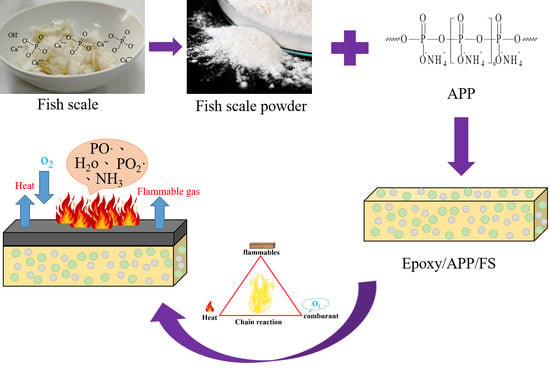
A Study on Circular Economy Material Using Fish Scales as a Natural Flame Retardant and the Properties of Its Composite Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Epoxy/APP/FS Composites
2.3. Measurements
2.4. Calculation of Experimental Data
3. Results and Discussion
3.1. Thermal Properties
3.2. Flame Retardant Property
3.3. Morphological Properties
3.4. Char Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiang, Q.; Xiao, F. Applications of epoxy materials in pavement engineering. Constr. Build. Mater. 2020, 235, 117529. [Google Scholar] [CrossRef]
- Jian, R.K.; Ai, Y.F.; Xia, L.; Zhao, L.J.; Zhao, H.B. Single component phosphamide-based intumescent flame retardant with potential reactivity towards low flammability and smoke epoxy resins. J. Hazard. Mater. 2019, 371, 529–539. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Li, S. A novel phosphorus−silicon containing epoxy resin with enhanced thermal stability, flame retardancy and mechanical properties. Polym. Degrad. Stab. 2019, 164, 36–45. [Google Scholar] [CrossRef]
- Zhang, W.; Fina, A.; Ferraro, G.; Yang, R. FTIR and GCMS analysis of epoxy resin decomposition products feeding the flame during UL 94 standard flammability test. Application to the understanding of the blowing-out effect in epoxy/polyhedral silsesquioxane formulations. J. Anal. Appl. Pyrolysis. 2018, 135, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Kashiwagi, T.; Du, F.; Douglas, J.F.; Winey, K.I.; Harris, R.H.; Shields, J.R. Nanoparticle networks reduce the flammability of polymer nanocomposites. Nat. Mater. 2005, 4, 928–933. [Google Scholar] [CrossRef]
- Birnbaum, L.S.; Staskal, D.F. Brominated flame retardants: Cause for concern? Environ. Health Perspect. 2004, 112, 9–17. [Google Scholar] [CrossRef]
- Xiao, J.; Hu, Y.; Yang, L.; Cai, Y.; Song, L.; Chen, Z.; Fan, W. Fire retardant synergism between melamine and triphenyl phosphate in poly(butylene terephthalate). Polym. Degrad. Stab. 2006, 91, 2093–2100. [Google Scholar] [CrossRef]
- Ciecierska, E.; Jurczyk-Kowalska, M.; Bazarnik, P.; Gloc, M.; Kulesza, M.; Kowalski, M.; Krauze, S.; Lewandowska, M. Flammability, mechanical properties and structure of rigid polyurethane foams with different types of carbon reinforcing materials. Compos. Struct. 2016, 140, 67–76. [Google Scholar] [CrossRef]
- Chen, M.J.; Shao, Z.B.; Wang, X.L.; Chen, L.; Wang, Y.Z. Halogen-free flame retardant flexible polyurethane foam with a novel nitrogen-phosphorus flame retardant. Ind. Eng. Chem. Res. 2012, 51, 9769–9776. [Google Scholar] [CrossRef]
- Tai, Q.; Yuen, R.K.K.; Yang, W.; Qiao, Z.; Song, L.; Hu, Y. Iron-montmorillonite and zinc borate as synergistic agents in flame-retardant glass fiber reinforced polyamide 6 composites in combination with melamine polyphosphate. Compos. Part A Appl. Sci. Manuf. 2012, 43, 415–422. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, M.; Zhang, L.; Zhang, X.; Zhu, S.; Wu, W. Adding the combination of CNTs and MoS2 into halogen-free flame retarding TPEE with enhanced the anti-dripping behavior and char forming properties. Thermochim. Acta 2015, 613, 87–93. [Google Scholar] [CrossRef]
- Lv, S.; Hu, L.; Xia, C.; Cabrera, M.B.; Guo, Y.; Liu, C.; You, L. Recycling fish scale powder in improving the performance of asphalt: A sustainable utilization of fish scale waste in asphalt. J. Clean. Prod. 2021, 288, 125682. [Google Scholar] [CrossRef]
- Antonić, B.; Dordević, D.; Jančíková, S.; Tremlova, B.; Kushkevych, I. Physicochemical characterization of home-made soap from waste-used frying oils. Processes 2020, 8, 1219. [Google Scholar] [CrossRef]
- Antonic, B.; Dordevic, D.; Jancikova, S.; Tremlova, B.; Nejezchlebova, M.; Goldová, K.; Treml, J. Reused plant fried oil: A case study with home-made soaps. Processes 2021, 9, 529. [Google Scholar] [CrossRef]
- Athinarayanan, J.; Periasamy, V.S.; Alshatwi, A.A. Simultaneous fabrication of carbon nanodots and hydroxyapatite nanoparticles from fish scale for biomedical applications. Mater. Sci. Eng. C 2020, 117, 111313. [Google Scholar] [CrossRef]
- Dai, X.; Li, P.; Sui, Y.; Zhang, C. Thermal and flame-retardant properties of intrinsic flame-retardant epoxy resin containing biphenyl structures and phosphorus. Eur. Polym. J. 2021, 147, 110319. [Google Scholar] [CrossRef]
- Wang, C.S.; Shieh, J.Y. Phosphorus-containing epoxy resin for an electronic application. J. Appl. Polym. Sci. 1999, 73, 353–361. [Google Scholar] [CrossRef]
- Qiu, L.; Xie, R.; Ding, P.; Qu, B. Preparation and characterization of Mg(OH)2 nanoparticles and flame-retardant property of its nanocomposites with EVA. Compos. Struct. 2003, 62, 391–395. [Google Scholar] [CrossRef]
- Paul, S.; Pal, A.; Choudhury, A.R.; Bodhank, S.; Balla, V.K.; Sinha, A.; Das, M. Effect of trace elements on the sintering effect of fish scale derived hydroxyapatite and its bioactivity. Ceram. Int. 2017, 43, 15678–15684. [Google Scholar] [CrossRef]
- Muhammad, N.; Gao, Y.; Iqbal, E.; Ahmad, P.; Ge, R.; Nishan, U.; Rahim, A.; Gonfa, G.; Ullah, Z. Extraction of biocompatible hydroxyapatite from fish scales using novel approach of ionic liquid pretreatment. Sep. Purif. Technol. 2016, 161, 129–135. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kozlowska, J. Fish scales as a biocomposite of collagen and calcium salts. Key Eng. Mater. 2014, 587, 185–190. [Google Scholar] [CrossRef]
- Guo, W.; Liu, J.; Zhang, P.; Song, L.; Wang, X.; Hu, Y. Multi-functional hydroxyapatite/polyvinyl alcohol composite aerogels with self-cleaning, superior fire resistance and low thermal conductivity. Compos. Sci. Technol. 2018, 158, 128–136. [Google Scholar] [CrossRef]
- Gou, X.; Zhao, X.; Singh, S.; Qiao, D. Tri-pyrolysis: A thermo-kinetic characterization of polyethylene, cornstalk, and anthracite coal using TGA-FTIR analysis. Fuel 2019, 252, 93–402. [Google Scholar] [CrossRef]
- Xiang, S.; Feng, L.; Bin, X.; Li, G.; Chen, X. Evaluation of PLA content in PLA/PBAT blends using TGA. Polym. Test. 2020, 81, 106211. [Google Scholar] [CrossRef]
- Laxmi; Khan, S.; Zafar, F.; Kareem, A.; Nami, S.A.A.; Alam, M.; Nishat, N. Development of coordination polyureas derived from amine terminated polyurea and metal ions having ‘d5’, ‘d7’, ‘d8’ and ‘d10’ orbitals: From synthesis to applications. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 219, 552–568. [Google Scholar] [CrossRef]
- Laxmi; Khan, S.; Kareem, A.; Zafar, F.; Nishat, N. Synthesis, vibrational spectrometry and thermal characterizations of coordination polymers derived from divalent metal ions and hydroxyl terminated polyurethane as ligand. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 188, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, W.H.; Cheng, W.T.; Chen, L.C.; Lin, S.Y. Non-isothermal dehydration kinetic study of aspartame hemihydrate using DSC, TGA and DSC-FTIR microspectroscopy. Asian J. Pharm. 2018, 13, 212–219. [Google Scholar] [CrossRef]
- Sun, J.; Li, L.; Li, J. Effects of furan-phosphamide derivative on flame retardancy and crystallization behaviors of poly(lactic acid). Chin. J. Chem. Eng. 2019, 369, 150–160. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Wang, X.; Li, H.; Sun, J.; Sun, W.; Yao, Y.; Gu, X.; Zhang, S. Surface coated rigid polyurethane foam with durable flame retardancy and improved mechanical property. Chin. J. Chem. Eng. 2020, 385, 123755. [Google Scholar] [CrossRef]
- Wang, J.; Ma, C.; Wang, P.; Qiu, S.; Cai, W.; Hu, Y. Ultra-low phosphorus loading to achieve the superior flame retardancy of epoxy resin. Polym. Degrad. Stab. 2018, 149, 119–128. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, M.; Chen, Z.; Chen, T.; Yu, Y.; Jiang, J. Preparation and characterization of a microencapsulated flame retardant and its flame-retardant mechanism in unsaturated polyester resins. Powder Technol. 2019, 354, 71–81. [Google Scholar] [CrossRef]
- Zhang, T.; Tao, Y.; Zhou, F.; Sheng, H.; Qiu, S.; Ma, C.; Hu, Y. Synthesis of a hyperbranched phosphorus-containing polyurethane as char forming agent combined with ammonium polyphosphate for reducing fire hazard of polypropylene. Polym. Degrad. Stab. 2019, 165, 207–219. [Google Scholar] [CrossRef]
- Guo, W.; Wang, X.; Zhang, P.; Liu, J.; Lei, S.; Hu, Y. Nano-fibrillated cellulose-hydroxyapatite based composite foams with excellent fire resistance. Carbohydr. Polym. 2018, 195, 71–78. [Google Scholar] [CrossRef]
- Liang, D.; Zhu, X.; Dai, P.; Lu, X.; Guo, H.; Que, H.; Wang, D.; He, T.; Xu, C.; Robin, H.M.; et al. Preparation of a novel lignin-based flame retardant for epoxy resin. Mater. Chem. Phys. 2021, 259, 124101. [Google Scholar] [CrossRef]
- Sui, Y.; Dai, X.; Li, P.; Zhang, C. Superior radical scavenging and catalytic carbonization capacities of bioderived assembly modified ammonium polyphosphate as a mono-component intumescent flame retardant for epoxy resin. Eur. Polym. J. 2021, 156, 110601. [Google Scholar] [CrossRef]
- Alttar, N.A.; Kopf, L.; Flavin, K.; Kennedy, E.; Giordani, S.; Rice, J.H. Surface-enhanced Raman scattering spectra of radial breathing and G band modes in functionalised nanotubes. Chem. Phys. Lett. 2013, 568–569, 95–100. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.; Zhang, X.; Ling, P.; Xu, K.; He, L.; Su, S.; Wang, Y.; Hu, S.; Xiang, J. Chemical imaging of coal in micro-scale with Raman mapping technology. Fuel 2020, 264, 116826. [Google Scholar] [CrossRef]










| Sample NO. | a Td5 (°C) | b Tmax (°C) | c Rmax (wt.%/min) | IPDT (°C) | C.Y (wt.%) |
|---|---|---|---|---|---|
| Pure epoxy | 397.1 | 432.7 | −24.5 | 685.6 | 16.45 |
| EP/APP/FS 10% | 288.7 | 392.4 | −19.6 | 802.7 | 23.56 |
| EP/APP/FS 20% | 260.4 | 384.9 | −19.3 | 866.1 | 26.44 |
| EP/APP/FS 30% | 258.2 | 378.7 | −17.2 | 980.4 | 30.80 |
| EP/APP/FS 40% | 238.5 | 376.9 | −13.9 | 1143.1 | 36.07 |
| α | EP | EP/FS/APP 40% | ||
|---|---|---|---|---|
| E (kJ/mole) | R Value | E (kJ/mole) | R Value | |
| 0.1 | 241 | 0.935 | 93 | 0.901 |
| 0.2 | 230 | 0.966 | 202 | 0.938 |
| 0.3 | 216 | 0.984 | 242 | 0.920 |
| 0.4 | 195 | 0.992 | 228 | 0.951 |
| 0.5 | 183 | 0.998 | 202 | 0.974 |
| 0.6 | 184 | 0.999 | 186 | 0.985 |
| 0.7 | 188 | 0.999 | 200 | 0.996 |
| 0.8 | 218 | 0.999 | 419 | 0.993 |
| 0.9 | 299 | 0.990 | 304 | 0.989 |
| ∆E(av) | 217 | 230 | ||
| Sample | UL-94 | LOI (%) | ∆LOI (%) | |
|---|---|---|---|---|
| Ranking | Dripping | |||
| Pure epoxy | Fail | NO | 21.2 ± 0.2 | 0 |
| EP/APP/FS 10% | Fail | NO | 21.3 ± 0.3 | 0.1 |
| EP/APP/FS 20% | Fail | NO | 23.1 ± 0.2 | 1.9 |
| EP/APP/FS 30% | V-0 | NO | 27.2 ± 0.3 | 6.0 |
| EP/APP/FS 40% | V-0 | NO | 36.2 ± 0.2 | 15.0 |
| Sample No. | FS:APP | LOI (%) |
|---|---|---|
| EP/APP 40% (experimental) | 0:3 | 46.2 ± 0.1 |
| EP/APP/FS 40% (experimental) | 1:2 | 47.3 ± 0.3 |
| EP/APP/FS 40% (experimental) | 2:1 | 36.2 ± 0.2 |
| EP/FS 40% (experimental) | 3:0 | 23.3 ± 0.2 |
| EP/APP/FS 40% (calculated) | 1:2 | 38.3 ± 0.2 |
| EP/APP/FS 40% (calculated) | 2:1 | 30.6 ± 0.3 |
| Sample No. | Elements | ||||
|---|---|---|---|---|---|
| C (wt.%) | N (wt.%) | O (wt.%) | P (wt.%) | Ca (wt.%) | |
| EP/APP/FS 10% (before burning) | 66.3 | 13.08 | 19.62 | 0.66 | 0.35 |
| EP/APP/FS 10% (after burning) | 56.03 | 14.77 | 22.86 | 4.01 | 2.34 |
| EP/APP/FS 40% (before burning) | 63.19 | 13.20 | 19.54 | 2.61 | 1.45 |
| EP/APP/FS 40% (after burning) | 41.95 | 11.42 | 32.17 | 12.13 | 2.33 |
| Sample No. | D-band | G-band | D/G | |
|---|---|---|---|---|
| 1350 cm−1 | 1850 cm−1 | |||
| EP/APP/FS 10% | 1 min | 723,254 | 220,353 | 3.282 |
| 5 min | 106,075 | 106,075 | 2.019 | |
| EP/APP/FS 40% | 1 min | 514,002 | 414,064 | 1.241 |
| 5 min | 176,812 | 322,428 | 0.548 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.-H.; Shen, M.-Y.; Yang, C.-Y.; Chiang, C.-L. A Study on Circular Economy Material Using Fish Scales as a Natural Flame Retardant and the Properties of Its Composite Materials. Polymers 2021, 13, 2446. https://doi.org/10.3390/polym13152446
Liu S-H, Shen M-Y, Yang C-Y, Chiang C-L. A Study on Circular Economy Material Using Fish Scales as a Natural Flame Retardant and the Properties of Its Composite Materials. Polymers. 2021; 13(15):2446. https://doi.org/10.3390/polym13152446
Chicago/Turabian StyleLiu, Shang-Hao, Ming-Yuan Shen, Cheng-You Yang, and Chin-Lung Chiang. 2021. "A Study on Circular Economy Material Using Fish Scales as a Natural Flame Retardant and the Properties of Its Composite Materials" Polymers 13, no. 15: 2446. https://doi.org/10.3390/polym13152446
APA StyleLiu, S. -H., Shen, M. -Y., Yang, C. -Y., & Chiang, C. -L. (2021). A Study on Circular Economy Material Using Fish Scales as a Natural Flame Retardant and the Properties of Its Composite Materials. Polymers, 13(15), 2446. https://doi.org/10.3390/polym13152446