Polycarbonate/Poly(vinylidene fluoride)-Blend-Based Nanocomposites—Effect of Adding Different Carbon Nanofillers/Organoclay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Characterization
3. Results and Discussion
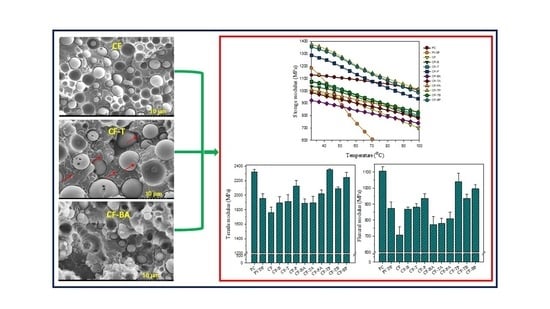
3.1. Phase Morphology and Selective Localization of Nanofillers
3.2. Crystal Structure
3.3. Crystallization and Melting Behavior
3.4. Thermal Stability
3.5. Mechanical Properties
3.6. Rheological and Electrical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Du, J.; Li, J.; Huang, X.; Kang, T.; Zhang, C.; Wang, S.; Ajao, O.O.; Wang, W.J.; Liu, P. Polymer nanocomposites with aligned two-dimensional materials. Prog. Polym. Sci. 2021, 114, 101360–101391. [Google Scholar] [CrossRef]
- Jen, Y.M.; Chang, H.H.; Lu, C.M.; Liang, S.Y. Temperature-dependent synergistic effect of multi-walled carbon nanotubes and graphene nanoplatelets on the tensile quasi-static and fatigue properties of epoxy nanocomposites. Polymers 2021, 13, 84. [Google Scholar] [CrossRef]
- Behera, K.; Chang, Y.H.; Chiu, F.C. Manufacturing poly(butylene adipate-co-terephthalate)/high density polyethylene blend-based nanocomposites with enhanced burning anti-dripping and physical properties—Effects of carbon nanofillers addition. Compos. B Eng. 2021, 217, 108878–108889. [Google Scholar] [CrossRef]
- Jin, X.; Wang, J.; Dai, L.; Wang, W.; Wu, H. Largely enhanced thermal conductive, dielectric, mechanical and anti-dripping performance in polycarbonate/boron nitride composites with graphene nanoplatelet and carbon nanotube. Compos. Sci. Technol. 2019, 184, 107862–107872. [Google Scholar] [CrossRef]
- Lin, H.M.; Behera, K.; Yadav, M.; Chiu, F.C. Polyamide 6/poly(vinylidene fluoride) blend-based nanocomposites with enhanced rigidity: Selective localization of carbon nanotube and organoclay. Polymers 2020, 12, 184. [Google Scholar] [CrossRef] [Green Version]
- Sieradzka, M.; Fryczkowski, R.; Binias, D.; Binias, W.; Janicki, J. A facile approach to obtaining PVDF/graphene fibers and the effect of nanoadditive on the structure and properties of nanocomposites. Polym. Test. 2020, 81, 106229–106238. [Google Scholar] [CrossRef]
- Kojima, Y.; Usuki, A.; Kawasumi, M.; Okada, A.; Kurauchi, T.; Kamigaito, O.J. Synthesis of nylon 6–clay hybrid by montmorillonite intercalated with ε-caprolactam. Polym. Sci. Polym. Chem. Ed. 1993, 31, 983–986. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F.; Ng, B.C. Directional alignment of carbon nanotubes in polymer matrices: Contemporary approaches and future advances. Compos. Part. A Appl. Sci. Manuf. 2014, 54, 103–126. [Google Scholar] [CrossRef]
- Bagotia, N.; Sharma, D.K. Ballistic behavior of multiwalled carbon nanotube reinforced toughened polycarbonate nanocomposites. Polym. Compos. 2020, 41, 1813–1819. [Google Scholar] [CrossRef]
- Netkueakul, W.; Fischer, B.; Walder, C.; Nüesch, F.; Rees, M.; Jovic, M.; Gaan, S.; Jacob, P.; Wang, J. Effects of combining graphene nanoplatelet and phosphorous flame retardant as additives on mechanical properties and flame retardancy of epoxy nanocomposite. Polymers 2020, 12, 2349. [Google Scholar] [CrossRef]
- Yaragalla, S.; Zahid, M.; Panda, J.K.; Tsagarakis, N.; Cingolani, R.; Athanassiou, A. Comprehensive enhancement in thermomechanical performance of melt-extruded PEEK filaments by graphene incorporation. Polymers 2021, 13, 1425. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, A.J.; Moy, P.; Beyer, F.L.; Madison, P.; Napadensky, E.; Ren, J.; Krishnamoorti, R. Mechanical response and rheological properties of polycarbonate layered-silicate nanocomposites. Polym. Eng. Sci. 2004, 44, 825–837. [Google Scholar] [CrossRef]
- Potschke, P.; Bhattacharyya, A.R.; Janke, A. Melt mixing of polycarbonate with multiwalled carbon nanotubes: Microscopic studies on the state of dispersion. Eur. Polym. J. 2004, 40, 137–148. [Google Scholar] [CrossRef]
- Lai, S.M.; Hsu, R.C.; Hsieh, C.Y.; Chiu, F.C. Comparisons of polycarbonate and polycarbonate/carbon nanotube nanocomposites and their microcellular foams prepared using supercritical carbon dioxide. J. Mater. Sci. 2015, 50, 2272–2283. [Google Scholar] [CrossRef]
- Mohammadi, H.M.; Majzoobi, G.H.; Payandehpeyman, J. Mechanical characterization of polycarbonate reinforced with nanoclay and graphene oxide. Polym. Compos. 2019, 40, 3947–3959. [Google Scholar] [CrossRef]
- Humphrey, J.S.; Sanayei, R.A. Vinylidene Fluoride Polymers; Wiley: New York, NY, USA, 2002. [Google Scholar]
- Lovinger, A.J. Crystallization of the β phase of poly(vinylidene fluoride) from the melt. Polymer 1981, 22, 412–413. [Google Scholar] [CrossRef]
- Mohajir, E.I.B.; Heymans, N. Changes in structural and mechanical behaviour of PVDF with processing and thermomechanical treatments. 1. Change in structure. Polymer 2001, 42, 5661–5667. [Google Scholar] [CrossRef]
- Priya, L.; Jog, J.P. Poly(vinylidene fluoride)/clay nanocomposites prepared by melt intercalation: Crystallization and dynamic mechanical behavior studies. J. Polym. Sci. Part. B Polym. Phys. 2002, 40, 1682–1689. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Zhang, Q.; Chen, F.; Deng, H.; Wang, H.D.K.; Fu, Q. Cooperative effect of shear and nanoclay on the formation of polar phase in poly(vinylidene fluoride) and the resultant properties. Polymer 2011, 52, 4970–4978. [Google Scholar] [CrossRef]
- Chiu, F.C. Comparisons of phase morphology and physical properties of PVDF nanocomposites filled with organoclay and/or multi-walled carbon nanotubes. Mater. Chem. Phys. 2014, 143, 681–692. [Google Scholar] [CrossRef]
- Anand, A.; Meena, D.; Dey, K.K.; Bhatnagar, M.C. Enhanced piezoelectricity properties of reduced graphene oxide (RGO) loaded polyvinylidene fluoride (PVDF) nanocomposite films for nanogenerator application. J. Polym. Res. 2020, 27, 358–369. [Google Scholar] [CrossRef]
- Chiu, F.C.; Chen, C.C.; Chen, Y.Z. Binary and ternary nanocomposites based on PVDF, PMMA, and PVDF/PMMA blends: Polymorphism, thermal, and rheological properties. J. Polym. Res. 2014, 21, 378–390. [Google Scholar] [CrossRef]
- Chiu, F.C.; Chen, Y.Z. Evaluation of thermal, mechanical, and electrical properties of PVDF/GNP binary and PVDF/PMMA/GNP ternary nanocomposites. Compos. Part. A Appl. Sci. Manuf. 2015, 68, 62–71. [Google Scholar] [CrossRef]
- Chiu, F.C.; Chuang, Y.C.; Liao, S.J.; Chang, Y.H. Comparison of PVDF/PVAc/GNP and PVDF/PVAc/CNT ternary nanocomposites: Enhanced thermal/electrical properties and rigidity. Polym. Test. 2018, 65, 197–205. [Google Scholar] [CrossRef]
- Cao, J.P.; Zhao, J.; Zhao, X.; You, F.; Yu, H.; Hu, G.H.; Dang, Z.M. High thermal conductivity and high electrical resistivity of poly(vinylidene fluoride)/polystyrene blends by controlling the localization of hybrid fillers. Compos. Sci. Technol. 2013, 89, 142–148. [Google Scholar] [CrossRef]
- Li, L.; Ruan, W.H.; Zhang, M.Q.; Rong, M.Z. Studies on the selective localization of multi-walled carbon nanotubes in blends of poly(vinylidene fluoride) and polycaprolactone. Express. Polym. Lett. 2015, 9, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Urtekin, G.; Aytac, A. The effects of multi-walled carbon nanotube additives with different functionalities on the properties of polycarbonate/poly (lactic acid) blend. J. Polym. Res. 2021, 28, 180–193. [Google Scholar] [CrossRef]
- Cao, J.P.; Zhao, J.Z.X.; Dang, Z.M. A facile route to prepare high-performance dielectric nanocomposites of poly(methyl methacrylate)/poly(vinylidene fluoride)/carbon nanotubes. Compos. Sci. Technol. 2021, 209, 108792–108800. [Google Scholar] [CrossRef]
- Kolonelou, E.; Loupou, E.; Klonos, P.A.; Sakellis, E.; Valadorou, D.; Kyritsis, A.; Papathanassiou, A.N. Thermal and electrical characterization of poly(vinyl)alcohol)/poly (vinylidene fluoride) blends reinforced with nano-graphene platelets. Polymer 2021, 224, 123731–123741. [Google Scholar] [CrossRef]
- Vozniak, I.; Hosseinnezhad, R.; Morawiec, J.; Galeski, A. Microstructural evolution of Poly(ε-caprolactone), its immiscible blend, and in situ generated nanocomposites. Polymers 2020, 12, 2587. [Google Scholar] [CrossRef]
- Chiu, F.C. Poly(vinylidene fluoride)/polycarbonate blend-based nanocomposites with enhanced rigidity—Selective localization of carbon nanofillers and organoclay. Polym. Test. 2017, 62, 115–123. [Google Scholar] [CrossRef]
- Mandal, A.; Nandi, A.K. Physical properties of poly(vinylidene fluoride) composites with polymer functionalized multiwalled carbon nanotubes using nitrene chemistry. J. Mater. Chem. 2011, 21, 15752–15763. [Google Scholar] [CrossRef]
- Kuzhir, P.; Paddubskaya, A.; Plyushch, N.; Volynets, S.; Maksimenko, J.; Macutkevic, I.; Kranauskaite, J.; Banys, E.; Ivanov, R.; Kotsilkova, A.; et al. Epoxy composites filled with high surface area-carbon fillers: Optimization of electromagnetic shielding, electrical, mechanical, and thermal properties. J. Appl. Phys. 2013, 114, 164304–164311. [Google Scholar] [CrossRef] [Green Version]









| Samples | Properties | ||||||
|---|---|---|---|---|---|---|---|
| Tp (°C) | Tm (°C) | Td5 (°C) | Td50 (°C) | TM * (MPa) | FM * (MPa) | ER (Ω-cm) | |
| PVDF | 138.8 | 167.2 | 447 | 471 | 1956 (67) | 873 (41) | >1013 |
| PC | ----- | ---- | 462 | 522 | 2325 (36) | 1107 (26) | >1013 |
| CF | 106.8 | 167.9 | 426 | 508 | 1760 (78) | 708 (51) | >1013 |
| CF-B | 106.2 | 168.1 | 427 | 504 | 1895 (82) | 869 (11) | 3.2 × 1010 |
| CF-T | 106.5 | 168.5 | 428 | 506 | 1916 (90) | 881 (23 | 6.0 × 109 |
| CF-P | 128.7 | 168.6 | 457 | 509 | 2126 (77) | 935 (29) | 1.2 × 1011 |
| CF-BA | 144.8 | 173.6 | 425 | 514 | 1889 (62) | 771 (50) | >1013 |
| CF-TA | 144.9 | 173.4 | 423 | 516 | 1897 (89) | 779 (33) | 2.4 × 1012 |
| CF-PA | 145.8 | 173.5 | 426 | 517 | 2021 (49) | 811 (38) | >1013 |
| CF-TP | 127.8 | 168.0 | 461 | 509 | 2351 (15) | 1039 (53) | 3.3 × 109 |
| CF-TB | 110.1 | 167.9 | 434 | 507 | 2093 (23) | 936 (27) | 6.4 × 108 |
| CF-BP | 128.3 | 167.7 | 459 | 510 | 2247 (69) | 995 (26) | 1.9 × 1010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, F.-C.; Behera, K.; Cai, H.-J.; Chang, Y.-H. Polycarbonate/Poly(vinylidene fluoride)-Blend-Based Nanocomposites—Effect of Adding Different Carbon Nanofillers/Organoclay. Polymers 2021, 13, 2626. https://doi.org/10.3390/polym13162626
Chiu F-C, Behera K, Cai H-J, Chang Y-H. Polycarbonate/Poly(vinylidene fluoride)-Blend-Based Nanocomposites—Effect of Adding Different Carbon Nanofillers/Organoclay. Polymers. 2021; 13(16):2626. https://doi.org/10.3390/polym13162626
Chicago/Turabian StyleChiu, Fang-Chyou, Kartik Behera, He-Jie Cai, and Yen-Hsiang Chang. 2021. "Polycarbonate/Poly(vinylidene fluoride)-Blend-Based Nanocomposites—Effect of Adding Different Carbon Nanofillers/Organoclay" Polymers 13, no. 16: 2626. https://doi.org/10.3390/polym13162626
APA StyleChiu, F. -C., Behera, K., Cai, H. -J., & Chang, Y. -H. (2021). Polycarbonate/Poly(vinylidene fluoride)-Blend-Based Nanocomposites—Effect of Adding Different Carbon Nanofillers/Organoclay. Polymers, 13(16), 2626. https://doi.org/10.3390/polym13162626