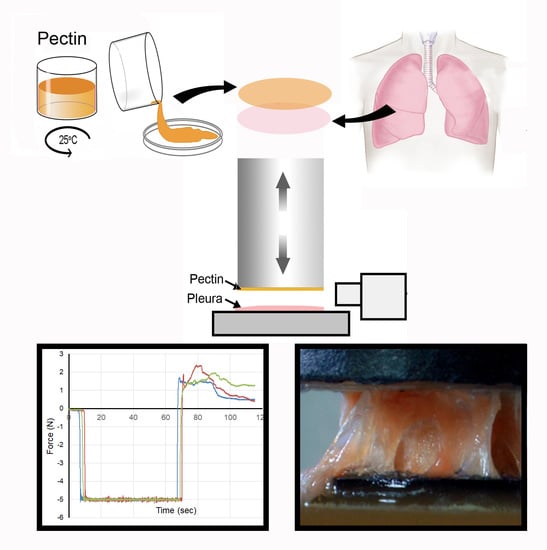
Functional Adhesion of Pectin Biopolymers to the Lung Visceral Pleura
Abstract
:1. Introduction
2. Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HMP | high-methoxyl pectin |
| LOS | length of stay |
| NCF | nanocellulose fibers |
| PSA | pressure sensitive adhesive |
| SEM | scanning electron microscopy |
References
- Torre, L.A.; Siegel, R.L.; Jemal, A. Lung Cancer Statistics. Adv. Exp. Med. Biol. 2016, 893, 1–19. [Google Scholar]
- Toumazis, I.; Bastani, M.; Han, S.S.; Plevritis, S.K. Risk-Based lung cancer screening: A systematic review. Lung Cancer 2020, 147, 154–186. [Google Scholar] [CrossRef]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef] [Green Version]
- Sugarbaker, D.J.; Bueno, R.; Krasna, M.K.; Mentzer, S.J.; Zellos, L. Adult Chest Surgery; McGraw-Hill: New York, NY, USA, 2009. [Google Scholar]
- Mentzer, S.J.; Tsuda, A.; Loring, S.H. Pleural mechanics and the pathophysiology of air leaks. J. Thorac. Cardiovasc. Surg. 2018, 155, 2182–2189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malapert, G.; Abou Hanna, H.; Pages, P.B.; Bernard, A. Surgical Sealant for the Prevention of Prolonged Air Leak After Lung Resection: Meta-Analysis. Ann. Thorac. Surg. 2010, 90, 1779–1785. [Google Scholar] [CrossRef]
- Petrella, F.; Borri, A.; Brambilla, D.; Calanca, G.; Vezzani, N.; Colantoni, A.; Gasparetto, A.; Spaggiari, L. Efficacy and safety of Innoseal for air leak after pulmonary resection: A case-control study. J. Surg. Res. 2016, 206, 22–26. [Google Scholar] [CrossRef]
- Pompili, C.; Miserocchi, G. Air leak after lung resection: Pathophysiology and patients’ implications. J. Thorac. Dis. 2016, 8, S46–S54. [Google Scholar]
- Bardell, T.; Petsikas, D. What keeps postpulmonary resection patients in hospital? Can. Respir. J. 2003, 10, 86–89. [Google Scholar] [CrossRef] [Green Version]
- Irshad, K.; Feldman, L.S.; Chu, V.F.; Dorval, J.F.; Baslaim, G.; Morin, J.E. Causes of increased length of hospitalization on a general thoracic surgery service: A prospective observational study. Can. J. Surg. 2002, 45, 264–268. [Google Scholar]
- Varela, G.; Jimenez, M.F.; Novoa, N.; Aranda, J.L. Estimating hospital costs attributable to prolonged air leak in pulmonary lobectomy. Eur. J. Cardio-Thorac. Surg. 2005, 27, 329–333. [Google Scholar] [CrossRef]
- Brunelli, A.; Monteverde, M.; Borri, A.; Salati, M.; Marasco, R.D.; Fianchini, A. Predictors of prolonged air leak after pulmonary lobectomy. Ann. Thorac. Surg. 2004, 77, 1205–1210. [Google Scholar] [CrossRef]
- Liang, S.Y.; Ivanovic, J.; Gilbert, S.; Maziak, D.E.; Shamji, F.M.; Sundaresan, R.S.; Seely, A.J.E. Quantifying the incidence and impact of postoperative prolonged alveolar air leak after pulmonary resection. J. Thorac. Cardiovasc. Surg. 2013, 145, 948–954. [Google Scholar] [CrossRef] [Green Version]
- Dordevic, D.; Jancikova, S.; Capikova, J.; Tremlova, B.; Kushkevych, I. Chemical and sensory properties of fruit jams affected by bamboo fiber fortification. Biointerface Res. Appl. Chem. 2020, 10, 5247–5251. [Google Scholar]
- Servais, A.B.; Kienzle, A.; Valenzuela, C.D.; Ysasi, A.B.; Wagner, W.L.; Tsuda, A.; Ackermann, M.; Mentzer, S.J. Structural heteropolysaccharide adhesion to the glycocalyx of visceral mesothelium. Tissue Eng. Part A 2018, 24, 199–206. [Google Scholar] [CrossRef]
- Servais, A.B.; Kienzle, A.; Ysasi, A.B.; Valenzuela, C.D.; Wagner, W.L.; Tsuda, A.; Ackermann, M.; Mentzer, S.J. Structural heteropolysaccharides as air-tight sealants of the human pleura. J. Biol. Mat. Res. 2018, 107, 799–806. [Google Scholar] [CrossRef]
- Servais, A.B.; Valenzuela, C.D.; Kienzle, A.; Ysasi, A.B.; Wagner, W.; Tsuda, A.; Ackermann, M.; Mentzer, S.J. Functional mechanics of a pectin-based pleural sealant after lung injury. Tissue Eng. Part A 2018, 24, 695–702. [Google Scholar] [CrossRef]
- Scheller, H.V.; Jensen, J.K.; Sorensen, S.O.; Harholt, J.; Geshi, N. Biosynthesis of pectin. Physiol. Plant 2007, 129, 283–295. [Google Scholar] [CrossRef]
- Monsoor, M.A.; Kalapathy, U.; Proctor, A. Determination of polygalacturonic acid content in pectin extracts by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem. 2001, 74, 233–238. [Google Scholar] [CrossRef]
- Nunes, C.; Silva, L.; Fernandes, A.P.; Guine, R.P.F.; Domingues, M.R.M.; Coimbra, M.A. Occurrence of cellobiose residues directly linked to galacturonic acid in pectic polysaccharides. Carbohydr. Polym. 2012, 87, 620–626. [Google Scholar] [CrossRef]
- Munarin, F.; Guerreiro, S.G.; Grellier, M.A.; Tanzi, M.C.; Barbosa, M.A.; Petrini, P.; Granja, P.L. Pectin-based injectable biomaterials for bone tissue engineering. Biomacromolecules 2011, 12, 568–577. [Google Scholar] [CrossRef]
- Smistad, G.; Boyum, S.; Alund, S.J.; Samuelsen, A.B.C.; Hiorth, M. The potential of pectin as a stabilizer for liposomal drug delivery systems. Carbohydr. Polym. 2012, 90, 1337–1344. [Google Scholar] [CrossRef]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Advances in biomedical applications of pectin gels. Int. J. Biol. Macromol. 2012, 51, 681–689. [Google Scholar] [CrossRef] [PubMed]
- ASNT. Nondestructive Testing Handbook: Leak Testing, 3rd ed.; American Society for Nondestructive Testing: Columbus, OH, USA, 1997. [Google Scholar]
- Servais, A.B.; Valenzuela, C.D.; Ysasi, A.B.; Wagner, W.L.; Kienzle, A.; Loring, S.H.; Tsuda, A.; Ackermann, M.; Mentzer, S.J. Pressure-decay testing of pleural air leaks in intact murine lungs: Evidence for peripheral airway regulation. Physiol. Rep. 2018, 6, 1–9. [Google Scholar] [CrossRef]
- Kuckelman, J.; Conner, J.R.; Zheng, Y.; Pierce, A.; Jones, I.; Lamers, D.; Cuadrado, D.; Eckert, M.; Mentzer, S.J. Improved outcomes utilizing a novel pectin-based pleural sealant following acute lung injury. J. Trauma Acute Care Surg. 2020, 89, 915–919. [Google Scholar] [CrossRef]
- Pierce, A.; Zheng, Y.; Wagner, W.L.; Scheller, H.V.; Mohnen, D.; Tsuda, A.; Ackermann, M.; Mentzer, S.J. Pectin biopolymer mechanics and microstructure associated with polysaccharide phase transitions. J. Biol. Mat. Res. Part A 2020, 108, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Panchev, I.N.; Slavov, A.; Nikolova, K.; Kovacheva, D. On the water-sorption properties of pectin. Food Hydrocoll. 2010, 24, 763–769. [Google Scholar] [CrossRef]
- Furmaniak, S.; Terzyk, A.P.; Gauden, P.A. The general mechanism of water sorption on foodstuffs—Importance of the multitemperature fitting of data and the hierarchy of models. J. Food Eng. 2007, 82, 528–535. [Google Scholar] [CrossRef]
- Zheng, Y.; Pierce, A.; Wagner, W.L.; Scheller, H.V.; Mohnen, D.; Ackermann, M.; Mentzer, S.J. Water-dependent blending of pectin films: The mechanics of conjoined biopolymers. Molecules 2020, 30, 11. [Google Scholar] [CrossRef]
- Pierce, A.; Zheng, Y.; Wagner, W.L.; Scheller, H.V.; Mohnen, D.; Tsuda, A.; Ackermann, M.; Mentzer, S.J. Visualizing pectin polymer-polymer entanglement produced by interfacial water movement. Carbohydr. Polym. 2020, 246, 116618. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Bennett, R.D.; Ackermann, M.; Ysasi, A.B.; Belle, J.M.; Valenzuela, C.D.; Pabst, A.M.; Tsuda, A.; Konerding, M.A.; Mentzer, S.J. Elastin cables define the axial connective tissue system in the murine lung. Anat. Rec. 2015, 298, 1960–1968. [Google Scholar] [CrossRef] [Green Version]
- Bodega, F.; Sironi, C.; Porta, C.; Zocchi, L.; Agostoni, E. Pleural mesothelium lubrication after phospholipase treatment. Respir. Physiol. Neurobiol. 2014, 194, 49–53. [Google Scholar] [CrossRef]
- Andrews, P.M.; Porter, K.R. Ultrastructural morphology and possible functional significance of mesothelial microvilli. Anat. Rec. 1973, 177, 409–426. [Google Scholar] [CrossRef]
- Mutsaers, S.E.; Bimie, K.; Lansley, S.; Herrick, S.E.; Lim, C.B.; Prele, C.M. Mesothelial cells in tissue repair and fibrosis. Front. Pharmacol. 2015, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Harholt, J.; Suttangkakul, A.; Scheller, H.V. Biosynthesis of Pectin. Plant Physiol. 2010, 153, 384–395. [Google Scholar] [CrossRef] [Green Version]
- Moulia, B. Plant biomechanics and mechanobiology are convergent paths to flourishing interdisciplinary research. J. Exp. Bot. 2013, 64, 4617–4633. [Google Scholar] [CrossRef] [Green Version]
- Rolin, C.; Nielsen, B.U.; Glahn, P.-E. Pectin. In Polysaccharides: Structural Diversity and Functional Versatility; Dumitriu, S., Ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Bernkop-Schnurch, A. Mucoadhesive Polymers Basics, Strategies, and Future Trends; CRC Press: Boca Raton, FL, USA, 2013; pp. 193–220. [Google Scholar]
- Puskas, J.D.; Mathisen, D.J.; Grillo, H.C.; Wain, J.C.; Wright, C.D.; Moncure, A.C. Treatment strategies for bronchopleural fistula. J. Thorac. Cardiovasc. Surg. 1995, 109, 989–996. [Google Scholar] [CrossRef] [Green Version]
- Wagner, W.L.; Zheng, Y.; Pierce, A.; Ackermann, M.; Horstmann, H.; Kuner, T.; Ronchi, P.; Schwab, Y.; Konietzke, P.; Wunnemann, F.; et al. Mesopolysaccharides: The extracellular surface layer of visceral organs. PLoS ONE 2020, 15, e0238798. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Pierce, A.F.; Wagner, W.L.; Khalil, H.A.; Chen, Z.; Servais, A.B.; Ackermann, M.; Mentzer, S.J. Functional Adhesion of Pectin Biopolymers to the Lung Visceral Pleura. Polymers 2021, 13, 2976. https://doi.org/10.3390/polym13172976
Zheng Y, Pierce AF, Wagner WL, Khalil HA, Chen Z, Servais AB, Ackermann M, Mentzer SJ. Functional Adhesion of Pectin Biopolymers to the Lung Visceral Pleura. Polymers. 2021; 13(17):2976. https://doi.org/10.3390/polym13172976
Chicago/Turabian StyleZheng, Yifan, Aidan F. Pierce, Willi L. Wagner, Hassan A. Khalil, Zi Chen, Andrew B. Servais, Maximilian Ackermann, and Steven J. Mentzer. 2021. "Functional Adhesion of Pectin Biopolymers to the Lung Visceral Pleura" Polymers 13, no. 17: 2976. https://doi.org/10.3390/polym13172976
APA StyleZheng, Y., Pierce, A. F., Wagner, W. L., Khalil, H. A., Chen, Z., Servais, A. B., Ackermann, M., & Mentzer, S. J. (2021). Functional Adhesion of Pectin Biopolymers to the Lung Visceral Pleura. Polymers, 13(17), 2976. https://doi.org/10.3390/polym13172976