Nanocellulose/Fullerene Hybrid Films Assembled at the Air/Water Interface as Promising Functional Materials for Photo-electrocatalysis
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characterization of Films at the Air/Water Interface
3.2. Characterization of LS Films
3.3. Main Features of CNCs Binding Process Highlighted by Competition with TPPS4
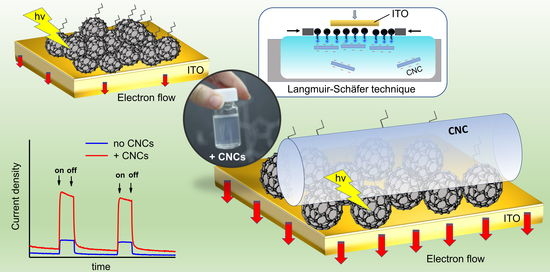
3.4. Photo-Electrochemical Characterization of FP/CNCs Hybrid Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manera, M.G.; Ferreiro-Vila, E.; García-Martín, J.M.; Cebollada, A.; García-Martín, A.; Giancane, G.; Valli, L.; Rella, R. Enhanced magneto-optical SPR platform for amine sensing based on Zn porphyrin dimers. Sens. Actuators B Chem. 2013, 182, 232–238. [Google Scholar] [CrossRef]
- Semeraro, P.; Syrgiannis, Z.; Bettini, S.; Giancane, G.; Guerra, F.; Fraix, A.; Bucci, C.; Sortino, S.; Prato, M.; Valli, L. Singlet oxygen photo-production by perylene bisimide derivative Langmuir-Schaefer films for photodynamic therapy applications. J. Colloid Interface Sci. 2019, 553, 390–401. [Google Scholar] [CrossRef]
- Manera, M.G.; Ferreiro-Vila, E.; Cebollada, A.; García-Martín, J.M.; García-Martín, A.; Giancane, G.; Valli, L.; Rella, R. Ethane-Bridged Zn Porphyrins Dimers in Langmuir–Schäfer Thin Films: Spectroscopic, Morphologic, and Magneto-Optical Surface Plasmon Resonance Characterization. J. Phys. Chem. C 2012, 116, 10734–10742. [Google Scholar] [CrossRef]
- Valli, L.; Giancane, G.; Mazzaglia, A.; Scolaro, L.M.; Conoci, S.; Sortino, S. Photoresponsive multilayer films by assembling cationic amphiphilic cyclodextrins and anionic porphyrins at the air/water interface. J. Mater. Chem. 2007, 17, 1660–1663. [Google Scholar] [CrossRef]
- Conoci, S.; Guldi, D.M.; Nardis, S.; Paolesse, R.; Kordatos, K.; Prato, M.; Ricciardi, G.; Vicente, M.G.H.; Zilbermann, I.; Valli, L. Langmuir–Shäfer Transfer of Fullerenes and Porphyrins: Formation, Deposition, and Application of Versatile Films. Chem. A Eur. J. 2004, 10, 6523–6530. [Google Scholar] [CrossRef]
- Valli, L.; Casilli, S.; Giotta, L.; Pignataro, B.; Conoci, S.; Borovkov, V.V.; Inoue, Y.; Sortino, S. Ethane-Bridged Zinc Porphyrin Dimers in Langmuir−Shäfer Thin Films: Structural and Spectroscopic Properties. J. Phys. Chem. B 2006, 110, 4691–4698. [Google Scholar] [CrossRef]
- Sgobba, V.; Giancane, G.; Cannoletta, D.; Operamolla, A.; Hassan Omar, O.; Farinola, G.M.; Guldi, D.M.; Valli, L. Langmuir–Schaefer Films for Aligned Carbon Nanotubes Functionalized with a Conjugate Polymer and Photoelectrochemical Response Enhancement. ACS Appl. Mater. Interfaces 2014, 6, 153–158. [Google Scholar] [CrossRef]
- Bodik, M.; Maxian, O.; Hagara, J.; Nadazdy, P.; Jergel, M.; Majkova, E.; Siffalovic, P. Langmuir–Scheaffer Technique as a Method for Controlled Alignment of 1D Materials. Langmuir 2020, 36, 4540–4547. [Google Scholar] [CrossRef]
- Valli, L.; Guldi, D.M. Langmuir Blodgett films of C60 and C60-materials. In Fullerenes: From Synthesis to Optoelectronic Properties; Guldi, D.M., Martin, N., Eds.; Springer Science+Business Media: Dordrecht, The Netherlands, 2002; pp. 327–385. [Google Scholar]
- Kouloumpis, A.; Vourdas, N.; Zygouri, P.; Chalmpes, N.; Potsi, G.; Kostas, V.; Spyrou, K.; Stathopoulos, V.N.; Gournis, D.; Rudolf, P. Controlled deposition of fullerene derivatives within a graphene template by means of a modified Langmuir-Schaefer method. J. Colloid Interface Sci. 2018, 524, 388–398. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Tang, Z.; Watkins, E.; Majewski, J.; Wang, H.-L. Synthesis and Characterization of Amphiphilic Fullerenes and Their Langmuir−Blodgett Films. Langmuir 2005, 21, 1416–1423. [Google Scholar] [CrossRef]
- Al-Hamadani, Y.A.J.; Chu, K.H.; Son, A.; Heo, J.; Her, N.; Jang, M.; Park, C.M.; Yoon, Y. Stabilization and dispersion of carbon nanomaterials in aqueous solutions: A review. Sep. Purif. Technol. 2015, 156, 861–874. [Google Scholar] [CrossRef]
- Giancane, G.; Ruland, A.; Sgobba, V.; Manno, D.; Serra, A.; Farinola, G.M.; Hassan Omar, O.; Guldi, D.M.; Valli, L. Aligning Single-Walled Carbon Nanotubes by Means of Langmuir–Blodgett Film Deposition: Optical, Morphological, and Photo-electrochemical Studies. Adv. Funct. Mater. 2010, 20, 2481–2488. [Google Scholar] [CrossRef]
- Hajian, A.; Lindström, S.B.; Pettersson, T.; Hamedi, M.M.; Wågberg, L. Understanding the Dispersive Action of Nanocellulose for Carbon Nanomaterials. Nano Lett. 2017, 17, 1439–1447. [Google Scholar] [CrossRef]
- Wang, Z.; Carlsson, D.O.; Tammela, P.; Hua, K.; Zhang, P.; Nyholm, L.; Strømme, M. Surface Modified Nanocellulose Fibers Yield Conducting Polymer-Based Flexible Supercapacitors with Enhanced Capacitances. ACS Nano 2015, 9, 7563–7571. [Google Scholar] [CrossRef]
- Malti, A.; Edberg, J.; Granberg, H.; Khan, Z.U.; Andreasen, J.W.; Liu, X.; Zhao, D.; Zhang, H.; Yao, Y.; Brill, J.W.; et al. An Organic Mixed Ion–Electron Conductor for Power Electronics. Adv. Sci. 2016, 3, 1500305. [Google Scholar] [CrossRef] [Green Version]
- Gopakumar, D.A.; Pai, A.R.; Pottathara, Y.B.; Pasquini, D.; Carlos de Morais, L.; Luke, M.; Kalarikkal, N.; Grohens, Y.; Thomas, S. Cellulose Nanofiber-Based Polyaniline Flexible Papers as Sustainable Microwave Absorbers in the X-Band. ACS Appl. Mater. Interfaces 2018, 10, 20032–20043. [Google Scholar] [CrossRef]
- Yoon, S.H.; Jin, H.J.; Kook, M.C.; Pyun, Y.R. Electrically conductive bacterial cellulose by incorporation of carbon nanotubes. Biomacromolecules 2006, 7, 1280–1284. [Google Scholar] [CrossRef]
- Hamedi, M.M.; Hajian, A.; Fall, A.B.; Håkansson, K.; Salajkova, M.; Lundell, F.; Wågberg, L.; Berglund, L.A. Highly Conducting, Strong Nanocomposites Based on Nanocellulose-Assisted Aqueous Dispersions of Single-Wall Carbon Nanotubes. ACS Nano 2014, 8, 2467–2476. [Google Scholar] [CrossRef]
- Song, L.; Li, Y.; Xiong, Z.; Pan, L.; Luo, Q.; Xu, X.; Lu, S. Water-Induced shape memory effect of nanocellulose papers from sisal cellulose nanofibers with graphene oxide. Carbohydr. Polym. 2018, 179, 110–117. [Google Scholar] [CrossRef]
- Nguyen Dang, L.; Seppälä, J. Electrically conductive nanocellulose/graphene composites exhibiting improved mechanical properties in high-moisture condition. Cellulose 2015, 22, 1799–1812. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, X.; Shen, Y.; Yoshino, K.; Feng, W. A mechanically strong, flexible and conductive film based on bacterial cellulose/graphene nanocomposite. Carbohydr. Polym. 2012, 87, 644–649. [Google Scholar] [CrossRef]
- Xiong, R.; Yu, S.; Smith, M.J.; Zhou, J.; Krecker, M.; Zhang, L.; Nepal, D.; Bunning, T.J.; Tsukruk, V.V. Self-Assembly of Emissive Nanocellulose/Quantum Dot Nanostructures for Chiral Fluorescent Materials. ACS Nano 2019, 13, 9074–9081. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, D.; Jiu, J.; Nge, T.T.; Sugahara, T.; Nagao, S.; Koga, H.; Nogi, M.; Suganuma, K.; Wang, X.; et al. Electrically conductive bacterial cellulose composite membranes produced by the incorporation of graphite nanoplatelets in pristine bacterial cellulose membranes. Express Polym. Lett. 2013, 7, 756–766. [Google Scholar] [CrossRef]
- Erbas Kiziltas, E.; Kiziltas, A.; Rhodes, K.; Emanetoglu, N.W.; Blumentritt, M.; Gardner, D.J. Electrically conductive nano graphite-filled bacterial cellulose composites. Carbohydr. Polym. 2016, 136, 1144–1151. [Google Scholar] [CrossRef]
- Rånby, B.G. Fibrous macromolecular systems. Cellulose and muscle. The colloidal properties of cellulose micelles. Discuss. Faraday Soc. 1951, 11, 158–164. [Google Scholar] [CrossRef]
- Battista, O.A. Hydrolysis and Crystallization of Cellulose. Ind. Eng. Chem. 1950, 42, 502–507. [Google Scholar] [CrossRef]
- Iwamoto, S.; Kai, W.; Isogai, A.; Iwata, T. Elastic Modulus of Single Cellulose Microfibrils from Tunicate Measured by Atomic Force Microscopy. Biomacromolecules 2009, 10, 2571–2576. [Google Scholar] [CrossRef]
- Nishino, T.; Takano, K.; Nakamae, K. Elastic modulus of the crystalline regions of cellulose polymorphs. J. Polym. Sci. Part B Polym. Phys. 1995, 33, 1647–1651. [Google Scholar] [CrossRef]
- Henriksson, M.; Berglund, L.A.; Isaksson, P.; Lindström, T.; Nishino, T. Cellulose Nanopaper Structures of High Toughness. Biomacromolecules 2008, 9, 1579–1585. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, S.; Jia, Z.; Parvinian, S.; Li, Y.; Vaaland, O.; Hu, L.; Li, T. Anomalous scaling law of strength and toughness of cellulose nanopaper. Proc. Natl. Acad. Sci. USA 2015, 112, 8971. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Fu, S.; Lavoine, N.; Lucia, L.A. Structural reconstruction strategies for the design of cellulose nanomaterials and aligned wood cellulose-based functional materials—A review. Carbohydr. Polym. 2020, 247, 116722. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose; De Gruyter: Berlin, Boston, 2013. [Google Scholar] [CrossRef]
- Sharma, P.R.; Joshi, R.; Sharma, S.K.; Hsiao, B.S. A Simple Approach to Prepare Carboxycellulose Nanofibers from Untreated Biomass. Biomacromolecules 2017, 18, 2333–2342. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Athira, K.B.; Rubiyah, M.H.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Lindström, T.; Hsiao, B.S. Nanocellulose-Enabled Membranes for Water Purification: Perspectives. Adv. Sustain. Syst. 2020, 4, 1900114. [Google Scholar] [CrossRef]
- Shatkin, J.A.; Wegner, T.H.; Bilek, E.M.; Cowie, J. Market projections of cellulose nanomaterial-enabled products—Part 1: Applications. TAPPI J. 2014, 13, 9–16. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Liang, H.-W.; Chen, L.-F.; Hu, B.-C.; Yu, S.-H. Bacterial Cellulose: A Robust Platform for Design of Three Dimensional Carbon-Based Functional Nanomaterials. Acc. Chem. Res. 2016, 49, 96–105. [Google Scholar] [CrossRef]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Tang, C.; Spinney, S.; Shi, Z.; Tang, J.; Peng, B.; Luo, J.; Tam, K.C. Amphiphilic Cellulose Nanocrystals for Enhanced Pickering Emulsion Stabilization. Langmuir 2018, 34, 12897–12905. [Google Scholar] [CrossRef]
- Grishkewich, N.; Mohammed, N.; Tang, J.; Tam, K.C. Recent advances in the application of cellulose nanocrystals. Curr. Opin. Colloid Interface Sci. 2017, 29, 32–45. [Google Scholar] [CrossRef]
- Golmohammadi, H.; Morales-Narváez, E.; Naghdi, T.; Merkoçi, A. Nanocellulose in Sensing and Biosensing. Chem. Mater. 2017, 29, 5426–5446. [Google Scholar] [CrossRef]
- Sunasee, R.; Hemraz, U.D.; Ckless, K. Cellulose nanocrystals: A versatile nanoplatform for emerging biomedical applications. Expert. Opin. Drug Deliv. 2016, 13, 1243–1256. [Google Scholar] [CrossRef]
- Zhan, C.; Sharma, P.R.; He, H.; Sharma, S.K.; McCauley-Pearl, A.; Wang, R.; Hsiao, B.S. Rice husk based nanocellulose scaffolds for highly efficient removal of heavy metal ions from contaminated water. Environ. Sci. Water Res. Technol. 2020, 6, 3080–3090. [Google Scholar] [CrossRef]
- Chen, H.; Sharma, S.K.; Sharma, P.R.; Yeh, H.; Johnson, K.; Hsiao, B.S. Arsenic(III) Removal by Nanostructured Dialdehyde Cellulose–Cysteine Microscale and Nanoscale Fibers. ACS Omega 2019, 4, 22008–22020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, C.; Li, Y.; Sharma, P.R.; He, H.; Sharma, S.K.; Wang, R.; Hsiao, B.S. A study of TiO(2) nanocrystal growth and environmental remediation capability of TiO(2)/CNC nanocomposites. RSC Adv. 2019, 9, 40565–40576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunetti, F.; Operamolla, A.; Castro-Hermosa, S.; Lucarelli, G.; Manca, V.; Farinola, G.M.; Brown, T.M. Printed Solar Cells and Energy Storage Devices on Paper Substrates. Adv. Funct. Mater. 2019, 29, 1806798. [Google Scholar] [CrossRef] [Green Version]
- Operamolla, A. Recent Advances on Renewable and Biodegradable Cellulose Nanopaper Substrates for Transparent Light-Harvesting Devices: Interaction with Humid Environment. Int. J. Photoenergy 2019, 2019, 16. [Google Scholar] [CrossRef] [Green Version]
- Giannelli, R.; Babudri, F.; Operamolla, A. Chapter 3—Nanocellulose-based functional paper. In Nanocellulose Based Composites for Electronics; Thomas, S., Pottathara, Y.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 31–72. [Google Scholar] [CrossRef]
- Roman, M. Toxicity of Cellulose Nanocrystals: A Review. Ind. Biotechnol. 2015, 11, 25–33. [Google Scholar] [CrossRef]
- Colombo, L.; Zoia, L.; Violatto, M.B.; Previdi, S.; Talamini, L.; Sitia, L.; Nicotra, F.; Orlandi, M.; Salmona, M.; Recordati, C.; et al. Organ Distribution and Bone Tropism of Cellulose Nanocrystals in Living Mice. Biomacromolecules 2015, 16, 2862–2871. [Google Scholar] [CrossRef]
- Cranston, E.D.; Gray, D.G. Morphological and Optical Characterization of Polyelectrolyte Multilayers Incorporating Nanocrystalline Cellulose. Biomacromolecules 2006, 7, 2522–2530. [Google Scholar] [CrossRef]
- Podsiadlo, P.; Choi, S.-Y.; Shim, B.; Lee, J.; Cuddihy, M.; Kotov, N.A. Molecularly Engineered Nanocomposites: Layer-by-Layer Assembly of Cellulose Nanocrystals. Biomacromolecules 2005, 6, 2914–2918. [Google Scholar] [CrossRef]
- Habibi, Y.; Hoeger, I.; Kelley, S.S.; Rojas, O.J. Development of Langmuir−Schaeffer Cellulose Nanocrystal Monolayers and Their Interfacial Behaviors. Langmuir 2010, 26, 990–1001. [Google Scholar] [CrossRef]
- Sawalha, S.; Milano, F.; Guascito, M.R.; Bettini, S.; Giotta, L.; Operamolla, A.; Da Ros, T.; Prato, M.; Valli, L. Improving 2D-organization of fullerene Langmuir-Schäfer thin films by interaction with cellulose nanocrystals. Carbon 2020, 167, 906–917. [Google Scholar] [CrossRef]
- Lin, J.; Zhong, Z.; Li, Q.; Tan, Z.; Lin, T.; Quan, Y.; Zhang, D. Facile Low-Temperature Synthesis of Cellulose Nanocrystals Carrying Buckminsterfullerene and Its Radical Scavenging Property in Vitro. Biomacromolecules 2017, 18, 4034–4040. [Google Scholar] [CrossRef] [PubMed]
- Herreros-López, A.; Carini, M.; Da Ros, T.; Carofiglio, T.; Marega, C.; La Parola, V.; Rapozzi, V.; Xodo, L.E.; Alshatwi, A.A.; Hadad, C.; et al. Nanocrystalline cellulose-fullerene: Novel conjugates. Carbohydr. Polym. 2017, 164, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Sgobba, V.; Giancane, G.; Conoci, S.; Casilli, S.; Ricciardi, G.; Guldi, D.M.; Prato, M.; Valli, L. Growth and Characterization of Films Containing Fullerenes and Water Soluble Porphyrins for Solar Energy Conversion Applications. J. Am. Chem. Soc. 2007, 129, 3148–3156. [Google Scholar] [CrossRef] [PubMed]
- Da Ros, T.; Prato, M.; Carano, M.; Ceroni, P.; Paolucci, F.; Roffia, S.; Valli, L.; Guldi, D. Synthesis, electrochemistry, Langmuir–Blodgett deposition and photophysics of metal-coordinated fullerene–porphyrin dyads. J. Organomet. Chem. 2000, 599, 62–68. [Google Scholar] [CrossRef]
- Kordatos, K.; Da Ros, T.; Bosi, S.; Vázquez, E.; Bergamin, M.; Cusan, C.; Pellarini, F.; Tomberli, V.; Baiti, B.; Pantarotto, D.; et al. Novel Versatile Fullerene Synthons. J. Org. Chem. 2001, 66, 4915–4920. [Google Scholar] [CrossRef]
- Operamolla, A.; Casalini, S.; Console, D.; Capodieci, L.; Di Benedetto, F.; Bianco, G.V.; Babudri, F. Tailoring water stability of cellulose nanopaper by surface functionalization. Soft Matter 2018, 14, 7390–7400. [Google Scholar] [CrossRef]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of Reaction Conditions on the Properties and Behavior of Wood Cellulose Nanocrystal Suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, X.; Eisenthal, K.B. Effects of Monolayer Density and Bulk Ionic Strength on Acid−Base Equilibria at the Air/Water Interface. J. Phys. Chem. B 2000, 104, 8855–8861. [Google Scholar] [CrossRef] [Green Version]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., McKelvy, M.L., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Surface chemistry, morphological analysis and properties of cellulose nanocrystals with gradiented sulfation degrees. Nanoscale 2014, 6, 5384–5393. [Google Scholar] [CrossRef] [PubMed]
- Benck, J.D.; Pinaud, B.A.; Gorlin, Y.; Jaramillo, T.F. Substrate Selection for Fundamental Studies of Electrocatalysts and Photoelectrodes: Inert Potential Windows in Acidic, Neutral, and Basic Electrolyte. PLoS ONE 2014, 9, e107942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szucs, A.; Loix, A.; Nagy, J.B.; Lamberts, L. Fullerene film electrodes in aqueous solutions Part 1. Preparation and electrochemical characterization. J. Electroanal. Chem. 1995, 397, 191–203. [Google Scholar] [CrossRef]
- Liu, S.-W.; Su, W.-C.; Lee, C.-C.; Lin, C.-F.; Yeh, S.-C.; Chen, C.-T.; Lee, J.-H. Comparison of short and long wavelength absorption electron donor materials in C60-based planar heterojunction organic photovoltaics. Org. Electron. 2012, 13, 2118–2129. [Google Scholar] [CrossRef]
- Ito, O.; D’Souza, F. Recent Advances in Photoinduced Electron Transfer Processes of Fullerene-Based Molecular Assemblies and Nanocomposites. Molecules 2012, 17, 5816. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Ichinohe, H.; Kakuta, S.; Nagai, K. Organic Photoanode of Fullerene/Phthalocyanine Working in the Water Phase with Respect to Preparation Methods of the Bilayer Film. Jpn. J. Appl. Phys. 2010, 49, 015101. [Google Scholar] [CrossRef]
- Muthu, S.; Maruthamuthu, P.; Vasudeva Rao, P.R. Fullerenes as Photocatalysts: Studies on Decomposition of Water and Oxalic Acid. Fuller. Sci. Technol. 1993, 1, 481–497. [Google Scholar] [CrossRef]
- Fernandez-Delgado, O.; Puente-Santiago, A.R.; Cano, M.; Giner-Casares, J.J.; Metta-Magaña, A.J.; Echegoyen, L. Facile synthesis of C60-nano materials and their application in high-performance water splitting electrocatalysis. Sustain. Energy Fuels 2020, 4, 2900–2906. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Meng, X.; Chen, T.; Kong, W.; Li, Y.; Zhao, Y.; Wang, D.; Zhu, S.; Cheema, W.A.; et al. Enhanced visible/near-infrared light harvesting and superior charge separation via 0D/2D all-carbon hybrid architecture for photocatalytic oxygen evolution. Carbon 2020, 167, 724–735. [Google Scholar] [CrossRef]
- Kuehnel, M.F.; Reisner, E. Solar Hydrogen Generation from Lignocellulose. Angew. Chem. Int. Ed. 2018, 57, 3290–3296. [Google Scholar] [CrossRef]
- Zhang, G.; Ni, C.; Huang, X.; Welgamage, A.; Lawton, L.A.; Robertson, P.K.J.; Irvine, J.T.S. Simultaneous cellulose conversion and hydrogen production assisted by cellulose decomposition under UV-light photocatalysis. Chem. Commun. 2016, 52, 1673–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| LS Film | Background Current Density 1 (nA/cm2) | Current Density Under Illumination (nA/cm2) | Photocurrent (nA/cm2) |
|---|---|---|---|
| Five-layer FP | 15 | 75 | 60 |
| Five-layer FP/CNCs | 30 | 260 | 230 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milano, F.; Guascito, M.R.; Semeraro, P.; Sawalha, S.; Da Ros, T.; Operamolla, A.; Giotta, L.; Prato, M.; Valli, L. Nanocellulose/Fullerene Hybrid Films Assembled at the Air/Water Interface as Promising Functional Materials for Photo-electrocatalysis. Polymers 2021, 13, 243. https://doi.org/10.3390/polym13020243
Milano F, Guascito MR, Semeraro P, Sawalha S, Da Ros T, Operamolla A, Giotta L, Prato M, Valli L. Nanocellulose/Fullerene Hybrid Films Assembled at the Air/Water Interface as Promising Functional Materials for Photo-electrocatalysis. Polymers. 2021; 13(2):243. https://doi.org/10.3390/polym13020243
Chicago/Turabian StyleMilano, Francesco, Maria Rachele Guascito, Paola Semeraro, Shadi Sawalha, Tatiana Da Ros, Alessandra Operamolla, Livia Giotta, Maurizio Prato, and Ludovico Valli. 2021. "Nanocellulose/Fullerene Hybrid Films Assembled at the Air/Water Interface as Promising Functional Materials for Photo-electrocatalysis" Polymers 13, no. 2: 243. https://doi.org/10.3390/polym13020243
APA StyleMilano, F., Guascito, M. R., Semeraro, P., Sawalha, S., Da Ros, T., Operamolla, A., Giotta, L., Prato, M., & Valli, L. (2021). Nanocellulose/Fullerene Hybrid Films Assembled at the Air/Water Interface as Promising Functional Materials for Photo-electrocatalysis. Polymers, 13(2), 243. https://doi.org/10.3390/polym13020243