Dextran-Based Edible Coatings to Prolong the Shelf Life of Blueberries
Abstract
:1. Introduction
2. Results and Discussion
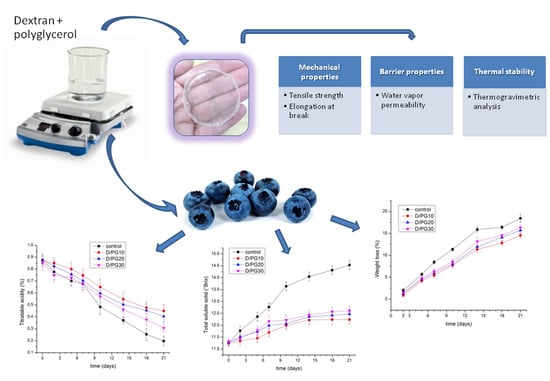
2.1. Mechanical Analysis
2.2. Water Vapor Permeability Analysis
2.3. Thermogravimetric Analysis
2.4. Quality of Blueberries
3. Materials and Methods
3.1. Preparation of Dextran Films
3.2. Characterization
3.2.1. Mechanical Analysis
3.2.2. Thermal Analysis
3.2.3. Water Vapor Permeability
3.2.4. Coating of Blueberry Samples
3.2.5. Quality of Blueberries
3.2.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mannozzi, C.; Tylewicz, U.; Chinnici, F.; Siroli, L.; Rocculi, P.; Dalla Rosa, M.; Romani, S. Effects of chitosan based coatings enriched with procyanidin by-product on quality of fresh blueberries during storage. Food Chem. 2018, 251, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.S.; Tomar, M.; Punia, S.; Kukula-Koch, W.; Kumar, M. Enhancing the functionality of chitosan- and alginate-based active edible coatings/films for the preservation of fruits and vegetables: A review. Int. J. Biol. Macromol. 2020, 164, 304–320. [Google Scholar] [CrossRef] [PubMed]
- Nešić, A.; Cabrera-Barjas, G.; Dimitrijević-Branković, S.; Davidović, S.; Radovanović, N.; Delattre, C. Prospect of polysaccharide-based materials as advanced food packaging. Molecules 2020, 25, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zibaei, R.; Hasanvand, S.; Hashami, Z.; Roshandel, Z.; Rouhi, M.; de Guimarães, J.T.; Mortazavian, A.M.; Sarlak, Z.; Mohammadi, R. Applications of emerging botanical hydrocolloids for edible films: A review. Carbohydr. Polym. 2021, 256, 117554. [Google Scholar] [CrossRef]
- Wang, H.; Ding, F.; Ma, L.; Zhang, Y. Edible films from chitosan-gelatin: Physical properties and food packaging application. Food Biosci. 2021, 40, 100871. [Google Scholar] [CrossRef]
- Kwak, H.; Shin, S.; Kim, J.; Kim, J.; Lee, D.; Lee, H.; Lee, E.J.; Hyun, J. Protective coating of strawberries with cellulose nanofibers. Carbohydr. Polym. 2021, 258, 117688. [Google Scholar] [CrossRef]
- Mannozzi, C.; Cecchini, J.P.; Tylewicz, U.; Siroli, L.; Patrignani, F.; Lanciotti, R.; Rocculi, P.; Dalla Rosa, M.; Romani, S. Study on the efficacy of edible coatings on quality of blueberry fruits during shelf-life. LWT—Food Sci. Technol. 2017, 85, 440–444. [Google Scholar] [CrossRef]
- Šešlija, S.; Nešić, A.; Ružić, J.; Kalagasidis Krušić, M.; Veličković, S.; Avolio, R.; Santagata, G.; Malinconico, M. Edible blend films of pectin and poly(ethylene glycol): Preparation and physico-chemical evaluation. Food Hydrocoll. 2017, 77, 494–501. [Google Scholar] [CrossRef]
- Francisco, C.B.; Pellá, M.G.; Silva, O.A.; Raimundo, K.F.; Caetano, J.; Linde, G.A.; Colauto, N.B.; Dragunski, D.C. Shelf-life of guavas coated with biodegradable starch and cellulose-based films. Int. J. Biol. Macromol. 2020, 152, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Abugoch, L.; Tapia, C.; Plasencia, D.; Pastor, A.; Castro-Mandujano, O.; López, L.; Escalona, V.H. Shelf-life of fresh blueberries coated with quinoa protein/chitosan/sunflower oil edible film. J. Sci. Food Agric. 2016, 96, 619–626. [Google Scholar] [CrossRef]
- Basaglia, R.R.; Pizato, S.; Santiago, N.G.; Maciel de Almeida, M.M.; Pinedo, R.A.; Cortez-Vega, W.R. Effect of edible chitosan and cinnamon essential oil coatings on the shelf life of minimally processed pineapple (Smooth cayenne). Food Biosci. 2021, 41, 100966. [Google Scholar] [CrossRef]
- Iñiguez-Moreno, M.; Ragazzo-Sánchez, J.A.; Barros-Castillo, J.C.; Sandoval-Contreras, T.; Calderón-Santoyo, M. Sodium alginate coatings added with Meyerozyma caribbica: Postharvest biocontrol of Colletotrichum gloeosporioides in avocado (Persea americana Mill. cv. Hass). Postharvest Biol. Technol. 2020, 163, 111123. [Google Scholar] [CrossRef]
- Sucharitha, K.V.; Beulah, A.M.; Ravikiran, K. Effect of chitosan coating on storage stability of tomatoes (Lycopersicon esculentum Mill). Int. Food Res. J. 2018, 25, 93–99. [Google Scholar]
- Tran, T.T.B.; Roach, P.; Nguyen, M.H.; Pristijono, P.; Vuong, Q.V. Development of biodegradable films based on seaweed polysaccharides and Gac pulp (Momordica cochinchinensis), the waste generated from Gac oil production. Food Hydrocoll. 2020, 99, 105322. [Google Scholar] [CrossRef]
- Vieira, J.M.; Flores-López, M.L.; de Rodríguez, D.J.; Sousa, M.C.; Vicente, A.A.; Martins, J.T. Effect of chitosan—Aloe vera coating on postharvest quality of blueberry (Vaccinium corymbosum) fruit. Postharvest Biol. Technol. 2016, 116, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Kraśniewska, K.; Pobiega, K.; Gniewosz, M. Pullulan—Biopolymer with Potential for Use as Food Packaging. Int. J. Food Eng. 2019, 15, 20190030. [Google Scholar] [CrossRef]
- Ferreira, A.R.V.; Alves, V.D.; Coelhoso, I.M. Polysaccharide-based membranes in food packaging applications. Membranes 2016, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Vettori, M.H.P.B.; Franchetti, S.M.M.; Contiero, J. Structural characterization of a new dextran with a low degree of branching produced by Leuconostoc mesenteroides FT045B dextransucrase. Carbohydr. Polym. 2012, 88, 1440–1444. [Google Scholar] [CrossRef] [Green Version]
- Goulas, A.K.; Fisher, D.A.; Grimble, G.K.; Grandison, A.S.; Rastall, R.A. Synthesis of isomaltooligosaccharides and oligodextrans by the combined use of dextransucrase and dextranase. Enzyme Microb. Technol. 2004, 35, 327–338. [Google Scholar] [CrossRef]
- Naessens, M.; Cerdobbel, A.; Soetaert, W.; Vandamme, E.J. Leuconostoc dextransucrase and dextran: Production, properties and applications. J. Chem. Technol. Biotechnol. 2005, 80, 845–860. [Google Scholar] [CrossRef]
- Ul-Qader, S.A.; Iqbal, L.; Rizvi, H.A.; Zuberi, R. Production of dextran from sucrose by a newly isolated strain of Leuconostoc mesenteroides (PCSIR-3) with reference to L. mesenteroides NRRL B-512F. Biotechnol. Appl. Biochem. 2003, 34, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Lazić, V.; Vivod, V.; Peršin, Z.; Stoiljković, M.; Ratnayake, I.S.; Ahrenkiel, P.S.; Nedeljković, J.M.; Kokol, V. Dextran-coated silver nanoparticles for improved barrier and controlled antimicrobial properties of nanocellulose films used in food packaging. Food Packag. Shelf Life 2020, 26, 100575. [Google Scholar] [CrossRef]
- Davidović, S.; Miljković, M.; Tomić, M.; Gordić, M.; Nešić, A.; Dimitrijević, S. Response surface methodology for optimisation of edible coatings based on dextran from Leuconostoc mesenteroides T3. Carbohydr. Polym. 2018, 184, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Nešić, A.; Onjia, A.; Davidović, S.; Dimitrijević, S.; Errico, M.E.; Santagata, G.; Malinconico, M. Design of pectin-sodium alginate based films for potential healthcare application: Study of chemico-physical interactions between the components of films and assessment of their antimicrobial activity. Carbohydr. Polym. 2017, 157, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Abbate, M.; Malinconico, M.; Santagata, G. Effect of polyglycerol and the crosslinking on the physical properties of a blend alginate-hydroxyethylcellulose. Carbohydr. Polym. 2010, 82, 1061–1067. [Google Scholar] [CrossRef]
- Ma, X.; Qiao, C.; Wang, X.; Yao, J.; Xu, J. Structural characterization and properties of polyols plasticized chitosan films. Int. J. Biol. Macromol. 2019, 135, 240–245. [Google Scholar] [CrossRef]
- Nešić, A.; Ružić, J.; Gordić, M.; Ostojić, S.; Micić, D.; Onjia, A. Pectin-polyvinylpyrrolidone films: A sustainable approach to the development of biobased packaging materials. Compos. Part B Eng. 2017, 110, 56–61. [Google Scholar] [CrossRef]
- Souza, V.C.; Monte, M.L.; Pinto, L.A.A. Effect of carp (Cyprinus carpio) oil incorporation on water vapour permeability, mechanical properties and transparency of chitosan films. Int. J. Food Sci. Tecnol. 2013, 48, 1309–1317. [Google Scholar] [CrossRef]
- Malagurski, I.; Levic, S.; Nesic, A.; Mitric, M.; Pavlovic, V.; Dimitrijevic-Brankovic, S. Mineralized agar-based nanocomposite films: Potential food packaging materials with antimicrobial properties. Carbohydr. Polym. 2017, 175, 55–62. [Google Scholar] [CrossRef]
- Díaz-Galindo, E.P.; Nesic, A.; Bautista-Baños, S.; García, O.D.; Cabrera-Barjas, G. Corn-Starch-Based Materials Incorporated with Cinnamon Oil Emulsion: Physico-Chemical Characterization and Biological Activity. Foods 2020, 9, 475. [Google Scholar] [CrossRef]
- Zhu, G.; Sheng, L.; Li, J.; Tong, Q. Preparation and characterisation of gellan / pullulan composite blend films. Int. J. Food Sci. Technol. 2013, 48, 2683–2687. [Google Scholar] [CrossRef]
- Gorrasi, G.; Bugatti, V. Edible bio-nano-hybrid coatings for food protection based on pectins and LDH-salicylate: Preparation and analysis of physical properties. LWT—Food Sci. Technol. 2016, 69, 139–145. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Miranda, K.W.E.; Rosa, M.F.; Nascimento, D.M.; de Moura, M.R. Edible films from alginate-acerola puree reinforced with cellulose whiskers. LWT—Food Sci. Technol. 2012, 46, 294–297. [Google Scholar] [CrossRef]
- Wang, Y.; Du, H.; Xie, M.; Ma, G.; Yang, W.; Hu, Q.; Pei, F. Characterization of the physical properties and biological activity of chitosan films grafted with gallic acid and caffeic acid: A comparison study. Food Packag. Shelf Life 2019, 22, 100401. [Google Scholar] [CrossRef]
- Mark, J.E. Physical Properties of Polymers Handbook, 2nd ed.; Springer: New York, NY, USA, 2007; ISBN 9780387690025. [Google Scholar]
- Shankar, S.; Reddy, J.P.; Rhim, J.W. Effect of lignin on water vapor barrier, mechanical, and structural properties of agar/lignin composite films. Int. J. Biol. Macromol. 2015, 81, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Z.; Kadouh, H.; Zhou, K. The antimicrobial, mechanical, physical and structural properties of chitosan-gallic acid films. LWT—Food Sci. Technol. 2014, 57, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Gekko, K. Thermal analysis of low molecular weight dextran. Agric. Biol. Chem. 1978, 42, 1287–1288. [Google Scholar] [CrossRef]
- Kothari, D.; Tingirikari, J.M.R.; Goyal, A. In vitro analysis of dextran from Leuconostoc mesenteroides NRRL B-1426 for functional food application. Bioact. Carbohydrates Diet. Fibre 2015, 6, 55–61. [Google Scholar] [CrossRef]
- Strbak, O.; Antal, I.; Khmara, I.; Koneracka, M.; Kubovcikova, M.; Zavisova, V.; Molcan, M.; Jurikova, A.; Hnilicova, P.; Gombos, J.; et al. Influence of dextran molecular weight on the physical properties of magnetic nanoparticles for hyperthermia and MRI applications. Nanomaterials 2020, 10, 2468. [Google Scholar] [CrossRef]
- Do Nunes, M.C.N. Correlations between subjective quality and physicochemical attributes of fresh fruits and vegetables. Postharvest Biol. Technol. 2015, 107, 43–54. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, B.; Qin, Y.; Li, F.; Yang, S.; Lu, P.; Wang, L.; Fan, J. Preparation and characterization of antifungal coating films composed of sodium alginate and cyclolipopeptides produced by Bacillus subtilis. Int. J. Biol. Macromol. 2020, 143, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Chiabrando, V.; Giacalone, G. Quality evaluation of blueberries coated with chitosan and sodium alginate during postharvest storage. Int. Food Res. J. 2017, 24, 1553–1561. [Google Scholar]




| Sample | TS, MPa | ε, % | WVP × 1012, g/m s Pa |
|---|---|---|---|
| D/PG10 | 4.60 ± 0.23 a | 122.5 ± 9.5 a | 3.45 ± 0.23 a |
| D/PG20 | 1.30 ± 0.15 b | 265 ± 24 b | 5.78 ± 0.46 b |
| D/PG30 | 0.19 ± 0.01 c | 602 ± 58 c | 8.81 ± 0.78 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davidović, S.; Miljković, M.; Gordic, M.; Cabrera-Barjas, G.; Nesic, A.; Dimitrijević-Branković, S. Dextran-Based Edible Coatings to Prolong the Shelf Life of Blueberries. Polymers 2021, 13, 4252. https://doi.org/10.3390/polym13234252
Davidović S, Miljković M, Gordic M, Cabrera-Barjas G, Nesic A, Dimitrijević-Branković S. Dextran-Based Edible Coatings to Prolong the Shelf Life of Blueberries. Polymers. 2021; 13(23):4252. https://doi.org/10.3390/polym13234252
Chicago/Turabian StyleDavidović, Slađana, Miona Miljković, Milan Gordic, Gustavo Cabrera-Barjas, Aleksandra Nesic, and Suzana Dimitrijević-Branković. 2021. "Dextran-Based Edible Coatings to Prolong the Shelf Life of Blueberries" Polymers 13, no. 23: 4252. https://doi.org/10.3390/polym13234252
APA StyleDavidović, S., Miljković, M., Gordic, M., Cabrera-Barjas, G., Nesic, A., & Dimitrijević-Branković, S. (2021). Dextran-Based Edible Coatings to Prolong the Shelf Life of Blueberries. Polymers, 13(23), 4252. https://doi.org/10.3390/polym13234252