid Films 2006, 515, 2644–2649. [Google Scholar] [CrossRef]
- Wang, C.Y.; Tsekouras, G.; Wagner, P.; Gambhir, S.; Too, C.O.; Officer, D.; Wallace, G.G. Functionalised polyterthiophenes as anode materials in polymer/polymer batteries. Synth. Met. 2010, 160, 76–82. [Google Scholar] [CrossRef]
- Czichy, M.; Wagner, P.; Lapkowski, M.; Officer, D.L. Effect of π-conjugation on electrochemical properties of poly(terthiophene)s 3′-substituted with fullerene C60. J. Electroanal. Chem. 2016, 772, 103–109. [Google Scholar] [CrossRef]
- Gallazzi, M.C.; Toscano, F.; Paganuzzi, D.; Bertarelli, C.; Farina, A.; Zotti, G. Polythiophenes with unusual electrical and optical properties based on donor acceptor alternance strategy. Macromol. Chem. Phys. 2001, 202, 2074–2085. [Google Scholar] [CrossRef]
- Mares, D.; Romagnoli, C.; Rossi, R.; Carpita, A.; Ciofalo, M.; Bruni, A. Antifungal activity of some 2,2′:5′,2″-terthiophene derivatives. Mycoses 1994, 37, 377–383. [Google Scholar] [CrossRef]
- Rossi, R.; Carpita, A.; Ciofalo, M.; Lippolis, V. Selective and efficient syntheses of phototoxic 2,2′:5′,2″-terthiophene derivatives bearing a functional substituent in the 3′- or the 5-position. Tetrahedron 1991, 47, 8443–8460. [Google Scholar] [CrossRef]
- Abdiryim, T.; Jamal, R.; Ubul, A.; Nurulla, I. Solid-state synthesis of poly(3′,4′-dimethoxy-2,2′:5′,2″-terthiophene): Comparison with poly(terthiophene) and poly(3′,4′-ethylenedioxy-2,2′:5′,2″-terthiophene). Molecules 2012, 17, 8647–8660. [Google Scholar] [CrossRef]
- Yigit, D.; Aykan, M.; Guellue, M. Substituent effect on supercapacitive performances of conducting polymer-based redox electrodes: Poly(3′,4′-bis(alkyloxy) 2,2’:5’,2’’-terthiophene) derivatives. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 480–495. [Google Scholar] [CrossRef]
- Lisak, G.; Wagner, K.; Wagner, P.; Barnsley, J.E.; Gordon, K.C.; Bobacka, J.; Wallace, G.G.; Ivaska, A.; Officer, D.L. A novel modified terpyridine derivative as a model molecule to study kinetic-based optical spectroscopic ion determination methods. Synth. Met. 2016, 219, 101–108. [Google Scholar] [CrossRef]
- Van Rijn, P.; Janeliunas, D.; Brizard, A.M.; Stuart, M.C.A.; Koper, G.J.M.; Eelkema, R.; van Esch, J.H. Self-assembly behaviour of conjugated terthiophene surfactants in water. New J. Chem. 2011, 35, 558–567. [Google Scholar] [CrossRef]
- Umeda, R.; Awaji, H.; Nakahodo, T.; Fujihara, H. Nanotube Composites Consisting of Metal Nanoparticles and Polythiophene from Electropolymerization of Terthiophene-Functionalized Metal (Au, Pd) Nanoparticles. J. Am. Chem. Soc. 2008, 130, 3240–3241. [Google Scholar] [CrossRef]
- Lee, J.U.; Huh, J.; Kim, K.H.; Park, C.; Jo, W.H. Aqueous suspension of carbon nanotubes via non-covalent functionalization with oligothiophene-terminated poly(ethylene glycol). Carbon N. Y. 2007, 45, 1051–1057. [Google Scholar] [CrossRef]
- Wang, Y.; Partridge, A.; Wu, Y. Comparison of a carboxylated terthiophene surface with carboxymethylated dextran layer for surface plasmon resonance detection of progesterone. Anal. Biochem. 2016, 508, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Partridge, A.; Wu, Y. Improving nanoparticle-enhanced surface plasmon resonance detection of small molecules by reducing steric hindrance via molecular linkers. Talanta 2019, 198, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Grant, D.K.; Jolley, K.W.; Officer, D.L.; Gordon, K.C.; Clarke, T.M. Towards functionalized poly(terthiophenes): Regioselective synthesis of oligoether-substituted bis(styryl)sexithiophenes. Org. Biomol. Chem. 2005, 3, 2008–2015. [Google Scholar] [CrossRef] [PubMed]
- Demeter, D.; Blanchard, P.; Allain, M.; Grosu, I.; Roncali, J. Synthesis and Metal Cation Complexing Properties of Crown-Annelated Terthiophenes Containing 3,4-Ethylenedioxythiophene. J. Org. Chem. 2007, 72, 5285–5290. [Google Scholar] [CrossRef]
- Yamamoto, T.; Omote, M.; Miyazaki, Y.; Kashiwazaki, A.; Lee, B.-L.; Kanbara, T.; Osakada, K.; Inoue, T.; Kubota, K. Poly(thiophene-2,5-diyl)s with a Crown Ethereal Subunit. Preparation, Optical Properties, and n-Doped State Stabilized against Air. Macromolecules 1997, 30, 7158–7165. [Google Scholar] [CrossRef]
- Berlin, A.; Zotti, G.; Zecchin, S.; Schiavon, G. EQCM analysis of the alkali metal ion coordination properties of novel poly(thiophene)s 3,4-functionalized with crown-ether moieties. Synth. Met. 2002, 131, 149–160. [Google Scholar] [CrossRef]
- Baeuerle, P.; Scheib, S. Synthesis and characterization of thiophenes, oligothiophenes and polythiophenes with crown ether units in direct π-conjugation. Acta Polym. 1995, 46, 124–129. [Google Scholar] [CrossRef]
- Lukovskaya, E.V.; Bobyleva, A.A.; Fedorova, O.A.; Fedorov, Y.V.; Anisimov, A.V.; Didane, Y.; Brisset, H.; Fages, F. Novel crown-containing 3-styryl derivatives of oligothiophenes: synthesis, structure, and optical and electrochemical characteristics. Russ. Chem. Bull. 2009, 58, 1509–1515. [Google Scholar] [CrossRef]
- Reddinger, J.L.; Reynolds, J.R. A Novel Polymeric Metallomacrocycle Sensor Capable of Dual-Ion Cocomplexation. Chem. Mater. 1998, 10, 3–5. [Google Scholar] [CrossRef]
- Goldoni, F.; Antolini, L.; Pourtois, G.; Schenning, A.P.H.J.; Janssen, R.A.J.; Lazzaroni, R.; Bredas, J.-L.; Meijer, E.W. Effect of ion coordination on the conformational and electronic structure of 3,4-bis(alkylthio)thiophenes. Eur. J. Inorg. Chem. 2001, 821–828. [Google Scholar] [CrossRef]
- Faye, D.; Duong, T.H.; Vieitez, I.; Gohier, F.F.; Brisset, H.; Frere, P.; Briand, J.-F.J.-F.; Leriche, P.; Bressy, C.; Frère, P.; et al. Electroactive polyacrylates bearing linear conjugated systems based on EDOT moieties. Polymer 2017, 117, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Barbarella, G.; Zambianchi, M.; Di Toro, R.; Colonna, M.J.; Iarossi, D.; Goldoni, F.; Bongini, A. Regioselective Oligomerization of 3-(Alkylsulfanyl)Thiophenes with Ferric Chloride. J. Org. Chem. 1996, 61, 8285–8292. [Google Scholar] [CrossRef]
- Rossi, R.; Ciofalo, M.; Carpita, A.; Ponterini, G. Singlet-triplet intersystem crossing in 2,2’:5’,2’’-terthiophene and some of its derivatives. J. Photochem. Photobiol. A Chem. 1993, 70, 59–67. [Google Scholar] [CrossRef]
- Spencer, H.J.; Skabara, P.J.; Giles, M.; McCulloch, I.; Coles, S.J.; Hursthouse, M.B. The first direct experimental comparison between the hugely contrasting properties of PEDOT and the all-sulfur analogue PEDTT by analogy with well-defined EDTT-EDOT copolymers. J. Mater. Chem. 2005, 15, 4783–4792. [Google Scholar] [CrossRef]
- Skabara, P.J.; Serebryakov, I.M.; Roberts, D.M.; Perepichka, I.F.; Coles, S.J.; Hursthouse, M.B. Novel Terthiophene and Bis(thienyl)furan Derivatives as Precursors to Highly Electroactive Polymers. J. Org. Chem. 1999, 64, 6418–6424. [Google Scholar] [CrossRef]
- Skabara, P.J.; Berridge, R.; Serebryakov, I.M.; Kanibolotsky, A.L.; Kanibolotskaya, L.; Gordeyev, S.; Perepichka, I.F.; Sariciftci, N.S.; Winder, C. cFluorene functionalised sexithiophenes-utilising intramolecular charge transfer to extend the photocurrent spectrum in organic solar cells. J. Mater. Chem. 2007, 17, 1055–1062. [Google Scholar] [CrossRef]
- Berridge, R.; Skabara, P.J.; Pozo-Gonzalo, C.; Kanibolotsky, A.; Lohr, J.; McDouall, J.J.W.; McInnes, E.J.L.; Wolowska, J.; Winder, C.; Sariciftci, N.S.; et al. Incorporation of Fused Tetrathiafulvalenes (TTFs) into Polythiophene Architectures: Varying the Electroactive Dominance of the TTF Species in Hybrid Systems. J. Phys. Chem. B 2006, 110, 3140–3152. [Google Scholar] [CrossRef] [PubMed]
- Skabara, P.J.; Roberts, D.M.; Serebryakov, I.M.; Pozo-Gonzalo, C. The development of an electropolymerizable unit for TTF-thiophene fused monomers. Chem. Commun. 2000, 1005–1006. [Google Scholar] [CrossRef]
- Skabara, P.J.; Serebryakov, I.M.; Perepichka, I.F.; Sariciftci, N.S.; Neugebauer, H.; Cravino, A. Toward Controlled Donor-Acceptor Interactions in Noncomposite Polymeric Materials: Synthesis and Characterization of a Novel Polythiophene Incorporating π-Conjugated 1,3-Dithiole-2-ylidenefluorene Units as Strong D-A Components. Macromolecules 2001, 34, 2232–2241. [Google Scholar] [CrossRef]
- Berridge, R.; Wright, S.P.; Skabara, P.J.; Dyer, A.; Steckler, T.; Argun, A.A.; Reynolds, J.R.; Harrington, R.W.; Clegg, W. Electrochromic properties of a fast switching, dual colour polythiophene bearing non-planar dithiinoquinoxaline units. J. Mater. Chem. 2007, 17, 225–231. [Google Scholar] [CrossRef]
- Forgie, J.C.; Kanibolotsky, A.L.; Skabara, P.J.; Coles, S.J.; Hursthouse, M.B.; Harrington, R.W.; Clegg, W. Electrochemical, Spectroelectrochemical, and Comparative Studies of Novel Organic Conjugated Monomers and Polymers Featuring the Redox-Active Unit Tetrathianaphthalene. Macromolecules 2009, 42, 2570–2580. [Google Scholar] [CrossRef]
- Ie, Y.; Yoshimura, A.; Takeuchi, D.; Osakada, K.; Aso, Y. Synthesis and properties of polymer having electronegative terthiophene pendants based on cyclopenta[c]thiophene. Chem. Lett. 2011, 40, 1039–1040. [Google Scholar] [CrossRef]
- Endou, M.; Ie, Y.; Aso, Y. Encapsulated oligothiophenes having electron-affinity characteristics. Chem. Commun. 2012, 48, 540–542. [Google Scholar] [CrossRef]
- Qian, D.; Ye, L.; Zhang, M.; Liang, Y.; Li, L.; Huang, Y.; Guo, X.; Zhang, S.; Tan, Z.; Hou, J. Design, Application, and Morphology Study of a New Photovoltaic Polymer with Strong Aggregation in Solution State. Macromolecules 2012, 45, 9611–9617. [Google Scholar] [CrossRef]
- Qian, D.; Ma, W.; Li, Z.; Guo, X.; Zhang, S.; Ye, L.; Ade, H.; Tan, Z.; Hou, J. Molecular Design toward Efficient Polymer Solar Cells with High Polymer Content. J. Am. Chem. Soc. 2013, 135, 8464–8467. [Google Scholar] [CrossRef]
- Bin, H.; Xiao, L.; Liu, Y.; Shen, P.; Li, Y. Effects of donor unit and π-bridge on photovoltaic properties of D-A copolymers based on benzo[1,2-b:4,5-c’]-dithiophene-4,8-dione acceptor unit. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1929–1940. [Google Scholar] [CrossRef]
- Zhang, G.; Guo, J.; Zhang, J.; Li, W.; Wang, X.; Lu, H.; Qiu, L. Benzodithiophenedione and diketopyrrolopyrrole based conjugated copolymers for organic thin-film transistors by structure modulation. Dyes Pigments 2016, 126, 20–28. [Google Scholar] [CrossRef]
- Liu, T.; Meng, D.; Cai, Y.; Sun, X.; Li, Y.; Huo, L.; Liu, F.; Wang, Z.; Russell, T.P.; Sun, Y. High-Performance Non-Fullerene Organic Solar Cells Based on a Selenium-Containing Polymer Donor and a Twisted Perylene Bisimide Acceptor. Adv. Sci. 2016, 3. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Weng, K.; Huo, L.; Fan, B.; Yang, C.; Sun, X.; Sun, Y. Effects of a heteroatomic benzothienothiophenedione acceptor on the properties of a series of wide-bandgap photovoltaic polymers. J. Mater. Chem. C Mater. Opt. Electron. Devices 2016, 4, 9052–9059. [Google Scholar] [CrossRef]
- Li, Z.; Weng, K.; Chen, A.; Sun, X.; Wei, D.; Yu, M.; Huo, L.; Sun, Y. Benzothiadiazole Versus Thiophene: Influence of the Auxiliary Acceptor on the Photovoltaic Properties of Donor-Acceptor-Based Copolymers. Macromol. Rapid Commun. 2018, 39. [Google Scholar] [CrossRef]
- Rehman, T.; Liu, Z.-X.; Lau, T.-K.; Yu, Z.; Shi, M.; Lu, X.; Li, C.-Z.; Chen, H. Influence of Bridging Groups on the Photovoltaic Properties of Wide-Bandgap Poly(BDTT-alt-BDD)s. ACS Appl. Mater. Interfaces 2019, 11, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- Lopes Graca, J.F.; Chane-Ching, K.I.; Yassar, A. A new polymer based on a conjugated terthiophene-β-diketone ligand: Electrochemical study and structural aspects. Electrochim. Acta 2005, 50, 1475–1480. [Google Scholar] [CrossRef]
- Fuse, S.; Asai, Y.; Sugiyama, S.; Matsumura, K.; Maitani, M.M.; Wada, Y.; Ogomi, Y.; Hayase, S.; Kaiho, T.; Takahashi, T. Synthesis of EDOT-containing organic dyes via one-pot, four-component Suzuki–Miyaura coupling and the evaluation of their photovoltaic properties. Tetrahedron 2014, 70, 8690–8695. [Google Scholar] [CrossRef]
- Istif, E.; Mantione, D.; Vallan, L.; Hadziioannou, G.; Brochon, C.; Cloutet, E.; Pavlopoulou, E. Thiophene-Based Aldehyde Derivatives for Functionalizable and Adhesive Semiconducting Polymers. ACS Appl. Mater. Interfaces 2020, 12, 8695–8703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, K.; Crowe, L.L.; Wagner, P.; Gambhir, S.; Partridge, A.C.; Earles, J.C.; Clarke, T.M.; Gordon, K.C.; Officer, D.L. Indanedione-Substituted Poly(terthiophene)s: Processable Conducting Polymers with Intramolecular Charge Transfer Interactions. Macromolecules 2010, 43, 3817–3827. [Google Scholar] [CrossRef] [Green Version]
- Collis, G.E.; Burrell, A.K.; Scott, S.M.; Officer, D.L. Toward Functionalized Conducting Polymers: Synthesis and Characterization of Novel β-(Styryl)terthiophenes. J. Org. Chem. 2003, 68, 8974–8983. [Google Scholar] [CrossRef]
- Collis, G.E.; Burrell, A.K.; Officer, D.L. β-Terthiophene aldehyde and phosphonate: Key building blocks for the synthesis of functionalized conducting polymers. Tetrahedron Lett. 2001, 42, 8733–8735. [Google Scholar] [CrossRef]
- Liao, Z.; Wang, Y.; An, Y.; Tan, Y.; Meng, X.; Wu, F.; Chen, L.; Chen, Y. Post-Treatment-Free Main Chain Donor and Side Chain Acceptor (D-s-A) Copolymer for Efficient Nonfullerene Solar Cells with a Small Voltage Loss. Macromol. Rapid Commun. 2018, 39. [Google Scholar] [CrossRef]
- Elmas, S.; Beelders, W.; Bradley, S.J.; Kroon, R.; Laufersky, G.; Andersson, M.; Nann, T. Platinum Terpyridine Metallopolymer Electrode as Cost-Effective Replacement for Bulk Platinum Catalysts in Oxygen Reduction Reaction and Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2017, 5, 10206–10214. [Google Scholar] [CrossRef]
- Lee, T.-Y.; Shim, Y.-B.; Shin, S.C. Simple preparation of terthiophene-3′-carboxylic acid and characterization of its polymer. Synth. Met. 2002, 126, 105–110. [Google Scholar] [CrossRef]
- Zanoni, M.; Coleman, S.; Fraser, K.J.; Byrne, R.; Wagner, K.; Gambhir, S.; Officer, D.L.; Wallace, G.G.; Diamond, D. Physicochemical study of spiropyran-terthiophene derivatives: Photochemistry and thermodynamics. Phys. Chem. Chem. Phys. 2012, 14, 9112–9120. [Google Scholar] [CrossRef] [Green Version]
- McTiernan, C.D.; Abbas, S.A.; Chahma, M. Organic surface modification using stable conducting materials. New J. Chem. 2012, 36, 2106–2111. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jeong, E.-D.; Ahn, C.W.; Lee, J.-W. Bioactive conducting scaffolds: Active ester-functionalized polyterthiophene. Synth. Met. 2013, 185–186, 66–70. [Google Scholar] [CrossRef]
- Yassar, A.; Moustrou, C.; Youssoufi, H.K.; Samat, A.; Guglielmetti, R.; Garnier, F. Synthesis and Characterization of Poly(thiophenes) Functionalized by Photochromic Spironaphthoxazine Groups. Macromolecules 1995, 28, 4548–4553. [Google Scholar] [CrossRef]
- Jang, S.-Y.; Sotzing, G.A.; Marquez, M. Intrinsically Conducting Polymer Networks of Poly(thiophene) via Solid-State Oxidative Cross-Linking of a Poly(norbornylene) Containing Terthiophene Moieties. Macromolecules 2002, 35, 7293–7300. [Google Scholar] [CrossRef]
- Destri, S.; Porzio, W.; Meinardi, F.; Tubino, R.; Salerno, G. Novel Erbium-Substituted Oligothiophene Chelates for Infrared Emission. Macromolecules 2003, 36, 273–275. [Google Scholar] [CrossRef]
- Destri, S.; Pasini, M.; Porzio, W.; Rizzo, F.; Dellepiane, G.; Ottonelli, M.; Musso, G.; Meinardi, F.; Veltri, L. New erbium complexes emitting in infrared region based on oligothiophene and thiophenefluorene carboxylate. J. Lumin. 2007, 127, 601–610. [Google Scholar] [CrossRef]
- Pokrop, R.; Pamula, K.; Deja-Drogomirecka, S.; Zagorska, M.; Borysiuk, J.; Reiss, P.; Pron, A. Electronic, electrochemical, and spectroelectrochemical properties of hybrid materials consisting of carboxylic acid derivatives of oligothiophene and CdSe semiconductor nanocrystals. J. Phys. Chem. C 2009, 113, 3487–3493. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, B.; Dong, L.; Sun, H.; Hu, D.; Xing, H.; Duan, X.; Chen, S.; Xu, J. Solvent effects on the synthesis, characterization and electrochromic properties of acetic acid modified polyterthiophene. Electrochim. Acta 2016, 220, 122–129. [Google Scholar] [CrossRef]
- Tiu, B.D.B.; Pernites, R.B.; Tiu, S.B.; Advincula, R.C. Detection of aspartame via microsphere-patterned and molecularly imprinted polymer arrays. Colloids Surfaces, A Physicochem. Eng. Asp. 2016, 495, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Zhang, L.; Spires, J.; Soeller, C.; Travas-Sejdic, J. Synthesis of a functionalized polythiophene as an active substrate for a label-free electrochemical genosensor. Polymer 2007, 48, 3413–3419. [Google Scholar] [CrossRef]
- Spires, J.B.; Peng, H.; Williams, D.; Travas-Sejdic, J. The solvent-induced collapse of a redox-active conducting polymer and the consequence on its DNA-sensing ability. J. Electroanal. Chem. 2011, 658, 1–9. [Google Scholar] [CrossRef]
- Bruns, C.J.; Herman, D.J.; Minuzzo, J.B.; Lehrman, J.A.; Stupp, S.I. Rationalizing Molecular Design in the Electrodeposition of Anisotropic Lamellar Nanostructures. Chem. Mater. 2013, 25, 4330–4339. [Google Scholar] [CrossRef]
- Baeuerle, P.; Hiller, M.; Scheib, S.; Sokolowski, M.; Umbach, E. Post-polymerization functionalization of conducting polymers. Novel poly(alkylthiophene)s substituted with easily replaceable activated ester groups. Adv. Mater. 1996, 8, 214–218. [Google Scholar] [CrossRef]
- Kim, D.-M.; Yoon, J.-H.; Won, M.-S.; Shim, Y.-B. Electrochemical characterization of newly synthesized polyterthiophene benzoate and its applications to an electrochromic device and a photovoltaic cell. Electrochim. Acta 2012, 67, 201–207. [Google Scholar] [CrossRef]
- Beouch, L.; Boileau, S.; Chevrot, C.; Tran-Van, F. Electropolymerization of hydrogen bond supramolecular associations between terthiophene-3-acetic acid and 4,4′-bipyridine. Polym. Int. 2017, 66, 1389–1394. [Google Scholar] [CrossRef]
- Badeva, D.; Tran-Van, F.; Beouch, L.; Chevrot, C.; Markova, I.; Racheva, T.; Froyer, G. Embedding and electropolymerization of terthiophene derivatives in porous n-type silicon. Mater. Chem. Phys. 2012, 133, 592–598. [Google Scholar] [CrossRef]
- Boopathi, M.; Won, M.-S.; Kim, Y.H.; Shin, S.C.; Shim, Y.-B. Electrocatalytic Reduction of Molecular Oxygen Using a Poly(terthiophene carboxylic acid) Appended by 1,5-Diaminonaphthalene Copper Complex. J. Electrochem. Soc. 2002, 149, E265–E271. [Google Scholar] [CrossRef]
- Destri, S.; Giovanella, U.; Fazio, A.; Porzio, W.; Gabriele, B.; Zotti, G. A new soluble poly(bithiophene)-co-3,4-di(methoxycarbonyl)methyl thiophene for LED. Org. Electron. 2002, 3, 149–156. [Google Scholar] [CrossRef]
- Fazio, A.; Gabriele, B.; Salerno, G.; Destri, S. Synthesis of 3,4-bis[(methoxycarbonyl)methylthiophene and bis-, ter- and pentathiophenes with alternating 3,4-bis[(methoxycarbonyl)methyl] substituted rings. Tetrahedron 1999, 55, 485–502. [Google Scholar] [CrossRef]
- Atilgan, N.; Cihaner, A.; Oenal, A.M. Electrochromic performance and ion sensitivity of a terthienyl based fluorescent polymer. React. Funct. Polym. 2010, 70, 244–250. [Google Scholar] [CrossRef]
- Taranekar, P.; Fulghum, T.; Baba, A.; Patton, D.; Advincula, R. Quantitative electrochemical and electrochromic behavior of terthiophene and carbazole containing conjugated polymer network film precursors: EC-QCM and EC-SPR. Langmuir 2007, 23, 908–917. [Google Scholar] [CrossRef]
- Allard, S.; Braun, L.; Brehmer, M.; Zentel, R. Oligothiophenes for pattern formation by stamping. Macromol. Chem. Phys. 2003, 204, 68–75. [Google Scholar] [CrossRef]
- Asil, D.; Cihaner, A.; Oenal, A.M. Electropolymerization and ion sensitivity of chemiluminescent thienyl systems. Electrochim. Acta 2009, 54, 6740–6746. [Google Scholar] [CrossRef]
- Atilgan, N.; Algi, F.; Oenal, A.M.; Cihaner, A. Synthesis and properties of a novel redox driven chemiluminescent material built on a terthienyl system. Tetrahedron 2009, 65, 5776–5781. [Google Scholar] [CrossRef]
- Watson, K.J.; Wolfe, P.S.; Nguyen, S.T.; Zhu, J.; Mirkin, C.A. Norbornenyl-Substituted Thiophenes and Terthiophenes: Novel Doubly Polymerizable Monomers. Macromolecules 2000, 33, 4628–4633. [Google Scholar] [CrossRef]
- Higgins, S.J.; Mouffouk, F.; Brown, S.J.; Williams, D.R.; Cossins, A.R. An electrogenerated polyterthiophene for binding and sensing polyadenosine-functionalised oligonucleotides. Sens. Actuators B Chem. 2007, 122, 253–258. [Google Scholar] [CrossRef]
- Gelmi, A.; Zanoni, M.; Higgins, M.J.; Gambhir, S.; Officer, D.L.; Diamond, D.; Wallace, G.G. Optical switching of protein interactions on photosensitive-electroactive polymers measured by atomic force microscopy. J. Mater. Chem. B Mater. Biol. Med. 2013, 1, 2162–2168. [Google Scholar] [CrossRef] [Green Version]
- Saitou, K.; Nishiyabu, R.; Iyoda, M.; Kubo, Y. Gold nanoparticle-templated assembly of oligothiophenes: Preparation and film properties. Tetrahedron 2011, 67, 9685–9689. [Google Scholar] [CrossRef]
- Maione, S.; Fabregat, G.; del Valle, L.J.; Bendrea, A.-D.; Cianga, L.; Cianga, I.; Estrany, F.; Aleman, C. Effect of the graft ratio on the properties of polythiophene-g-poly(ethylene glycol). J. Polym. Sci. Part B Polym. Phys. 2015, 53, 239–252. [Google Scholar] [CrossRef] [Green Version]
- Sakakibara, K.; Rosenau, T. Polythiophene-cellulose composites: synthesis, optical properties and homogeneous oxidative co-polymerization. Holzforschung 2012, 66, 9–19. [Google Scholar] [CrossRef]
- Jiang, G.; Ponnapati, R.; Pernites, R.; Felipe, M.J.; Advincula, R. Surface-Initiated Ring-Opening Metathesis Polymerization (SI-ROMP): Synthesis and Electropolymerization of Terthiophene-Functionalized Olefin Peripheral Dendrons. Macromolecules 2010, 43, 10262–10274. [Google Scholar] [CrossRef]
- Nicoletta, F.P.; Chidichimo, G.; Cupelli, D.; De Filpo, G.; De Benedittis, M.; Gabriele, B.; Salerno, G.; Fazio, A. Electrochromic polymer-dispersed liquid-crystal film: A new bifunctional device. Adv. Funct. Mater. 2005, 15, 995–999. [Google Scholar] [CrossRef]
- Asil, D.; Cihaner, A.; Algi, F.; Oenal, A.M. A novel conducting polymer based on terthienyl system bearing strong electron-withdrawing substituents and its electrochromic device application. J. Electroanal. Chem. 2008, 618, 87–93. [Google Scholar] [CrossRef]
- McTiernan, C.D.; Chahma, M. Synthesis and characterization of alanine functionalized oligo/polythiophenes. New J. Chem. 2010, 34, 1417–1423. [Google Scholar] [CrossRef]
- Kim, D.-S.; Ahn, K.H. Fluorescence “turn-on” sensing of carboxylate anions with oligothiophene-based o-(carboxamido)trifluoroacetophenones. J. Org. Chem. 2008, 73, 6831–6834. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, C.D.; Omri, K.; Chahma, M. Chiral Conducting Surfaces via Electrochemical Oxidation of L-Leucine-Oligothiophenes. J. Org. Chem. 2010, 75, 6096–6103. [Google Scholar] [CrossRef] [PubMed]
- Chahma, M.; McTiernan, C.D.; Abbas, S.A. Characterization of phenomena occurring at the interface of chiral conducting surfaces. New J. Chem. 2014, 38, 3379–3385. [Google Scholar] [CrossRef]
- Kaewtong, C.; Niamsa, N.; Wanno, B.; Morakot, N.; Pulpoka, B.; Tuntulani, T. Optical chemosensors for Hg2+ from terthiophene appended rhodamine derivatives: FRET based molecular and in situ hybrid gold nanoparticle sensors. New J. Chem. 2014, 38, 3831–3839. [Google Scholar] [CrossRef]
- Guo, H.; Liu, M.; Han, Y.; Han, S.; Chen, Y. Synthesis and characterization of S,N-heteroacenes by Bischler-Napieralski reaction. Chin. J. Polym. Sci. 2016, 34, 1319–1329. [Google Scholar] [CrossRef]
- Guo, X.; Ortiz, R.P.; Zheng, Y.; Kim, M.-G.; Zhang, S.; Hu, Y.; Lu, G.; Facchetti, A.; Marks, T.J. Thieno[3,4-c]pyrrole-4,6-dione-Based Polymer Semiconductors: Toward High-Performance, Air-Stable Organic Thin-Film Transistors. J. Am. Chem. Soc. 2011, 133, 13685–13697. [Google Scholar] [CrossRef]
- Najari, A.; Beaupre, S.; Berrouard, P.; Zou, Y.; Pouliot, J.-R.; Lepage-Perusse, C.; Leclerc, M. Synthesis and characterization of new thieno[3,4-c]pyrrole-4,6-dione derivatives for photovoltaic applications. Adv. Funct. Mater. 2011, 21, 718–728. [Google Scholar] [CrossRef]
- Wen, S.; Cheng, W.; Li, P.; Yao, S.; Xu, B.; Li, H.; Gao, Y.; Wang, Z.; Tian, W. Synthesis and Photovoltaic Properties of Thieno[3,4-c]pyrrole-4,6-dione-based donor-acceptor Copolymers. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 3758–3766. [Google Scholar] [CrossRef]
- Zhang, G.; Fu, Y.; Xie, Z.; Zhang, Q. Low bandgap polymers with benzo [1,2-b:4,5-b’] dithiophene and bisthiophene-dioxopyrrolothiophene units for photovoltaic applications. Polymer 2011, 52, 415–421. [Google Scholar] [CrossRef]
- Lu, Y.; Lei, Y.; Wu, B.; Xu, X.; Zhu, F.; Hu, X.; Ong, B.S.; Ng, S.C. Synthesis and properties of benzo[c]-, pyrrolo[3,4-c]-, and thieno[3,4-c]-pyrrole-4,6-dione copolymers. New J. Chem. 2015, 39, 2642–2650. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Z.; Hu, Q.; Liu, F.; Russell, T.P.; Zhu, X. 1,3-Bis(thieno[3,4-b]thiophen-6-yl)-4H-thieno[3,4-c]pyrrole-4,6(5H)-dione-Based Small-Molecule Donor for Efficient Solution-Processed Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 6213–6219. [Google Scholar] [CrossRef]
- Kim, J.; Lee, W.-H.; Park, J.B.; Hwang, D.-H.; Kang, I.-N. Synthesis and characterization of the fluorinated thieno[3,4-c]pyrrole-4,6-dione-based donor-acceptor polymers for organic solar cells. Dyes Pigments 2019, 160, 403–409. [Google Scholar] [CrossRef]
- Sonmez, G.; Meng, H.; Wudl, F. Very Stable Low Band Gap Polymer for Charge Storage Purposes and Near-Infrared Applications. Chem. Mater. 2003, 15, 4923–4929. [Google Scholar] [CrossRef]
- Blanco, R.; Gomez, R.; Seoane, C.; Segura, J.L.; Mena-Osteritz, E.; Baeuerle, P. An Ambipolar Peryleneamidine Monoimide-Fused Polythiophene with Narrow Band Gap. Org. Lett. 2007, 9, 2171–2174. [Google Scholar] [CrossRef]
- Raimundo, J.-M.; Blanchard, P.; Brisset, H.; Akoudad, S.; Roncali, J. Proquinoid acceptors as building blocks for the design of efficient π-conjugated fluorophores with high electron affinity. Chem. Commun. 2000, 939–940. [Google Scholar] [CrossRef]
- Vangeneugden, D.L.; Kiebooms, R.H.L.; Vanderzande, D.J.M.; Gelan, J.M.J. V A general synthetic route towards soluble poly(1,3-dithienylisothianaphthene) derivatives. Synth. Met. 1999, 101, 120–121. [Google Scholar] [CrossRef]
- Vangeneugden, D.; Kiebooms, R.; Adriaensens, P.; Vanderzande, D.; Gelan, J.; Desmet, J.; Huyberechts, G. “Formal” copolymers based on 1,3-dithienylisothianaphthene derivatives. Promising materials for electronic devices. Acta Polym. 1998, 49, 687–692. [Google Scholar] [CrossRef]
- Kiebooms, R.; Adriaensens, P.; Vanderzande, D.; Gelan, J.; Swann, M.J.; Bloor, D.; Drury, C.J.; Brooke, G.M. Poly(tetrafluorobenzo[c]thiophene). Structure Analysis of Oligomers and Model Compound Based on 1D and 2D NMR Spectroscopy. Macromolecules 1996, 29, 5981–5989. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ie, Y.; Nitani, M.; Tohnai, N.; Kakiuchi, F.; Zhang, K.; Pisula, W.; Asadi, K.; Blom, P.W.M.; Aso, Y. Oligothiophene quinoids containing a benzo[c]thiophene unit for the stabilization of the quinoidal electronic structure. J. Mater. Chem. C Mater. Opt. Electron. Devices 2018, 6, 7493–7500. [Google Scholar] [CrossRef] [Green Version]
- Kiebooms, R.; Hoogmartens, I.; Adriaensens, P.; Vanderzande, D.; Gelan, J. Low-Band-Gap Conjugated Polymers. Improved Model Compounds for the Structural Analysis of Poly(isothianaphthene). Macromolecules 1995, 28, 4961–4969. [Google Scholar] [CrossRef]
- Hoogmartens, I.; Adriaensens, P.; Carleer, R.; Vanderzande, D.; Martens, H.; Gelan, J. An investigation into the electronic structure of poly(isothianaphthene). Synth. Met. 1992, 51, 219–228. [Google Scholar] [CrossRef]
- Kawabata, K.; Takeguchi, M.; Goto, H. Optical Activity of Heteroaromatic Conjugated Polymer Films Prepared by Asymmetric Electrochemical Polymerization in Cholesteric Liquid Crystals: Structural Function for Chiral Induction. Macromolecules 2013, 46, 2078–2091. [Google Scholar] [CrossRef]
- Musmanni, S.; Ferraris, J.P. Preparation and characterization of conducting polymers based on 1,3-di(2-thienyl)benzo[c]thiophene. J. Chem. Soc. Chem. Commun. 1993, 172–174. [Google Scholar] [CrossRef]
- D’Auria, M.; Guarnaccio, A.; Racioppi, R.; Santagata, A.; Teghil, R. Synthesis and photophysical properties of some dithienylbenzo[c]thiophene derivatives. Heterocycles 2015, 91, 313–331. [Google Scholar] [CrossRef]
- Baeuerle, P.; Goetz, G.; Emerle, P.; Port, H. Synthesis and characterization of new annulated terheterocycles. Adv. Mater. 1992, 4, 564–568. [Google Scholar] [CrossRef]
- Lakshmikantham, M.V.; Lorcy, D.; Scordilis-Kelley, C.; Wu, X.L.; Parakka, J.P.; Metzger, R.M.; Cava, M.P. Poly(naphtho[2,3-c]thiophene-alt-bithiophene): a novel low band gap polymer. Adv. Mater. 1993, 5, 723–726. [Google Scholar] [CrossRef]
- Qin, Y.; Kim, J.Y.; Frisbie, C.D.; Hillmyer, M.A. Distannylated Isothianaphthene: A Versatile Building Block for Low Bandgap Conjugated Polymers. Macromolecules 2008, 41, 5563–5570. [Google Scholar] [CrossRef]
- Clement, J.A.; Mohanakrishnan, A.K. Synthesis and characterization of naphth-annelated thiophene analogs. Tetrahedron 2010, 66, 2340–2350. [Google Scholar] [CrossRef]
- Raj, M.R.; Anandan, S. Donor conjugated polymers-based on alkyl chain substituted oligobenzo[c]thiophene derivatives with well-balanced energy levels for bulk heterojunction solar cells. RSC Adv. 2013, 3, 14595–14608. [Google Scholar] [CrossRef]
- Lorcy, D.; Cava, M.P. Poly(isothianaphthene-bithiophene): a new regularly structured polythiophene analog. Adv. Mater. 1992, 4, 562–564. [Google Scholar] [CrossRef]
- Wu, Y.; Jing, Y.; Guo, X.; Zhang, S.; Zhang, M.; Huo, L.; Hou, J. A thieno[3,4-f]isoindole-5,7-dione based copolymer for polymer solar cells. Polym. Chem. 2013, 4, 536–541. [Google Scholar] [CrossRef]
- Paulussen, H.; Vanderzande, D.; Gelan, J. The synthesis of methoxy substituted model compounds for structural analysis of poly(isothianaphthene) derivatives. Synth. Met. 1995, 69, 569–570. [Google Scholar] [CrossRef]
- Tuennermann, M.; Rehsies, P.; Floerke, U.; Bauer, M. A Straightforward Synthesis to Novel 1,10-Phenanthrolines with Fused Thiophene Structure. Synlett 2018, 29, 2638–2642. [Google Scholar] [CrossRef] [Green Version]
- Karsten, B.P.; Viani, L.; Gierschner, J.; Cornil, J.; Janssen, R.A.J. An Oligomer Study on Small Band Gap Polymers. J. Phys. Chem. A 2008, 112, 10764–10773. [Google Scholar] [CrossRef]
- Perzon, E.; Wang, X.; Zhang, F.; Mammo, W.; Delgado, J.L.; de la Cruz, P.; Inganaes, O.; Langa, F.; Andersson, M.R. Design, Synthesis and Properties of Low Band Gap Polyfluorenes for Photovoltaic Devices. Synth. Met. 2005, 154, 53–56. [Google Scholar] [CrossRef]
- Mak, C.S.K.; Leung, Q.Y.; Chan, W.K.; Djurisic, A.B. Optically tunable intramolecular charge transfer dyes for vacuum deposited bulk heterojunction solar cells. Nanotechnology 2008, 19, 424008/1–424008/8. [Google Scholar] [CrossRef]
- Petersen, M.H.; Gevorgyan, S.A.; Krebs, F.C. Thermocleavable Low Band Gap Polymers and Solar Cells Therefrom with Remarkable Stability toward Oxygen. Macromolecules 2008, 41, 8986–8994. [Google Scholar] [CrossRef]
- Yue, W.; Larsen-Olsen, T.T.; Hu, X.; Shi, M.; Chen, H.; Hinge, M.; Fojan, P.; Krebs, F.C.; Yu, D. Synthesis and photovoltaic properties from inverted geometry cells and roll-to-roll coated large area cells from dithienopyrrole-based donor-acceptor polymers. J. Mater. Chem. A Mater. Energy Sustain. 2013, 1, 1785–1793. [Google Scholar] [CrossRef]
- Sonmez, G.; Sonmez, H.B.; Shen, C.K.F.; Jost, R.W.; Rubin, Y.; Wudl, F. A Processable Green Polymeric Electrochromic. Macromolecules 2005, 38, 669–675. [Google Scholar] [CrossRef]
- Keshtov, M.L.; Godovsky, D.Y.; Khokhlov, A.R.; Mizobe, T.; Fujita, H.; Goto, E.; Hiyoshi, J.; Nakamura, S.; Kawauchi, S.; Higashihara, T.; et al. Synthesis and photovoltaic properties of thieno[3,4-b]pyrazine or dithieno[3′,2′:3,4;2″,3″:5,6]benzo[1,2-d]imidazole-containing conjugated polymers. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1067–1075. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, L.; Du, H.; Zhao, J.; Xie, Y. Three novel donor-acceptor type electrochromic polymers containing 2,3-bis(5-methylfuran-2-yl)thieno[3,4-b]pyrazine acceptor and different thiophene donors: Low-band-gap, neutral green-colored, fast-switching materials. J. Electroanal. Chem. 2018, 830–831, 7–19. [Google Scholar] [CrossRef]
- Mak, C.S.K.; Cheung, W.K.; Leung, Q.Y.; Chan, W.K. Conjugated Copolymers Containing Low Bandgap Rhenium(I) Complexes. Macromol. Rapid Commun. 2010, 31, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Mak, C.S.K.; Leung, Q.Y.; Li, C.H.; Chan, W.K. Tuning the electronic properties of conjugated polymer by tethering low-bandgap rhenium(I) complex on the main chain. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2311–2319. [Google Scholar] [CrossRef]
- Esmer, E.N.; Tarkuc, S.; Udum, Y.A.; Toppare, L. Near infrared electrochromic polymers based on phenazine moieties. Mater. Chem. Phys. 2011, 131, 519–524. [Google Scholar] [CrossRef]
- de Echegaray, P.; Mancheno, M.J.; Arrechea-Marcos, I.; Juarez, R.; Lopez-Espejo, G.; Lopez Navarrete, J.T.; Ramos, M.M.; Seoane, C.; Ortiz, R.P.; Segura, J.L. Synthesis of Perylene Imide Diones as Platforms for the Development of Pyrazine Based Organic Semiconductors. J. Org. Chem. 2016, 81, 11256–11267. [Google Scholar] [CrossRef] [PubMed]
- Keshtov, M.L.; Kuklin, S.A.; Konstantinov, I.O.; Peregudov, A.S.; Muranov, A.V.; Khokhlov, A.R. New monomer based on thienopyrazine with fluorocarbazole substituents as a promising building block for organic electronics. Dokl. Chem. 2017, 472, 25–29. [Google Scholar] [CrossRef]
- Sonmez, G.; Shen, C.K.F.; Rubin, Y.; Wudl, F. The unusual effect of bandgap lowering by C60 on a conjugated polymer. Adv. Mater. 2005, 17, 897–900. [Google Scholar] [CrossRef]
- Zhang, L.; Lo, K.C.; Chan, W.K. A new route to the synthesis of near-infrared absorbing pyrazinopyrazine bridged dyes with intramolecular charge transfer character. Chem. Commun. 2014, 50, 4245–4247. [Google Scholar] [CrossRef] [PubMed]
- Mikroyannidis, J.A.; Tsagkournos, D.V.; Sharma, S.S.; Vijay, Y.K.; Sharma, G.D. Synthesis and application of low band gap conjugated small molecules containing benzobisthiadiazole and thienothiadiazole central units for bulk heterojunction solar cells. J. Mater. Chem. 2011, 21, 4679–4688. [Google Scholar] [CrossRef]
- Shao, J.; Wang, G.; Wang, K.; Yang, C.; Wang, M. Direct arylation polycondensation for efficient synthesis of narrow-bandgap alternating D–A copolymers consisting of naphthalene diimide as an acceptor. Polym. Chem. 2015, 6, 6836–6844. [Google Scholar] [CrossRef]
- Gao, J.; He, D.; Zhang, W.; Xiao, Z.; Zuo, Q.; Shi, Z.; Ding, L. Synthesis, characterization and photovoltaic properties of conjugated copolymers based on 2-alkyl-thieno[3,4-b]imidazole. Synth. Met. 2012, 162, 1694–1700. [Google Scholar] [CrossRef]
- Shi, Z.; Neo, W.T.; Lin, T.T.; Zhou, H.; Xu, J. Solution-processable low-bandgap 3-fluorothieno[3,4-b]thiophene-2-carboxylate-based conjugated polymers for electrochromic applications. RSC Adv. 2015, 5, 96328–96335. [Google Scholar] [CrossRef]
- Lee, G.B.; Kim, R.; Cha, H.-J.; Park, C.E.; Kim, J.H.; Kim, Y.-H. New donor-acceptor copolymer containing dialkoxy naphthalene and carbonylated thieno[3,4-b]thiophene for OTFT and OPV. Macromol. Res. 2014, 22, 569–573. [Google Scholar] [CrossRef]
- Chen, L.; Cai, S.; Wang, X.; Chen, Y. Novel Donor-Acceptor Copolymers Based on Dithienosilole and Ketone Modified Thieno[3,4-b]thiophene for Photovoltaic Application. Chin. J. Chem. 2013, 31, 1455–1462. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, X.; Liu, F.; Huo, L.; Chen, Y.; Russell, T.P.; Han, C.C.; Li, Y.; Hou, J. Improving the Ordering and Photovoltaic Properties by Extending π-Conjugated Area of Electron-Donating Units in Polymers with D-A Structure. Adv. Mater. 2012, 24, 3383–3389. [Google Scholar] [CrossRef]
- Al-Taweel, S.A.; Al-Saraierh, H.F. Synthesis of thiophene oligomers via organotin compounds. Phosphorus Sulfur Silicon Relat. Elem. 1999, 155, 47–57. [Google Scholar] [CrossRef]
- Truong, M.A.; Fukuta, S.; Koganezawa, T.; Shoji, Y.; Ueda, M.; Higashihara, T. Synthesis, characterization, and application to polymer solar cells of polythiophene derivatives with ester- or ketone-substituted phenyl side groups. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 875–887. [Google Scholar] [CrossRef]
- Seol, H.; Shin, S.C.; Shim, Y.-B. Trace analysis of Al (III) ions based on the redox current of a conducting polymer. Electroanalysis 2004, 16, 2051–2057. [Google Scholar] [CrossRef]
- Dragonetti, C.; Righetto, S.; Roberto, D.; Valore, A.; Benincori, T.; Sannicolo, F.; Angelis, F.; Fantacci, S. Cationic cyclometallated iridium(III) complexes with substituted 1,10-phenanthrolines: The role of the cyclometallated moiety on this new class of complexes with interesting luminescent and second order non linear optical properties. J. Mater. Sci. Mater. Electron. 2009, 20, 460–464. [Google Scholar] [CrossRef]
- Noh, H.-B.; Won, M.-S.; Hwang, J.; Kwon, N.-H.; Shin, S.C.; Shim, Y.-B. Conjugated polymers and an iron complex as electrocatalytic materials for an enzyme-based biofuel cell. Biosens. Bioelectron. 2010, 25, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.-B.; Shim, Y.-B. Catalytic activity of polymerized self-assembled artificial enzyme nanoparticles: Applications to microfluidic channel-glucose biofuel cells and sensors. J. Mater. Chem. A Mater. Energy Sustain. 2016, 4, 2720–2728. [Google Scholar] [CrossRef]
- Tovar, J.D.; Swager, T.M. Cofacially constrained organic semiconductors. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3693–3702. [Google Scholar] [CrossRef]
- Mitschke, U.; Bauerle, P. Synthesis, characterization, and electrogenerated chemiluminescence of phenyl-substituted, phenyl-annulated, and spirofluorenyl-bridged oligothiophenes. J. Chem. Soc. Perkin Trans. 1 2001, 740–753. [Google Scholar] [CrossRef]
- Briehn, C.A.; Kirschbaum, T.; Baeuerle, P. Polymer-Supported Synthesis of Regioregular Head-to-Tail-Coupled Oligo(3-arylthiophene)s Utilizing a Traceless Silyl Linker. J. Org. Chem. 2000, 65, 352–359. [Google Scholar] [CrossRef]
- Olejnik, E.; Herzog-Ronen, C.; Eichen, Y.; Ehrenfreund, E. Recombination kinetics of polarons in films of alkylator-sensing co-polymers. Synth. Met. 2009, 159, 1024–1027. [Google Scholar] [CrossRef]
- Dinsdale, D.R.; Lough, A.J.; Lemaire, M.T. Structure and magnetic properties of an unusual homoleptic iron(III) thiocyanate dimer. Dalt. Trans. 2015, 44, 11077–11082. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, D.; Peng, L.; Li, H.; Shen, P.; Xiang, N.; Liu, Y.; Tan, S. Effect of oxadiazole side chains based on alternating fluorene-thiophene copolymers for photovoltaic cells. Eur. Polym. J. 2009, 45, 2079–2086. [Google Scholar] [CrossRef]
- Huang, M.H.; Tian, Z.F.; Huang, H. Synthesis and photovoltaic properties of poly(p-phenylenevinylene) derivatives modified by thiophene derivatives. Adv. Mater. Res. 2013, 643, 13–16. [Google Scholar] [CrossRef]
- Nagarjuna, G.; Yurt, S.; Jadhav, K.G.; Venkataraman, D. Impact of Pendant 1,2,3-Triazole on the Synthesis and Properties of Thiophene-Based Polymers. Macromolecules 2010, 43, 8045–8050. [Google Scholar] [CrossRef]
- Algi, F.; Cihaner, A. A novel terthienyl based polymer electrochrome with peripheral BODIPY. Polymer 2012, 53, 3469–3475. [Google Scholar] [CrossRef]
- Clarke, T.M.; Gordon, K.C.; Wagner, P.; Officer, D.L. Modulation of Electronic Properties in Neutral and Oxidized Oligothiophenes Substituted with Conjugated Polyaromatic Hydrocarbons. J. Phys. Chem. A 2007, 111, 2385–2397. [Google Scholar] [CrossRef] [PubMed]
- Wagner, P.; Officer, D.L. Structural and electronic properties of substituted terthiophenes. Synth. Met. 2005, 154, 325–328. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, Y.; Zhong, C.; Yu, G.; Liu, Y.; Qin, J. Two-dimensional copolymers with D-A type side chains for organic thin-film transistors: synthesis and properties. Polym. Chem. 2011, 2, 2842–2849. [Google Scholar] [CrossRef]
- Clarke, T.M.; Gordon, K.C.; Officer, D.L.; Grant, D.K. The effect of oxidation on the structure of styryl-substituted sexithiophenes: A resonance Raman spectroscopy and density functional theory study. J. Chem. Phys. 2006, 124, 164501/1–164501/11. [Google Scholar] [CrossRef] [PubMed]
- Grant, D.K.; Officer, D.L. Towards processable polyether-functionalized poly(3′-styrylterthiophenes). Synth. Met. 2005, 154, 93–96. [Google Scholar] [CrossRef]
- Cutler, C.A.; Burrell, A.K.; Collis, G.E.; Dastoor, P.C.; Officer, D.L.; Too, C.O.; Wallace, G.G. Photoelectrochemical cells based on polymers and copolymers from terthiophene and nitrostyrylterthiophene. Synth. Met. 2001, 123, 225–237. [Google Scholar] [CrossRef]
- Loire, G.; Schouteeten, S.; Andrioletti, B.; Prim, D.; Tranchier, J.-P.; Rose-Munch, F.; Rose, E.; Persoons, A. Oligothiophene-substituted arenetricarbonylchromium complexes. Comptes Rendus Chim. 2003, 6, 223–230. [Google Scholar] [CrossRef]
- Kuo, C.-Y.; Huang, Y.-C.; Hsiow, C.-Y.; Yang, Y.-W.; Huang, C.-I.; Rwei, S.-P.; Wang, H.-L.; Wang, L. Effect of Side-Chain Architecture on the Optical and Crystalline Properties of Two-Dimensional Polythiophenes. Macromolecules 2013, 46, 5985–5997. [Google Scholar] [CrossRef]
- Mei, S.; Wu, F.; Huang, Y.; Zhao, B.; Tan, S. Synthesis and photovoltaic properties of the copolymers based on 3-ethylrhodanine side group. Eur. Polym. J. 2015, 67, 31–39. [Google Scholar] [CrossRef]
- Chen, J.; Burrell, A.K.; Collis, G.E.; Officer, D.L.; Swiegers, G.F.; Too, C.O.; Wallace, G.G. Preparation, characterization and biosensor application of conducting polymers based on ferrocene substituted thiophene and terthiophene. Electrochim. Acta 2002, 47, 2715–2724. [Google Scholar] [CrossRef]
- O’Sullivan, T.J.; Djukic, B.; Dube, P.A.; Lemaire, M.T. A conducting metallopolymer featuring valence tautomerism. Chem. Commun. 2009, 1903–1905. [Google Scholar] [CrossRef]
- Manca, P.; Pilo, M.I.; Sanna, G.; Bergamini, G.; Ceroni, P.; Boaretto, R.; Caramori, S. Heteroleptic Ru(II)-terpyridine complex and its metal-containing conducting polymer: Synthesis and characterization. Synth. Met. 2015, 200, 109–116. [Google Scholar] [CrossRef]
- Murata, Y.; Suzuki, M.; Komatsu, K. Synthesis and electropolymerization of fullerene-terthiophene dyads. Org. Biomol. Chem. 2003, 1, 2624–2625. [Google Scholar] [CrossRef]
- Frankevich, V.E.; Dashtiev, M.; Zenobi, R.; Kitagawa, T.; Lee, Y.; Murata, Y.; Yamazaki, T.; Gao, Y.; Komatsu, K.; Oliva, J.M. MALDI-Fourier transform mass spectrometric and theoretical studies of donor-acceptor and donor-bridge-acceptor fullerenes. Phys. Chem. Chem. Phys. 2005, 7, 1036–1042. [Google Scholar] [CrossRef]
- Manca, P.; Pilo, M.I.; Sanna, G.; Zucca, A.; Bergamini, G.; Ceroni, P. Ru2+ complexes comprising terpyridine ligands appended with terthiophene chromophores: Energy transfer and energy reservoir effect. Chem. Commun. 2011, 47, 3413–3415. [Google Scholar] [CrossRef]
- Visy, C.; Lukkari, J.; Kankare, J. Electrochemically Polymerized Terthiophene Derivatives Carrying Aromatic Substituents. Macromolecules 1994, 27, 3322–3329. [Google Scholar] [CrossRef]
- Amir, E.; Rozen, S. Synthesis of [all]-S,S-Dioxide Oligothiophenes Using HOF⋅CH3CN. Angew. Chemie Int. Ed. 2005, 44, 7374–7378. [Google Scholar] [CrossRef]
- Miller, R.W.; Dodge, N.J.; Dyer, A.M.; Fortner-Buczala, E.M.; Whalley, A.C. A one-pot method for the preparation of 2,5-diarylthiophene-1-oxides from arylacetylenes. Tetrahedron Lett. 2016, 57, 1860–1862. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.-H.; Chirdon, D.N.; Maurer, A.B.; Bernhard, S.; Noonan, K.J.T. Synthesis of Thiophene 1,1-Dioxides and Tuning Their Optoelectronic Properties. Org. Lett. 2013, 15, 5230–5233. [Google Scholar] [CrossRef] [PubMed]
- Barbarella, G.; Favaretto, L.; Sotgiu, G.; Zambianchi, M.; Arbizzani, C.; Bongini, A.; Mastragostino, M. Controlling the Electronic Properties of Polythiophene through the Insertion of Nonaromatic Thienyl S, S -dioxide Units. Chem. Mater. 1999, 11, 2533–2541. [Google Scholar] [CrossRef]
- Leclerc, N.; Michaud, A.; Sirois, K.; Morin, J.-F.; Leclerc, M. Synthesis of 2,7-Carbazolenevinylene-Based Copolymers and Characterization of Their Photovoltaic Properties. Adv. Funct. Mater. 2006, 16, 1694–1704. [Google Scholar] [CrossRef]
- Melucci, M.; Frère, P.; Allain, M.; Levillain, E.; Barbarella, G.; Roncali, J. Molecular engineering of hybrid π-conjugated oligomers combining 3,4-ethylenedioxythiophene (EDOT) and thiophene-S,S-dioxide units. Tetrahedron 2007, 63, 9774–9783. [Google Scholar] [CrossRef]
- Barbarella, G.; Favaretto, L.; Sotgiu, G.; Zambianchi, M.; Bongini, A.; Arbizzani, C.; Mastragostino, M.; Anni, M.; Gigli, G.; Cingolani, R. Tuning Solid-State Photoluminescence Frequencies and Efficiencies of Oligomers Containing One Central Thiophene- S, S -dioxide Unit. J. Am. Chem. Soc. 2000, 122, 11971–11978. [Google Scholar] [CrossRef]
- Anni, M.; Gigli, G.; Paladini, V.; Cingolani, R.; Barbarella, G.; Favaretto, L.; Sotgiu, G.; Zambianchi, M. Color engineering by modified oligothiophene blends. Appl. Phys. Lett. 2000, 77, 2458–2460. [Google Scholar] [CrossRef]
- Berlin, A.; Zotti, G.; Zecchin, S.; Schiavon, G.; Cocchi, M.; Virgili, D.; Sabatini, C. 3,4-Ethylenedioxy-substituted bithiophene-alt-thiophene-S,S-dioxide regular copolymers. Synthesis and conductive, magnetic and luminescence properties. J. Mater. Chem. 2003, 13, 27–33. [Google Scholar] [CrossRef]
- Kuchison, A.M.; Wolf, M.O.; Patrick, B.O. Conjugated ligand-based tribochromic luminescence. Chem. Commun. 2009, 7387–7389. [Google Scholar] [CrossRef]
- Moore, S.A.; Davies, D.L.; Karim, M.M.; Nagle, J.K.; Wolf, M.O.; Patrick, B.O. Photophysical behaviour of cyclometalated iridium(iii) complexes with phosphino(terthiophene) ligands. Dalt. Trans. 2013, 42, 12354–12363. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.A.; Nagle, J.K.; Wolf, M.O.; Patrick, B.O. Coordination Mode Dependent Excited State Behavior in Group 8 Phosphino(terthiophene) Complexes. Inorg. Chem. 2011, 50, 5113–5122. [Google Scholar] [CrossRef] [PubMed]
- Clot, O.; Wolf, M.O.; Patrick, B.O. Electropolymerization of a cyclometalated terthiophene: A hybrid material with a palladium-carbon bond to the backbone. J. Am. Chem. Soc. 2000, 122, 10456–10457. [Google Scholar] [CrossRef]
- Clot, O.; Wolf, M.O.; Patrick, B.O. Electropolymerization of Pd(II) complexes containing phosphinoterthiophene ligands. J. Am. Chem. Soc. 2001, 123, 9963–9973. [Google Scholar] [CrossRef] [PubMed]
- Kuchison, A.M.; Wolf, M.O.; Patrick, B.O. Photophysical and electrochemical properties of Ru(II) complexes containing tridentate bisphosphino-oligothiophene ligands. Dalt. Trans. 2011, 40, 6912–6921. [Google Scholar] [CrossRef]
- Cao, Y.; Wolf, M.O.; Patrick, B.O. Dual-Emissive Platinum(II) Metallacycles with Thiophene-Containing Bisacetylide Ligands. Inorg. Chem. 2016, 55, 8985–8993. [Google Scholar] [CrossRef]
- Manca, P.; Scanu, R.; Zucca, A.; Sanna, G.; Spano, N.; Pilo, M.I. Electropolymerization of a Ru(II)-terpyridine complex ethynyl-terthiophene functionalized originating different metallopolymers. Polymer 2013, 54, 3504–3509. [Google Scholar] [CrossRef]
- Scanu, R.; Manca, P.; Zucca, A.; Sanna, G.; Spano, N.; Seeber, R.; Zanardi, C.; Pilo, M.I. Homoleptic Ru(II) complex with terpyridine ligands appended with terthiophene moieties: Synthesis, characterization and electropolymerization. Polyhedron 2013, 49, 24–28. [Google Scholar] [CrossRef]
- Zöllner, M.J.; Becker, E.; Jahn, U.; Kowalsky, W.; Johannes, H.H. New versatile strategy towards zinc(II)-, copper(II)- and cobalt(II)metallated thiophene/porphyrin-hybrids. Eur. J. Org. Chem. 2010, 4426–4435. [Google Scholar] [CrossRef]
- Zoellner, M.J.; Fraehmcke, J.S.; Elstner, M.; Jahn, U.; Jones, P.G.; Becker, E.; Kowalsky, W.; Johannes, H.-H. A New Synthetic Approach to Thiophene-Nickel(II)porphyrin Hybrid Molecules and their Electrochemical and Computational Investigation. Macromol. Chem. Phys. 2010, 211, 359–371. [Google Scholar] [CrossRef]
- Collis, G.E.; Campbell, W.M.; Officer, D.L.; Burrell, A.K. The design and synthesis of porphyrin/oligiothiophene hybrid monomers. Org. Biomol. Chem. 2005, 3, 2075–2084. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Jones, R.A.; Holliday, B.J. Direct synthesis of CdSe nanocrystals within a conducting metallopolymer: Toward improving charge transfer in hybrid nanomaterials. Chem. Commun. 2016, 52, 13112–13115. [Google Scholar] [CrossRef] [PubMed]
- Reddinger, J.L.; Reynolds, J.R. Tunable Redox and Optical Properties Using Transition Metal-Complexed Polythiophenes. Macromolecules 1997, 30, 673–675. [Google Scholar] [CrossRef]
- Kim, J.S.J.J.; Kang, D.M.; Shin, S.C.; Choi, M.Y.; Kim, J.S.J.J.; Lee, S.S.; Kim, J.S.J.J. Functional polyterthiophene-appended uranyl-salophen complex: Electropolymerization and ion-selective response for monohydrogen phosphate. Anal. Chim. Acta 2008, 614, 85–92. [Google Scholar] [CrossRef]
- Pozo-Gonzalo, C.; Berridge, R.; Skabara, P.J.; Cerrada, E.; Laguna, M.; Coles, S.J.; Hursthouse, M.B. A new family of conjugated metallopolymers from electropolymerised bis[(terthiophene)dithiolene] complexes. Chem. Commun. 2002, 2408–2409. [Google Scholar] [CrossRef]
- Kang, B.S.; Kim, D.H.; Jung, T.S.; Jang, E.K.; Pak, Y.; Shin, S.C.; Park, D.S.; Shim, Y.B. Polyterthiophene appended by transition-metal cluster: Electropolymerization of 3′-[CCo3(CO)9]-5,2′:5′,2″-terthiophene. Synth. Met. 1999, 105, 9–12. [Google Scholar] [CrossRef]
- Hyun, D.; Park, D.; Shim, Y.; Chul, S. Polyterthiophene p -conjugated by organomolybdenum complex. J. Organomet. Chem. 2000, 608, 133–138. [Google Scholar]
- Burrell, A.K.; Chen, J.; Collis, G.E.; Grant, D.K.; Officer, D.L.; Too, C.O.; Wallace, G.G. Functionalised poly(terthiophenes). Synth. Met. 2003, 135–136, 97–98. [Google Scholar] [CrossRef]
- Bäuerle, P.; Gaudl, K.U. New functionalized polythiophenes. Synth. Met. 1991, 43, 3037–3042. [Google Scholar] [CrossRef]
- Özenler, S.; Kaya, H.; Elmaci, N.; Yildiz, U.H. Transition-Metal-Free Direct C-H Arylation of Thiophene in Aqueous Media via Potassium Peroxymonosulfate. ChemistrySelect 2019, 4, 8516–8521. [Google Scholar] [CrossRef]
- Havinga, E.E.; van Horssen, L.W.; ten Hoeve, W.; Wynberg, H.; Meijer, E.W. Self-doped water-soluble conducting polymers. Polym. Bull. 1987, 18, 277–281. [Google Scholar] [CrossRef] [Green Version]
- Dufil, G.; Parker, D.; Gerasimov, J.Y.; Nguyen, T.Q.; Berggren, M.; Stavrinidou, E. Enzyme-assistedin vivopolymerisation of conjugated oligomer based conductors. J. Mater. Chem. B 2020, 8, 4221–4227. [Google Scholar] [CrossRef] [Green Version]
- Volkov, A.V.; Singh, S.K.; Stavrinidou, E.; Gabrielsson, R.; Franco-Gonzalez, J.F.; Cruce, A.; Chen, W.M.; Simon, D.T.; Berggren, M.; Zozoulenko, I.V. Spectroelectrochemistry and Nature of Charge Carriers in Self-Doped Conducting Polymer. Adv. Electron. Mater. 2017, 3, 1700096. [Google Scholar] [CrossRef]
- Stavrinidou, E.; Gabrielsson, R.; Nilsson, K.P.R.; Singh, S.K.; Franco-Gonzalez, J.F.; Volkov, A.V.; Jonsson, M.P.; Grimoldi, A.; Elgland, M.; Zozoulenko, I.V.; et al. In vivo polymerization and manufacturing of wires and supercapacitors in plants. Proc. Natl. Acad. Sci. 2017, 114, 2807–2812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujimoto, N.; Nakahodo, T.; Fujihara, H. Synthesis of anionic sulfonate-functionalized conducting polymer nanotubes and selective confinement of cationic gold nanoparticles in their inner cavities via electrostatic interaction. Chem. Lett. 2013, 42, 1394–1396. [Google Scholar] [CrossRef]
- Tanaka, S.; Kumei, M. A new polythiophene prepared by the electropolymerization of a branched sexithienyl. J. Chem. Soc. Chem. Commun. 1995, 815. [Google Scholar] [CrossRef]
- Jeong, S.; Kong, M.S.; Kim, J.H.; Kim, K.H.; Cho, Y.; Han, Y.S. Synthesis of a thiophene derivative and its effects as an additive on the performance of solar cells. Mol. Cryst. Liq. Cryst. 2019, 678, 121–130. [Google Scholar] [CrossRef]
- Moriyama, Y.; Matsuda, K.; Tanifuji, N.; Irie, S.; Irie, M. Electrochemical Cyclization/Cycloreversion Reactions of Diarylethenes. Org. Lett. 2005, 7, 3315–3318. [Google Scholar] [CrossRef]
- Bolduc, A.; Lachapelle, V.; Skene, W.G. Snap Together Bonds for Amine Capturing—New Spectroscopic and Amperometric Sensors. Macromol. Symp. 2010, 297, 87–93. [Google Scholar] [CrossRef]
- Apodaca, D.C.; Pernites, R.B.; Ponnapati, R.R.; Del Mundo, F.R.; Advincula, R.C. Electropolymerized Molecularly Imprinted Polymer Films of a Bis-Terthiophene Dendron: Folic Acid Quartz Crystal Microbalance Sensing. ACS Appl. Mater. Interfaces 2011, 3, 191–203. [Google Scholar] [CrossRef]
- Park, J.Y.; Advincula, R.C. Electroluminescent Behaviors of Electrochemically Cross-Linkable Poly(benzyl ether) Terthiophene Dendrimers. Macromol. Chem. Phys. 2016, 217, 1948–1954. [Google Scholar] [CrossRef]
- Postigo, A.; Bulacio, L.; Sortino, M. Photodynamic inactivation of oropharyngeal Candida strains. Phytomedicine 2014, 21, 1424–1431. [Google Scholar] [CrossRef]
- Postigo, A.; Funes, M.; Petenatti, E.; Bottai, H.; Pacciaroni, A.; Sortino, M. Antifungal photosensitive activity of Porophyllum obscurum (Spreng.) DC.: Correlation of the chemical composition of the hexane extract with the bioactivity. Photodiagn. Photodyn. Ther. 2017, 20, 263–272. [Google Scholar] [CrossRef]
- Zhou, Z.; Ergene, C.; Lee, J.Y.; Shirley, D.J.; Carone, B.R.; Caputo, G.A.; Palermo, E.F. Sequence and Dispersity Are Determinants of Photodynamic Antibacterial Activity Exerted by Peptidomimetic Oligo(thiophene)s. ACS Appl. Mater. Interfaces 2019, 11, 1896–1906. [Google Scholar] [CrossRef]
- Luo, Z.-G.; Liu, Z.-Y.; Yang, Z.-H. The synthesis and photoactivated cytotoxicity of novel 5-phenyl-3-(2,2′:5′,2″-terthien-5-yl)-4,5-dihydro-1H-pyrazoles. Chin. Chem. Lett. 2014, 25, 333–336. [Google Scholar] [CrossRef]
- Chen, X.; Xu, H.; Wang, Y.; Hu, S.; Zhang, Z.; Zhang, Y. Study on active oxygen quantum yield, insecticidal activities and stability of diphenylthiophene. Agric. Sci. China 2007, 6, 458–465. [Google Scholar] [CrossRef]
- Huang, Q.; Yun, X.; Rao, W.; Xiao, C. Antioxidative cellular response of lepidopteran ovarian cells to photoactivated alpha-terthienyl. Pestic. Biochem. Physiol. 2017, 137, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ahmad, S.; Wang, L.-Y.; Han, Q.; Zhang, J.-C.; Luo, Y.-P. Cell death induced by α-terthienyl via reactive oxygen species-mediated mitochondrial dysfunction and oxidative stress in the midgut of Aedes aegypti larvae. Free Radic. Biol. Med. 2019, 137, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.-C.; Liu, Y.; Zhan, T.-S.; Deng, Y.-F.; He, Y. Comparable Susceptibilities of Human 293 Cells and Insect Tn-5B1-4 Cells to Photoactivated α-Terthienyl. J. Agric. Food Chem. 2010, 58, 2637–2642. [Google Scholar] [CrossRef] [PubMed]
- Weidenhamer, J.D.; Montgomery, T.M.; Cipollini, D.F.; Weston, P.A.; Mohney, B.K. Plant Density and Rhizosphere Chemistry: Does Marigold Root Exudate Composition Respond to Intra- and Interspecific Competition? J. Chem. Ecol. 2019, 45, 525–533. [Google Scholar] [CrossRef]
- Zhao, B.; Huo, J.; Zhang, J.; Zhao, B.; Liu, N.; Dong, J. Transketolase Is Identified as a Target of Herbicidal Substance α-Terthienyl by Proteomics. Toxins 2018, 10, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, H.; Cantrell, C.L.; Mamonov, L.K.; Osbrink, W.L.A.; Ross, S.A. Echinopsacetylenes A and B, New Thiophenes from Echinops transiliensis. Org. Lett. 2011, 13, 6228–6231. [Google Scholar] [CrossRef]
- Marques, M.M.M.; Morais, S.M.; Vieira, I.G.P.; Vieira, M.G.S.; Silva, A.R.A.; de Almeida, R.R.; Guedes, M.I.F. Larvicidal activity of Tagetes erecta against Aedes Aegypti. J. Am. Mosq. Control Assoc. 2011, 27, 156–158. [Google Scholar] [CrossRef]
- Faizi, S.; Fayyaz, S.; Bano, S.; Yawar Iqbal, E. Isolation of Nematicidal Compounds from Tagetes patula L. Yellow Flowers: Structure-Activity Relationship Studies against Cyst Nematode Heterodera zeae Infective Stage Larvae. J. Agric. Food Chem. 2011, 59, 9080–9093. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Man, L.; Wang, X.; Ying, L. Research on the antimicrobial activity of α-triple thiophene in the marigold. Adv. J. Food Sci. Technol. 2015, 7, 936–939. [Google Scholar]
- Chow, C.-F. Two-photon induced emissive thiophene donor–acceptor systems as molecular probes for in vitro bio-imaging: synthesis, crystal structure, and spectroscopic properties. RSC Adv. 2013, 3, 18835–18843. [Google Scholar] [CrossRef]
- Guo, Z.; Hu, T.; Sun, T.; Li, T.; Chi, H.; Niu, Q. A colorimetric and fluorometric oligothiophene-indenedione-based sensor for rapid and highly sensitive detection of cyanide in real samples and bioimaging in living cells. Dyes Pigments 2019, 163, 667–674. [Google Scholar] [CrossRef]
- Guo, Z.; Niu, Q.; Yang, Q.; Li, T.; Chi, H. Highly selective and sensitive dual-mode sensor for colorimetric and turn-on fluorescent detection of cyanide in water, agro-products and living cells. Anal. Chim. Acta 2019, 1065, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Niu, Q.; Yang, Q.; Lan, L.; Li, T. A new “naked-eye” colorimetric and ratiometric fluorescent sensor for imaging Hg2+ in living cells. Tetrahedron 2019, 75, 130687. [Google Scholar] [CrossRef]
- Liu, Q.; Mukherjee, S.; Huang, R.; Liu, K.; Liu, T.; Liu, K.; Miao, R.; Peng, H.; Fang, Y.; Liu, Q. Naphthyl End-Capped Terthiophene-Based Chemiresistive Sensors for Biogenic Amine Detection and Meat Spoilage Monitoring. Chem. Asian J. 2019, 14, 2751–2758. [Google Scholar] [CrossRef]
- Akhtar, M.H.; Hussain, K.K.; Gurudatt, N.G.; Chandra, P.; Shim, Y.-B. Ultrasensitive dual probe immunosensor for the monitoring of nicotine induced-brain derived neurotrophic factor released from cancer cells. Biosens. Bioelectron. 2018, 116, 108–115. [Google Scholar] [CrossRef]
- Preya, U.H.; Lee, K.-T.; Jang, D.S.; Kim, N.-J.; Lee, J.-Y.; Choi, J.-H. The natural terthiophene α-terthienylmethanol induces S phase cell cycle arrest of human ovarian cancer cells via the generation of ROS stress. Chem. Biol. Interact. 2017, 272, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ding, C.; Li, L.; Gao, C.; Jiang, Y.; Tan, C.; Hua, R. Synthesis and antiproliferative activity of RITA and its analogs. Tetrahedron Lett. 2014, 55, 6635–6638. [Google Scholar] [CrossRef]
- Jin, W.; Shi, Q.; Hong, C.; Cheng, Y.; Ma, Z.; Qu, H. Cytotoxic properties of thiophenes from Echinops grijissi Hance. Phytomedicine 2008, 15, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Kim, H.M.; Ryu, B.; Lee, J.-S.; Choi, J.-H.; Jang, D.S. Constituents of the aerial parts of Eclipta prostrata and their cytotoxicity on human ovarian cancer cells in vitro. Arch. Pharmacal Res. 2015, 38, 1963–1969. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.K.; Seki, M.; Tabata, H. Self-organized ZnO nanorod with photooxidative cell membrane perforation enables large-scale cell manipulation. Anal. Bioanal. Chem. 2008, 391, 2513–2519. [Google Scholar] [CrossRef]
- Noh, H.-B.; Revin, S.B.; Shim, Y.-B. Voltammetric analysis of anti-arthritis drug, ascorbic acid, tyrosine, and uric acid using a graphene decorated-functionalized conductive polymer electrode. Electrochim. Acta 2014, 139, 315–322. [Google Scholar] [CrossRef]
- Jamal, R.; Liu, Y.; Abdurexit, A.; Sawut, N.; Yan, Y.; Ali, A.; Abdiryim, T. Electrochemical Sensor for Detection of Paracetamol Based on Pendent Nitrogen Heterocyclic Ring-Functionalized Polyterthiophene Derivatives. ChemistrySelect 2021, 6, 4473–4481. [Google Scholar] [CrossRef]
- Jo, H.; Her, J.; Lee, H.; Shim, Y.-B.; Ban, C. Highly sensitive amperometric detection of cardiac troponin I using sandwich aptamers and screen-printed carbon electrodes. Talanta 2017, 165, 442–448. [Google Scholar] [CrossRef]
- Kim, D.-M.; Shim, Y.-B. Disposable Amperometric Glycated Hemoglobin Sensor for the Finger Prick Blood Test. Anal. Chem. 2013, 85, 6536–6543. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.-B.; Chandra, P.; Moon, J.O.; Shim, Y.-B. In vivo detection of glutathione disulfide and oxidative stress monitoring using a biosensor. Biomaterials 2012, 33, 2600–2607. [Google Scholar] [CrossRef]
- Das, D.; Kim, D.-M.; Park, D.-S.; Shim, Y.-B. A Glucose Sensor Based on an Aminophenyl Boronic Acid Bonded Conducting Polymer. Electroanalysis 2011, 23, 2036–2041. [Google Scholar] [CrossRef]
- Lee, W.-C.; Gurudatt, N.G.; Park, D.-S.; Kim, K.B.; Choi, C.S.; Shim, Y.-B. Microneedle array sensor for monitoring glucose in single cell using glucose oxidase-bonded polyterthiophene coated on AuZn oxide layer. Sens. Actuators B Chem. 2020, 320, 128416. [Google Scholar] [CrossRef]
- Noh, H.-B.; Rahman, M.A.; Yang, J.E.; Shim, Y.-B. Ag(I)-cysteamine complex based electrochemical stripping immunoassay: Ultrasensitive human IgG detection. Biosens. Bioelectron. 2011, 26, 4429–4435. [Google Scholar] [CrossRef]
- Chandra, P.; Koh, W.C.A.; Noh, H.-B.; Shim, Y.-B. In vitro monitoring of i-NOS concentrations with an immunosensor: The inhibitory effect of endocrine disruptors on i-NOS release. Biosens. Bioelectron. 2012, 32, 278–282. [Google Scholar] [CrossRef]
- Koh, W.-C.A.; Chandra, P.; Kim, D.-M.; Shim, Y.-B. Electropolymerized Self-Assembled Layer on Gold Nanoparticles: Detection of Inducible Nitric Oxide Synthase in Neuronal Cell Culture. Anal. Chem. 2011, 83, 6177–6183. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-Y.; Naveen, M.H.; Gurudatt, N.G.; Shim, Y.-B. Detection of Nitric Oxide from Living Cells Using Polymeric Zinc Organic Framework-Derived Zinc Oxide Composite with Conducting Polymer. Small 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, A.A.; Shim, Y.-B. Nonenzymatic H2O2 sensing based on silver nanoparticles capped polyterthiophene/MWCNT nanocomposite. Sens. Actuators, B 2014, 201, 51–58. [Google Scholar] [CrossRef]
- Noh, H.-B.; Gurudatt, N.G.; Won, M.-S.; Shim, Y.-B. Analysis of Phthalate Esters in Mammalian Cell Culture Using a Microfluidic Channel Coupled with an Electrochemical Sensor. Anal. Chem. 2015, 87, 7069–7077. [Google Scholar] [CrossRef]
- Pernites, R.B.; Santos, C.M.; Maldonado, M.; Ponnapati, R.R.; Rodrigues, D.F.; Advincula, R.C. Tunable Protein and Bacterial Cell Adsorption on Colloidally Templated Superhydrophobic Polythiophene Films. Chem. Mater. 2012, 24, 870–880. [Google Scholar] [CrossRef]
- Quigley, A.F.; Wagner, K.; Kita, M.; Gilmore, K.J.; Higgins, M.J.; Breukers, R.D.; Moulton, S.E.; Clark, G.M.; Penington, A.J.; Wallace, G.G.; et al. In vitro growth and differentiation of primary myoblasts on thiophene based conducting polymers. Biomater. Sci. 2013, 1, 983–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevenson, G.; Moulton, S.E.; Innis, P.C.; Wallace, G.G. Polyterthiophene as an electrostimulated controlled drug release material of therapeutic levels of dexamethasone. Synth. Met. 2010, 160, 1107–1114. [Google Scholar] [CrossRef]
- Margalith, I.; Suter, C.; Ballmer, B.; Schwarz, P.; Tiberi, C.; Sonati, T.; Falsig, J.; Nystroem, S.; Hammarstroem, P.; Aslund, A.; et al. Polythiophenes Inhibit Prion Propagation by Stabilizing Prion Protein (PrP) Aggregates. J. Biol. Chem. 2012, 287, 18872–18887. [Google Scholar] [CrossRef] [Green Version]



















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallan, L.; Istif, E.; Gómez, I.J.; Alegret, N.; Mantione, D. Thiophene-Based Trimers and Their Bioapplications: An Overview. Polymers 2021, 13, 1977. https://doi.org/10.3390/polym13121977
Vallan L, Istif E, Gómez IJ, Alegret N, Mantione D. Thiophene-Based Trimers and Their Bioapplications: An Overview. Polymers. 2021; 13(12):1977. https://doi.org/10.3390/polym13121977
Chicago/Turabian StyleVallan, Lorenzo, Emin Istif, I. Jénnifer Gómez, Nuria Alegret, and Daniele Mantione. 2021. "Thiophene-Based Trimers and Their Bioapplications: An Overview" Polymers 13, no. 12: 1977. https://doi.org/10.3390/polym13121977
APA StyleVallan, L., Istif, E., Gómez, I. J., Alegret, N., & Mantione, D. (2021). Thiophene-Based Trimers and Their Bioapplications: An Overview. Polymers, 13(12), 1977. https://doi.org/10.3390/polym13121977