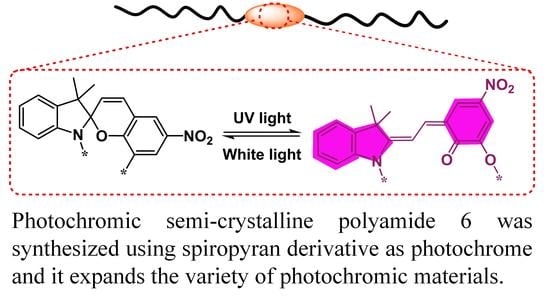
Photochromic Polyamide 6 Based on Spiropyran Synthesized via Hydrolyzed Ring-Opening Polymerization
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Carboxyl-Terminated Spiropyran (HOOC-SP-COOH)
2.3. Synthesis of Photochromic PA6
3. Characterization Methods
3.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.2. 1H Nuclear Magnetic Resonance Spectroscopy (1H NMR)
3.3. Liquid Chromatograph-Mass Spectrometry (LC-MS)
3.4. Melting Point
3.5. 13C Nuclear Magnetic Resonance Spectroscopy (13C NMR)
3.6. Differential Scanning Calorimetry (DSC)
3.7. Thermogravimetric Analysis (TGA)
3.8. X-ray Diffraction (XRD)
3.9. Mechanical Properties
3.10. Fluorescence Spectrophotometer
3.11. UV-Visible Spectrophotometer
3.12. RGB Analysis
3.13. UV-Vis-Near Infrared Spectrometer
3.14. Gel Penetration Chromatography (GPC)
4. Results and Discussion
4.1. Characterization of Carboxyl-Terminated Spiropyran (HOOC-SP-COOH)
4.2. Structural Characterization of Polymers
4.3. Thermal and Mechanical Properties
4.4. Photochromic Properties of SP-Modified PA6
4.5. Photoresponse Reversibility and Fatigue Resistance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, J.; Wang, Y.; Yao, Y.; Wang, Y.; Fei, X.; Qi, P.; Lin, S.; Kaplan, D.L.; Buehler, M.J.; Ling, S. Biological Material Interfaces as Inspiration for Mechanical and Optical Material Designs. Chem. Rev. 2019, 119, 12279–12336. [Google Scholar] [CrossRef] [PubMed]
- Genovese, M.E.; Abraham, S.; Caputo, G.; Nanni, G.; Kumaran, S.K.; Montemagno, C.D.; Athanassiou, A.; Fragouli, D. Photochromic Paper Indicators for Acidic Food Spoilage Detection. ACS Omega 2018, 3, 13484–13493. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Ji, Y.; Huang, X.; Yang, X.; Gouma, P.-I.; Dudley, M. Fabrication and Characterization of Polycrystalline WO3 Nanofibers and Their Application for Ammonia Sensing. J. Phys. Chem. B 2006, 110, 23777–23782. [Google Scholar] [CrossRef]
- Lin, S.; Gutierrez-Cuevas, K.G.; Zhang, X.; Guo, J.; Li, Q. Fluorescent Photochromic α-Cyanodiarylethene Molecular Switches: An Emerging and Promising Class of Functional Diarylethene. Adv. Funct. Mater. 2021, 31, 2007957. [Google Scholar] [CrossRef]
- Abdollahi, A.; Roghani-Mamaqani, H.; Razavi, B.; Salami-Kalajahi, M. Photoluminescent and Chromic Nanomaterials for Anticounterfeiting Technologies: Recent Advances and Future Challenges. ACS Nano 2020, 14, 14417–14492. [Google Scholar] [CrossRef]
- Ramlow, H.; Andrade, K.L.; Immich, A.P.S. Smart textiles: An Overview of Recent Progress on Chromic Textiles. J. Text. Inst. 2021, 112, 152–171. [Google Scholar] [CrossRef]
- Li, M.; Fu, S. Photochromic Holo-Cellulose Wood-Based Aerogel Grafted Azobenzene Derivative by SI-ATRP. Carbohydr. Polym. 2021, 259, 117736. [Google Scholar] [CrossRef]
- Kortekaas, L.; Chen, J.; Jacquemin, D.; Browne, W.R. Proton-Stabilized Photochemically Reversible E/Z Isomerization of Spiropyrans. J. Phys. Chem. B 2018, 122, 6423–6430. [Google Scholar] [CrossRef] [PubMed]
- Saes, B.W.; Wienk, M.M.; Janssen, R.A. Photochromic Organic Solar Cells Based on Diarylethenes. RSC Adv. 2020, 10, 30176–30185. [Google Scholar] [CrossRef]
- Lachmann, D.; Studte, C.; Männel, B.; Hübner, H.; Gmeiner, P.; König, B. Photochromic Dopamine Receptor Ligands Based on Dithienylethenes and Fulgides. Adv. Opt. Mater. 2017, 23, 13423–13434. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fan, W.; Liu, Z.; Yu, A.; Jiang, X. Advances on Tungsten Oxide Based Photochromic Materials: Strategies to Improve Their Photochromic Properties. J. Mater. Chem. C 2018, 6, 191–212. [Google Scholar] [CrossRef]
- Nie, H.; Self, J.L.; Kuenstler, A.S.; Hayward, R.C.; Read de Alaniz, J. Multiaddressable Photochromic Architectures: From Molecules to Materials. Chem. Eur. J. 2019, 7, 1900224. [Google Scholar] [CrossRef]
- Klajn, R. Spiropyran-based dynamic materials. Chem. Soc. Rev. 2014, 43, 148–184. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zhang, Q.; Zhou, Y.N.; Zhu, S. Let Spiropyran Help Polymers Feel Force! Prog. Polym. Sci. 2018, 79, 26–39. [Google Scholar] [CrossRef]
- Potisek, S.L.; Davis, D.A.; Sottos, N.R.; White, S.R.; Moore, J.S. Mechanophore-linked addition polymers. J. Am. Chem. Soc. 2007, 129, 13808–13809. [Google Scholar] [CrossRef]
- Davis, D.A.; Hamilton, A.; Yang, J.; Cremar, L.D.; Van Gough, D.; Potisek, S.L.; Ong, M.T.; Braun, P.V.; Martínez, T.J.; White, S.R. Force-induced activation of covalent bonds in mechanoresponsive polymeric materials. Nature 2009, 459, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Bao, B.; Wang, Z.; Xu, R.; Wang, W.; Yu, D. High Tri-stimulus Response Photochromic Cotton Fabrics Based on Spiropyran Dye by Thiol-Ene Click Chemistry. Cellulose 2020, 27, 493–510. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, T.; Yan, J.; Fu, L.; Xiang, H.; Cui, Y.; Su, J.; Liu, X. Preparation and Photochromic Behavior of Spiropyran-Containing Fuorinated Polyacrylate Hydrophobic Coatings. Langmuir 2018, 34, 15812–15819. [Google Scholar] [CrossRef]
- Nam, Y.S.; Yoo, I.; Yarimaga, O.; Park, I.S.; Park, D.H.; Song, S.; Kim, J.M.; Lee, C.W. Photochromic Spiropyran-Embedded PDMS for Highly Sensitive and Tunable Optochemical Gas Sensing. Chem. Commun. 2014, 50, 4251–4254. [Google Scholar] [CrossRef] [PubMed]
- Campos, P.P.; Dunne, A.; Delaney, C.; Moloney, C.; Moulton, S.E.; Benito-Lopez, F.; Ferreira, M.; Diamond, D.; Florea, L. Photoswitchable Layer-by-Layer Coatings Based on Photochromic Polynorbornenes Bearing Spiropyran Side Groups. Langmuir 2018, 34, 4210–4216. [Google Scholar] [CrossRef]
- Krohm, F.; Kind, J.; Savka, R.; Alcaraz Janβen, M.; Herold, D.; Plenio, H.; Thiele, C.M.; Andrieu-Brunsen, A. Photochromic Spiropyran- and Spirooxazine-Homopolymers in Mesoporous Thin Films by Surface Initiated ROMP. J. Mater. Chem. C 2016, 4, 4067–4076. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Scheiger, J.M.; Levkin, P.A. Design and Applications of Photoresponsive Hydrogels. Adv. Mater. 2019, 31, 1807333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Ren, B.; Yang, F.; Cai, Y.; Chen, H.; Wang, T.; Feng, Z.; Tang, J.; Xu, J.; Zheng, J. Micellar-Incorporated Hydrogels with Highly Tough, Mechanoresponsive, and Self-Recovery Properties for Strain-Induced Color Sensors. J. Mater. Chem. C 2018, 6, 11536–11551. [Google Scholar] [CrossRef]
- Miao, W.; Wang, S.; Liu, M. Reversible Quadruple Switching with Optical, Chiroptical, Helicity, and Macropattern in Self-Assembled Spiropyran Gels. Adv. Funct. Mater. 2017, 27, 1701368. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Z.; Liu, H.; Wang, S.; Zhang, Y.; Li, C.; Wu, Y. Preparation of Reversible Photoresponsive Poly(SPA-co-MMA) Films by Electrospinning: A Possible Route to Smart Materials for Regulating Wettability and Humidity. Adv. Mater. Technol. 2019, 4, 1900039. [Google Scholar] [CrossRef]
- Keyvan Rad, J.; Ghomi, A.R.; Karimipour, K.; Mahdavian, A.R. Progressive Readout Platform Based on Photoswitchable Polyacrylic Nanofibers Containing Spiropyran in Photopatterning with Instant Responsivity to Acid–Base Vapors. Macromolecules 2020, 53, 1613–1622. [Google Scholar] [CrossRef]
- De Sousa, F.B.; Guerreiro, J.D.T.; Ma, M.; Anderson, D.G.; Drum, C.L.; Sinisterra, R.D.; Langer, R. Photo-Response Behavior of Electrospun Nanofibers Based on Spiropyran-Cyclodextrin Modified Polymer. J. Mater. Chem. 2010, 20, 9910–9917. [Google Scholar] [CrossRef]
- Das, R.; Kuehnert, M.; Sadat Kazemi, A.; Abdi, Y.; Schulze, A. Water Softening Using a Light-Responsive, Spiropyran-Modified Nanofiltration Membrane. Polymers 2019, 11, 344. [Google Scholar] [CrossRef] [Green Version]
- Yaghini, N.; Peters, G.W.M. Modeling Crystallization Kinetics and Resulting Properties of Polyamide 6. Macromolecules 2021, 54, 1894–1904. [Google Scholar] [CrossRef]
- Ghosh, S.; Hall, J.; Joshi, V. Study of chameleon nylon and polyester fabrics using photochromic ink. J. Text. Inst. 2018, 109, 723–729. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Fang, X.; Lin, Y.; Xu, Y.; Weng, W. Mechanical activation of mechanophore enhanced by strong hydrogen bonding interactions. ACS Macro Lett. 2014, 3, 141–145. [Google Scholar] [CrossRef]
- Aurbach, D.; Turgeman, R.; Chusid, O.; Gofer, Y. Spectroelectrochemical studies of magnesium deposition by in situ FTIR spectroscopy. Electrochem. Commun. 2001, 3, 252–261. [Google Scholar] [CrossRef]
- Assifaoui, A.; Loupiac, C.; Chambin, O.; Cayot, P. Structure of calcium and zinc pectinate films investigated by FTIR spectroscopy. Carbohydr. Res. 2010, 345, 929–933. [Google Scholar] [CrossRef]
- Hong, G.; Zhang, H.; Lin, Y.; Chen, Y.; Xu, Y.; Weng, W.; Xia, H. Mechanoresponsive healable metallosupramolecular polymers. Macromolecules 2013, 46, 8649–8656. [Google Scholar] [CrossRef]
- Mouanni, S.; Amitouche, D.; Mazari, T.; Rabia, C. Transition metal-substituted Keggin-type polyoxometalates as catalysts for adipic acid production. Appl. Petrochem. Res. 2019, 9, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Fornes, T.D.; Yoon, P.J.; Paul, D.R. Polymer Matrix Degradation and Color Formation in Melt Processed Nylon 6/Clay Nanocomposites. Polymer 2003, 44, 7545–7556. [Google Scholar] [CrossRef]
- Miri, V.; Elkoun, S.; Peurton, F.; Vanmansart, C.; Lefebvre, J.M.; Krawczak, P.; Seguela, R. Crystallization Kinetics and Crystal Structure of Nylon 6-Clay Nanocomposites: Combined Effects of Thermomechanical History, Clay Content, and Cooling Conditions. Macromolecules 2008, 41, 9234–9244. [Google Scholar] [CrossRef]
- He, Z.; Bao, B.; Fan, J.; Wang, W.; Yu, D. Photochromic cotton fabric based on microcapsule technology with anti-fouling properties. Colloids Surf. A Physicochem. Eng. Asp. 2020, 594, 124661. [Google Scholar] [CrossRef]









| Samples | CPL(g) | H3PO4(g) | H2O(g) | HDA(g) | HOOC-SP-COOH | |||
|---|---|---|---|---|---|---|---|---|
| SP Diol (mg) | Adipic Acid (mg) | DCC (mg) | DMAP (mg) | |||||
| PA6 | 50.012 | 1.002 | 0.503 | 0 | 0 | 0 | 0 | 0 |
| SP-PA6-1 | 50.002 | 1.002 | 0.501 | 0.25 | 25.0 | 47.45 | 26.9 | 7.9 |
| SP-PA6-2 | 50.003 | 1.001 | 0.501 | 0.25 | 30.0 | 57.67 | 32.3 | 9.6 |
| SP-PA6-3 | 50.004 | 1.001 | 0.500 | 0.25 | 40.0 | 76.65 | 43.3 | 12.3 |
| Samples | Mna (g/mol) | Mwa (g/mol) | Mw/Mna | Tcb (°C) | Tmb (°C) | Toughness c (MJ/m3) |
|---|---|---|---|---|---|---|
| PA6 | 16,200 | 26,200 | 1.62 | 177 | 217 | 106.6 ± 13.2 |
| SP-PA6-1 | 17,600 | 23,700 | 1.35 | 168 | 216 | 103.7 ± 15.2 |
| SP-PA6-2 | 18,900 | 28,300 | 1.50 | 166 | 218 | 91.0 ± 9.3 |
| SP-PA6-3 | 31,100 | 58,400 | 1.87 | 163 | 217 | 81.6 ± 8.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, S.; Zhang, J.; Zhou, Q.; Shi, L.; Wang, W.; Wang, D. Photochromic Polyamide 6 Based on Spiropyran Synthesized via Hydrolyzed Ring-Opening Polymerization. Polymers 2021, 13, 2496. https://doi.org/10.3390/polym13152496
Tian S, Zhang J, Zhou Q, Shi L, Wang W, Wang D. Photochromic Polyamide 6 Based on Spiropyran Synthesized via Hydrolyzed Ring-Opening Polymerization. Polymers. 2021; 13(15):2496. https://doi.org/10.3390/polym13152496
Chicago/Turabian StyleTian, Shiyou, Jicong Zhang, Qiong Zhou, Limei Shi, Wenwen Wang, and Dong Wang. 2021. "Photochromic Polyamide 6 Based on Spiropyran Synthesized via Hydrolyzed Ring-Opening Polymerization" Polymers 13, no. 15: 2496. https://doi.org/10.3390/polym13152496
APA StyleTian, S., Zhang, J., Zhou, Q., Shi, L., Wang, W., & Wang, D. (2021). Photochromic Polyamide 6 Based on Spiropyran Synthesized via Hydrolyzed Ring-Opening Polymerization. Polymers, 13(15), 2496. https://doi.org/10.3390/polym13152496