Poly-ε-Caprolactone/Halloysite Nanotube Composites for Resorbable Scaffolds: Effect of Processing Technology on Homogeneity and Electrospinning
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Halloysite Content
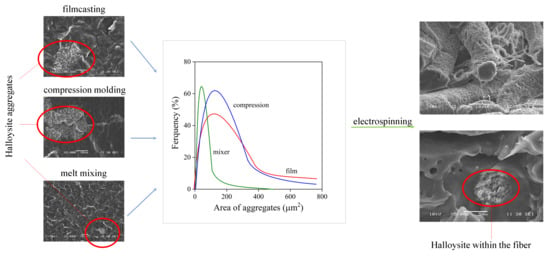
3.2. Homogeneity, Distribution
3.3. Thermal Analysis, Crystalline Structure
3.4. Tensile Properties
3.5. Fiber Spinning
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- George, A.; Sanjay, M.R.; Srisuk, R.; Parameswaranpillai, J.; Siengchin, S. A comprehensive review on chemical properties and applications of biopolymers and their composites. Int. J. Biol. Macromol. 2020, 154, 329–338. [Google Scholar] [CrossRef]
- Niaounakis, M. Biopolymers: Applications and Trends, 1st ed.; Elsevier: Oxford, UK, 2015. [Google Scholar]
- Elvers, D.; Song, C.H.; Steinbüchel, A.; Leker, J. Technology trends in biodegradable polymers: Evidence from patent analysis. Polym. Rev. 2016, 56, 584–606. [Google Scholar] [CrossRef]
- Udayakumar, G.P.; Muthusamy, S.; Selvaganesh, B.; Sivarajasekar, N.; Rambabu, K.; Banat, F.; Sivamani, S.; Sivakumar, N.; Hosseini-Bandegharaei, A.; Show, P.L. Bipolymers and composites: Properties, characterization and their applications in food, medical and pharmaceutical industries. J. Environ. Chem. Eng. 2021, 9, 105322. [Google Scholar] [CrossRef]
- Park, S.B.; Lih, E.; Park, K.S.; Joung, Y.K.; Han, D.K. Biopolymer-based functional composites for medical applications. Prog. Polym. Sci. 2017, 67, 77–105. [Google Scholar] [CrossRef]
- Sodhi, A.S.; Sharma, N.; Bhatia, S.; Verma, A.; Soni, S.; Batra, N. Insights on sustainable approaches for production and applications of value added products. Chemosphere 2021, 286, 131623. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.C.; Ananthakrishna, R. A review of bioresorbable scaffolds: Hype or hope? Singap. Med. J. 2017, 58, 512–515. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, G.; Vernekar, V.N.; Kuyinu, E.L.; Laurencin, C.T. Poly(lactic-acid)-based biomaterials for orthopaedic regenerative engineering. Adv. Drug Deliv. Rev. 2016, 107, 247–276. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Khademhosseini, A.; Mironov, V. The synergy of scaffold-based and scaffold-free tissue engineering strategies. Trends Biotechnol. 2018, 36, 348–357. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmatcher, D.W. The return of a forgotten polymer- Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Manoukian, O.S.; Sardashti, N.; Stedman, T.; Gailiunas, K.; Ojha, A.; Penalosa, A.; Mancuso, C.; Hobert, M.; Kumbar, S.G. Biomaterials for Tissue Engineering and Regenerative Medicine. Ency. Biom. Eng. 2019, 462–482. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Adhikari, R. Biodegradable synthetic polymers for tissue engineering. Eur. Cell Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef]
- Pastorino, L.; Pioli, F.; Zilli, M.; Converti, A.; Nicolini, C. Lipase-catalyzed degradation of poly(ε-caprolactione). Enzyme Microb. Technol. 2004, 35, 321–326. [Google Scholar] [CrossRef]
- Aris, M.H.; Annuar, M.S.M.; Ling, T.C. Lipase-mediated degradation of poly-ε-caprolactone in toluene: Behavior and its action mechanism. Polym. Degrad. Stab. 2016, 133, 182–191. [Google Scholar] [CrossRef]
- Hegyesi, N.; Hodosi, E.; Polyák, P.; Faludi, G.; Balogh-Weiser, D.; Pukánszky, B. Controlled degradation of poly-ε-caprolactone for resorbable scaffolds. Colloids Surf. B Biointerfaces 2020, 186, 110678. [Google Scholar] [CrossRef]
- Liu, M.; Duan, X.-P.; Li, Y.-M.; Yang, D.-P.; Long, Y.-Z. Electrospun nanofibers for wound healing. Mater. Sci. Eng. C 2017, 76, 1413–1423. [Google Scholar] [CrossRef]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.O.; Jafari, S.H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Thakkar, S.; Misra, M. Electrospun polymeric nanofibers: New horizons in drug delivery. Eur. J. Pharm. Sci. 2017, 107, 148–167. [Google Scholar] [CrossRef]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Zamani, M.; Prabhakaran, M.P.; Ramakrishna, S. Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int. J. Nanomed. 2013, 8, 2997–3017. [Google Scholar] [CrossRef] [Green Version]
- Amatulu, R.; Khan, W.S. Introduction to electrospun nanofibers. In Synthesis and Applications of Electrospun Nanofibers; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–15. [Google Scholar] [CrossRef]
- Chou, S.F.; Carson, D.; Woodrow, K.A. Current strategies for sustaining drug release from electrospun nanofibers. J. Control. Release 2015, 220, 584–591. [Google Scholar] [CrossRef] [Green Version]
- Chou, S.F.; Woodrow, K.A. Relationships between mechanical properties and drug release from electrospun fibers of PCL and PLGA blends. J. Mech. Behav. Biomed. Mater. 2017, 65, 724–733. [Google Scholar] [CrossRef] [Green Version]
- Zhai, R.; Zhang, B.; Liu, L.; Xie, Y.; Zhang, H.; Liu, J. Immobilization of enzyme biocatalyst on natural halloysite nanotubes. Catal. Commun. 2010, 12, 259–263. [Google Scholar] [CrossRef]
- Chao, C.; Liu, J.; Wang, J.; Zhang, Y.; Zhang, B.; Zhang, Y.; Xiang, X.; Chen, R. Surface modification of halloysite nanotubes with dopamine for enzyme immobilization. ACS Appl. Mater. Interfaces 2013, 5, 10559–10564. [Google Scholar] [CrossRef]
- Tully, J.; Yendluri, R.; Lvov, Y. Halloysite clay nanotubes for enzyme immobilization. Biomacromolecules 2016, 17, 615–621. [Google Scholar] [CrossRef]
- Machado, G.S.; de Freitas Castro, K.A.D.; Wypych, F.; Nakagaki, S. Immobilization of metalloporphyrins into nanotubes of natural halloysite toward selective catalysts for oxidation reactions. J. Mol. Catal. A Chem. 2008, 283, 99–107. [Google Scholar] [CrossRef]
- Santos, A.C.; Ferreira, C.; Veiga, F.; Ribeiro, A.J.; Panchal, A.; Lvov, Y.; Agarwal, A. Halloysite clay nanotubes for life sciences applications: From drug encapsulation to bioscaffold. Adv. Colloid Interface Sci. 2018, 257, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Colijn, I.; Schroën, K. Thermoplastic bio-nanocomposites: From measurement of fundamental properties to practical application. Adv. Colloid Interface Sci. 2021, 292, 102419. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Wang, B.; Dutta, P. Nanoparticle processing: Understanding and controlling aggregation. Adv. Colloid Interface Sci. 2020, 279, 102162. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jia, Z.; Jia, D.; Zhou, C. Recent advance in research on halloysite nanotubes-polymer nanocomposite. Prog. Polym. Sci. 2014, 39, 1498–1525. [Google Scholar] [CrossRef]
- Iyer, S.; Schiraldi, D.A. Role of specific interactions and solubility in the reinforcement of bisphenol A polymers with polyhedral oligomeric silsesquioxanes. Macromolecules 2007, 40, 4942–4952. [Google Scholar] [CrossRef]
- Schmidt, R.G.; Gordon, G.V.; Dreiss, C.A.; Cosgrove, T.; Krukonis, V.J.; Williams, K.; Wetmore, P.M. A critical size ratio for viscosity reduction in poly(dimethylsiloxane)-polysilicate nanocomposites. Macromolecules 2010, 43, 10143–10151. [Google Scholar] [CrossRef]
- Sun, H.; Jiao, R.; An, G.; Xu, H.; Wang, D. Influence of particle size on the aggregation behavior of nanoparticles: Role of structural hydration layer. J. Environ. Sci. 2021, 103, 33–42. [Google Scholar] [CrossRef]
- Krzysko, A.J.; Nakouzi, E.; Zhang, X.; Graham, T.R.; Rosso, K.M.; Schenter, G.K.; Ilavsky, J.; Kuzmenko, I.; Frith, M.G.; Ivory, C.F.; et al. Correlating inter-particle forces and particle shape to shear-induced aggregation/fragmentation and rheology for dilute anisotropic particle suspensions: A complementary study via capillary rheometry and in-situ small and ultra-small angle X-ray scattering. J. Colloid Interface Sci. 2020, 576, 47–58. [Google Scholar] [CrossRef]
- Usune, S.; Kubo, M.; Tsukada, T.; Koike, O.; Tatsumi, R.; Fujita, M.; Takami, S.; Adschiri, T. Numerical simulations of dispersion and aggregation behavior of surface-modified nanoparticles under shear flow. Powder Technol. 2019, 343, 113–121. [Google Scholar] [CrossRef]
- Yang, Y.T.; Cheng, Y.; Len, F.; Huang, L.; Wang, Z.J.; Tian, W.Q. Recent advances on modification of halloysite nanotubes for multifunctional applications. Appl. Sci. 2017, 7, 1215. [Google Scholar] [CrossRef] [Green Version]
- Lvov, Y.; Guo, B.; Fakhrullin, R.F. Functional Polymer Composites with Nanoclays; RSC: Cambridge, UK, 2017. [Google Scholar]
- Tharmavaram, M.; Pandey, G.; Rawtani, D. Surface modified halloysite nanotubes: A flexible interface for biological, environmental and catalytic applications. Adv. Colloid Interface Sci. 2018, 261, 82–101. [Google Scholar] [CrossRef] [PubMed]
- Móczó, J.; Fekete, E.; László, K.; Pukánszky, B. Aggregation of particulate fillers: Factors, determination, properties. Macromol. Symp. 2003, 194, 111–124. [Google Scholar] [CrossRef]
- Shandiz, S.A.; Moradi, M.A.; Babaluo, A.A.; Jalili, A.H. A comparative experimental and molecular simulation study on the mechanical and morphological behaviors of adamantane-based polypropylene composites. Comput. Mater. Sci. 2015, 109, 341–349. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, X.Y.; Ma, Y.M.; Ke, Y.C.; Zhang, J.K.; Wang, F.S. The effects of particle size and content on the thermal conductivity and mechanical properties of Al2O3/high density polyethylene (HDPE) composites. Express Polym. Lett. 2011, 5, 581–590. [Google Scholar] [CrossRef]
- Müller, P.; Imre, B.; Bere, J.; Móczó, J.; Pukánszky, B. Physical ageing and molecular mobility in PLA blends and composites. J. Therm. Anal. Calorim. 2015, 122, 1423–1433. [Google Scholar] [CrossRef]
- Pukánszky, B.; Fekete, E. Aggregation tendency of particulate fillers: Determination and consequences. Period. Polytech. Chem. Eng. 1998, 42, 167–187. [Google Scholar]
- Nielsen, L.E. Mechanical Properties of Polymers and Composites, 2nd ed.; Marcel Dekker: New York, NY, USA, 1974. [Google Scholar]












| Technology | Halloysite (vol%) | Aggregate Area (μm) | |
|---|---|---|---|
| Average | Most Frequent | ||
| Film casting | 5 | 499.9 ± 652.3 | 88.5 |
| Compression | 391.8 ± 687.1 | 98.1 | |
| Melt mixing | 83.2 ± 117.8 | 9.6 | |
| Film casting | 10 | 547.9 ± 874.1 | 162.4 |
| Compression | 1078.1 ± 2348.4 | 443.3 | |
| Melt mixing | 322.2 ± 398.4 | 137.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Józó, M.; Várdai, R.; Hegyesi, N.; Móczó, J.; Pukánszky, B. Poly-ε-Caprolactone/Halloysite Nanotube Composites for Resorbable Scaffolds: Effect of Processing Technology on Homogeneity and Electrospinning. Polymers 2021, 13, 3772. https://doi.org/10.3390/polym13213772
Józó M, Várdai R, Hegyesi N, Móczó J, Pukánszky B. Poly-ε-Caprolactone/Halloysite Nanotube Composites for Resorbable Scaffolds: Effect of Processing Technology on Homogeneity and Electrospinning. Polymers. 2021; 13(21):3772. https://doi.org/10.3390/polym13213772
Chicago/Turabian StyleJózó, Muriel, Róbert Várdai, Nóra Hegyesi, János Móczó, and Béla Pukánszky. 2021. "Poly-ε-Caprolactone/Halloysite Nanotube Composites for Resorbable Scaffolds: Effect of Processing Technology on Homogeneity and Electrospinning" Polymers 13, no. 21: 3772. https://doi.org/10.3390/polym13213772
APA StyleJózó, M., Várdai, R., Hegyesi, N., Móczó, J., & Pukánszky, B. (2021). Poly-ε-Caprolactone/Halloysite Nanotube Composites for Resorbable Scaffolds: Effect of Processing Technology on Homogeneity and Electrospinning. Polymers, 13(21), 3772. https://doi.org/10.3390/polym13213772