Surface Morphology and Mechanical Properties of Polyether Ether Ketone (PEEK) Nanocomposites Reinforced by Nano-Sized Silica (SiO2) for Prosthodontics and Restorative Dentistry
Abstract
:1. Introduction
2. Materials and Methods or Experimental
2.1. Materials
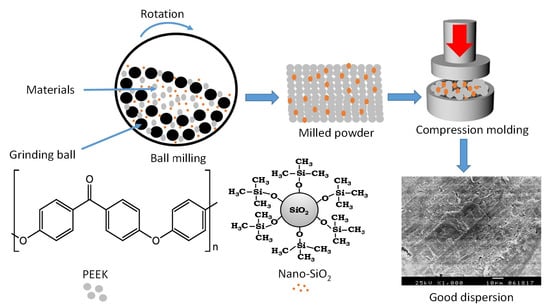
2.2. Preparation of PEEK/SiO2 Nanocomposites
2.3. Characterization Methods
2.3.1. X-ray Diffraction (XRD)
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Energy-Dispersive X-ray Spectroscopy (EDX)
2.3.4. Surface Roughness Analyses
2.3.5. Contact Angle Measurement
2.3.6. Microhardness Measurement
2.3.7. Mechanical Tests
2.4. Statistical Analysis
3. Results and Discussion
3.1. Fabrication of Nanocomposites
3.2. Structural Analysis
3.3. Morphological Observation
3.4. Compatibilization of Hydrophobic Polymer and Nanofiller
3.5. Surface Roughness (Ra)
3.6. Contact Angle Measurement
3.7. Microhardness
3.8. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehra, M.; Vahidi, F.; Berg, R.W. A complete denture impression technique survey of postdoctoral prosthodontic programs in the United States. J. Prosthodont. 2014, 23, 320–327. [Google Scholar] [CrossRef]
- Saavedra, G.; Valandro, L.F.; Leite, F.P.P.; Amaral, R.; Özcan, M.; Bottino, M.A.; Kimpara, E.T. Bond strength of acrylic teeth to denture base resin after various surface conditioning methods before and after thermocycling. Int. J. Prosthodont. 2007, 20, 199–201. [Google Scholar]
- Fischer, N.G.; Münchow, E.A.; Tamerler, C.; Bottino, M.C.; Aparicio, C. Harnessing biomolecules for bioinspired dental biomaterials. J. Mater. Chem. B 2020, 8, 8713–8747. [Google Scholar] [CrossRef] [PubMed]
- Ferrando-Magraner, E.; Bellot-Arcís, C.; Paredes-Gallardo, V.; Almerich-Silla, J.M.; García-Sanz, V.; Fernández-Alonso, M. Antibacterial properties of nanoparticles in dental restorative materials. A systematic review and meta-analysis. Medicina 2020, 56, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komabayashi, T.; Colmenar, D.; Cvach, N.; Bhat, A.; Primus, C.; Imai, Y. Comprehensive review of current endodontic sealers. Dent. Mater. J. 2020, 39, 703–720. [Google Scholar] [CrossRef] [Green Version]
- Galante, R.; Figueiredo-Pina, C.G.; Serro, A.P. Additive manufacturing of ceramics for dental applications: A review. Dent. Mater. 2019, 35, 825–846. [Google Scholar] [CrossRef]
- Chen, H.; Wang, R.; Zhang, J.; Hua, H.; Zhu, M. Synthesis of core-shell structured ZnO@ m-SiO2 with excellent reinforcing effect and antimicrobial activity for dental resin composites. Dent. Mater. 2018, 34, 1846–1855. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gan, K.; Liu, H.; Song, X.; Chen, T.; Liu, C. Antibacterial properties of nano-silver coated PEEK prepared through magnetron sputtering. Dent. Mater. 2017, 33, e348–e360. [Google Scholar] [CrossRef]
- Hanafy, R.A.; Mostafa, D.; Abd El-Fattah, A.; Kandil, S. Biomimetic chitosan against bioinspired nanohydroxyapatite for repairing enamel surfaces. Bioinspired Biomim. Nanobiomater. 2019, 9, 85–94. [Google Scholar] [CrossRef]
- Alghazzawi, T.F. The effect of extended aging on the optical properties of different zirconia materials. J. Prosthodont. Res. 2017, 61, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.C.; Sokolonski, A.R.; Fonseca, M.S.; Stanisic, D.; Araújo, D.B.; Azevedo, V.; Portela, R.D.; Tasic, L. Applications of Silver Nanoparticles in Dentistry: Advances and Technological Innovation. Int. J. Mol. Sci. 2021, 22, 2485. [Google Scholar] [CrossRef]
- Liu, F.; Hong, T.; Xie, J.; Zhan, X.; Wang, Y. Application of Reactive Oxygen Species-Based Nanomaterials in Dentistry: A Review. Crystals 2021, 11, 266. [Google Scholar] [CrossRef]
- Balbaa, A.O.; El-Fattah, A.A.; Awad, N.M.; Abdellatif, A. Effects of nanoscale electric fields on the histology of liver cell dysplasia. Nanomedicine 2019, 14, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Fattah, A.; Nageeb Hassan, M.; Rashad, A.; Marei, M.; Kandil, S. Viscoelasticity, mechanical properties, and in vivo biocompatibility of injectable polyvinyl alcohol/bioactive glass composite hydrogels as potential bone tissue scaffolds. Int. J. Polym. Anal. Charact. 2020, 25, 362–373. [Google Scholar] [CrossRef]
- Abd El-Fattah, A.; Mansour, A. Viscoelasticity, mechanical properties, and in vitro biodegradation of injectable chitosan-poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/nanohydroxyapatite composite hydrogel. Bull. Mater. Sci. 2018, 41, 1–10. [Google Scholar] [CrossRef] [Green Version]
- El-Fattah, A.A.; El Demerdash, A.G.M.; Alim Sadik, W.A.; Bedir, A. The effect of sugarcane bagasse fiber on the properties of recycled high density polyethylene. J. Compos. Mater. 2015, 49, 3251–3262. [Google Scholar] [CrossRef]
- Parvinzadeh, M.; Moradian, S.; Rashidi, A.; Yazdanshenas, M.-E. Surface characterization of polyethylene terephthalate/silica nanocomposites. Appl. Surf. Sci. 2010, 256, 2792–2802. [Google Scholar] [CrossRef]
- Zhong, F.; Xie, P.; Hou, R.; Niu, W.; Huang, J.; Hu, F.; Zheng, G.; Liu, H.; Qu, T.; Zhu, Y. Improved performance of sulfonated poly ether ether ketone/three-dimensional hierarchical molybdenum disulfide nanoflower composite proton exchange membrane for fuel cells. J. Mater. Sci. 2021, 56, 6531–6548. [Google Scholar] [CrossRef]
- Dunlop, M.J.; Bissessur, R. Nanocomposites based on graphene analogous materials and conducting polymers: A review. J. Mater. Sci. 2020, 55, 6721–6753. [Google Scholar] [CrossRef]
- Basgul, C.; Yu, T.; MacDonald, D.W.; Siskey, R.; Marcolongo, M.; Kurtz, S.M. Structure–property relationships for 3D-printed PEEK intervertebral lumbar cages produced using fused filament fabrication. J. Mater. Res. 2018, 33, 2040–2051. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, J.; Yan, M.; Tian, X. Poly ether ether ketone and its composite powder prepared by thermally induced phase separation for high temperature selective laser sintering. Mater. Des. 2021, 201, 109510. [Google Scholar] [CrossRef]
- Akhtar, F.H.; Abdulhamid, M.A.; Vovusha, H.; Ng, K.C.; Schwingenschlögl, U.; Szekely, G. Defining sulfonation limits of poly (ether-ether-ketone) for energy-efficient dehumidification. J. Mater. Chem. A 2021, 9, 17740–17748. [Google Scholar] [CrossRef]
- Abdulhamid, M.A.; Park, S.H.; Vovusha, H.; Akhtar, F.H.; Ng, K.C.; Schwingenschlögl, U.; Szekely, G. Molecular engineering of high-performance nanofiltration membranes from intrinsically microporous poly (ether-ether-ketone). J. Mater. Chem. A 2020, 8, 24445–24454. [Google Scholar] [CrossRef]
- Yogarathinam, L.T.; Jaafar, J.; Ismail, A.F.; Goh, P.S.; Gangasalam, A.; Hanifah, M.F.R.; Wong, K.C.; Subramaniam, M.N.; Peter, J. Functionalized boron nitride embedded sulfonated poly (ether ether ketone) proton exchange membrane for direct methanol fuel cell applications. J. Environ. Chem. Eng. 2021, 9, 105876. [Google Scholar] [CrossRef]
- Hao, L.; Hu, Y.; Zhang, Y.; Wei, W.; Hou, X.; Guo, Y.; Hu, X.; Jiang, D. Enhancing the mechanical performance of poly (ether ether ketone)/zinc oxide nanocomposites to provide promising biomaterials for trauma and orthopedic implants. RSC Adv. 2018, 8, 27304–27317. [Google Scholar] [CrossRef] [Green Version]
- Santing, H.J.; Meijer, H.J.; Raghoebar, G.M.; Özcan, M. Fracture strength and failure mode of maxillary implant-supported provisional single crowns: A comparison of composite resin crowns fabricated directly over PEEK abutments and solid titanium abutments. Clin. Implant. Dent. Relat. Res. 2012, 14, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.T.; Koak, J.Y.; Lim, Y.J.; Kim, S.K.; Kwon, H.B.; Kim, M.J. Stress shielding and fatigue limits of poly-ether-ether-ketone dental implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Chowdhary, R. PEEK materials as an alternative to titanium in dental implants: A systematic review. Clin. Implant. Dent. Relat. Res. 2019, 21, 208–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Nano-TiO2 reinforced PEEK/PEI blends as biomaterials for load-bearing implant applications. ACS Appl. Mater. Interfaces 2015, 7, 5561–5573. [Google Scholar] [CrossRef]
- Schmidlin, P.R.; Stawarczyk, B.; Wieland, M.; Attin, T.; Hämmerle, C.H.; Fischer, J. Effect of different surface pre-treatments and luting materials on shear bond strength to PEEK. Dent. Mater. 2010, 26, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.-H.; Kuo, M.; Huang, J.; Chen, M. On the PEEK composites reinforced by surface-modified nano-silica. Mater. Sci. Eng. A 2007, 458, 158–169. [Google Scholar] [CrossRef] [Green Version]
- Lümkemann, N.; Eichberger, M.; Stawarczyk, B. Bonding to different PEEK compositions: The impact of dental light curing units. Materials 2017, 10, 67. [Google Scholar] [CrossRef] [Green Version]
- Silthampitag, P.; Chaijareenont, P.; Tattakorn, K.; Banjongprasert, C.; Takahashi, H.; Arksornnukit, M. Effect of surface pretreatments on resin composite bonding to PEEK. Dent. Mater. J. 2016, 35, 668–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sproesser, O.; Schmidlin, P.R.; Uhrenbacher, J.; Roos, M.; Gernet, W.; Stawarczyk, B. Effect of sulfuric acid etching of polyetheretherketone on the shear bond strength to resin cements. J. Adhes Dent. 2014, 16, 465–472. [Google Scholar]
- Zhang, M.; Matinlinna, J.P. E-glass fiber reinforced composites in dental applications. Silicon 2012, 4, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Hedayati, M.; Salehi, M.; Bagheri, R.; Panjepour, M.; Maghzian, A. Ball milling preparation and characterization of poly (ether ether ketone)/surface modified silica nanocomposite. Powder Technol. 2011, 207, 296–303. [Google Scholar] [CrossRef]
- Dinari, M.; Soltani, R.; Mohammadnezhad, G. Kinetics and thermodynamic study on novel modified–mesoporous silica MCM-41/polymer matrix nanocomposites: Effective adsorbents for trace CrVI removal. J. Chem. Eng. Data 2017, 62, 2316–2329. [Google Scholar] [CrossRef]
- Kuo, M.; Kuo, J.; Yang, M.; Huang, J. On the crystallization behavior of the nano-silica filled PEEK composites. Mater. Chem. Phys. 2010, 123, 471–480. [Google Scholar] [CrossRef]
- Gashti, M.P.; Moradian, S.; Rashidi, A.; Yazdanshenas, M.-E. Dispersibility of hydrophilic and hydrophobic nano-silica particles in polyethylene terephthalate films: Evaluation of morphology and thermal properties. Polym. Polym. Compos. 2015, 23, 285–296. [Google Scholar] [CrossRef]
- Bose, S.; Robertson, S.F.; Bandyopadhyay, A. Surface modification of biomaterials and biomedical devices using additive manufacturing. Acta Biomater. 2018, 66, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Mekuria, T.D.; Chunhong, Z.; Yingnan, L.; Fouad, D.E.D.; Lv, K.; Yang, M.; Zhou, Y. Surface modification of nano-silica by diisocyanates and their application in polyimide matrix for enhanced mechanical, thermal and water proof properties. Mater. Chem. Phys. 2019, 225, 358–364. [Google Scholar] [CrossRef]
- Monich, P.R.; Berti, F.V.; Porto, L.M.; Henriques, B.; de Oliveira, A.P.N.; Fredel, M.C.; Souza, J.C. Physicochemical and biological assessment of PEEK composites embedding natural amorphous silica fibers for biomedical applications. Mater. Sci. Eng. C 2017, 79, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Zhang, C.; Tang, Y.; Liu, Y.; Niu, L.; Ding, T.; Li, X.; Zhang, Z. Preparation of hexamethyl disilazane-surface functionalized nano-silica by controlling surface chemistry and its “agglomeration-collapse” behavior in solution polymerized styrene butadiene rubber/butadiene rubber composites. Compos. Sci. Technol. 2021, 201, 108482. [Google Scholar] [CrossRef]
- Tran, N.T.; Patterson, B.A.; Harris, D.E.; Napadensky, E.; Lenhart, J.L.; Knorr, D.B., Jr.; Bain, E.D. Influence of Interfacial Bonding on the Mechanical and Impact Properties Ring-Opening Metathesis Polymer (ROMP) Silica Composites. ACS Appl. Mater. Interfaces 2020, 12, 53342–53355. [Google Scholar] [CrossRef] [PubMed]
- Gladson, T.F.; Ramesh, R.; Kavitha, C. Experimental investigation of mechanical, tribological and dielectric properties of alumina nano wire-reinforced PEEK/PTFE composites. Mater. Res. Express 2019, 6, 115327. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, G.; Wang, D.; Zhao, F.; Wang, T.; Wang, Q. Significance of combined functional nanoparticles for enhancing tribological performance of PEEK reinforced with carbon fibers. Compos. Part A Appl. Sci. Manuf. 2017, 102, 400–413. [Google Scholar] [CrossRef]
- Duongthipthewa, A.; Su, Y.; Zhou, L. Electrical conductivity and mechanical property improvement by low-temperature carbon nanotube growth on carbon fiber fabric with nanofiller incorporation. Compos. Part B Eng. 2020, 182, 107581. [Google Scholar] [CrossRef]
- Peng, C.; Li, X. The mechanical properties of PEEK/CF composites reinforced with ZrO 2 nanoparticles. Mech. Compos. Mater. 2014, 49, 679–684. [Google Scholar] [CrossRef]
- Kim, I.Y.; Sugino, A.; Kikuta, K.; Ohtsuki, C.; Cho, S.B. Bioactive composites consisting of PEEK and calcium silicate powders. J. Biomater. Appl. 2009, 24, 105–118. [Google Scholar]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Silica Content | ||||
|---|---|---|---|---|
| Code | Sample | PEEK (wt%) | BS (wt%) | LS (wt%) |
| PK | Unfilled PEEK | 100 | 0 | 0 |
| PKBS-10 | PEEK/BS 10 wt% | 90 | 10 | 0 |
| PKBS-20 | PEEK/BS 20 wt% | 80 | 20 | 0 |
| PKBS-30 | PEEK/BS 30 wt% | 70 | 30 | 0 |
| PKLS-10 | PEEK/LS 10 wt% | 90 | 0 | 10 |
| PKLS-20 | PEEK/LS 20 wt% | 80 | 0 | 20 |
| PKLS-30 | PEEK/LS 30 wt% | 70 | 0 | 30 |
| Code | Sample (n = 7) | Surface Roughness (Ra) (μm) | Contact Angle (◦) |
|---|---|---|---|
| PK | Unfilled PEEK | 1.45 ± 0.35 | 93.71 ± 1.52 |
| PKBS-10 | PEEK/BS 10 wt% | 1.47 ± 0.23 | 122.40 ± 2.16 |
| PKBS-20 | PEEK/BS 20 wt% | 2.03 ± 0.35 | 94.90 ± 1.10 |
| PKBS-30 | PEEK/BS 30 wt% | 2.36 ± 0.32 | 60.59 ± 0.52 |
| PKLS-10 | PEEK/LS 10 wt% | 1.52 ± 0.24 | 98.52 ± 1.75 |
| PKLS-20 | PEEK/LS 20 wt% | 2.13 ± 0.16 | 113.10 ± 1.33 |
| PKLS-30 | PEEK/LS 30 wt% | 3.32 ± 0.22 | 117.54 ± 1.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd El-Fattah, A.; Youssef, H.; Gepreel, M.A.H.; Abbas, R.; Kandil, S. Surface Morphology and Mechanical Properties of Polyether Ether Ketone (PEEK) Nanocomposites Reinforced by Nano-Sized Silica (SiO2) for Prosthodontics and Restorative Dentistry. Polymers 2021, 13, 3006. https://doi.org/10.3390/polym13173006
Abd El-Fattah A, Youssef H, Gepreel MAH, Abbas R, Kandil S. Surface Morphology and Mechanical Properties of Polyether Ether Ketone (PEEK) Nanocomposites Reinforced by Nano-Sized Silica (SiO2) for Prosthodontics and Restorative Dentistry. Polymers. 2021; 13(17):3006. https://doi.org/10.3390/polym13173006
Chicago/Turabian StyleAbd El-Fattah, Ahmed, Heba Youssef, Mohamed Abdel Hady Gepreel, Rafik Abbas, and Sherif Kandil. 2021. "Surface Morphology and Mechanical Properties of Polyether Ether Ketone (PEEK) Nanocomposites Reinforced by Nano-Sized Silica (SiO2) for Prosthodontics and Restorative Dentistry" Polymers 13, no. 17: 3006. https://doi.org/10.3390/polym13173006
APA StyleAbd El-Fattah, A., Youssef, H., Gepreel, M. A. H., Abbas, R., & Kandil, S. (2021). Surface Morphology and Mechanical Properties of Polyether Ether Ketone (PEEK) Nanocomposites Reinforced by Nano-Sized Silica (SiO2) for Prosthodontics and Restorative Dentistry. Polymers, 13(17), 3006. https://doi.org/10.3390/polym13173006