Expanding Poly(lactic acid) (PLA) and Polyhydroxyalkanoates (PHAs) Applications: A Review on Modifications and Effects
Abstract
:1. Introduction
1.1. PLA and Its Properties
1.2. PHAs and Their Properties
2. PLA’s Modifications
2.1. Plasticizers’ Effect
2.2. Impact Modifiers’ Effect
2.3. Belnding’s Effect
2.3.1. PLA/PHAs Blends
2.3.2. PLA/PCL Blends
2.3.3. Blends of PLA with Other Biodegradable/Renewable Resource-Based Polymers
2.3.4. Features of Various PLA Blends
2.4. Composites’/Nanocomposites’ Effect
3. PHAs’ Modifications
3.1. Blending’s Effect
3.2. Composites’/Nanocomposites’ Effect
3.3. Features of Various PHAs Blends and Nanocomposites
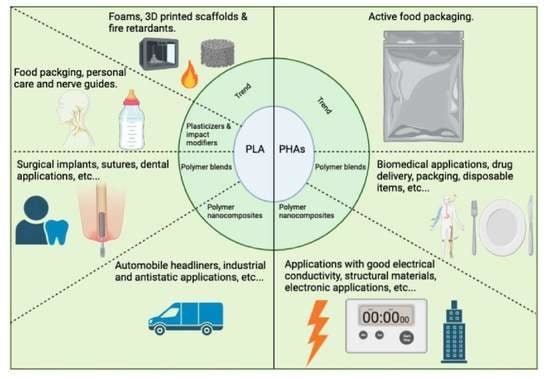
4. Trends of PLA and PHAs Applications
4.1. PLA Foams, 3D-printed Scaffolds and Flame Retardancy
4.2. PHAs in Active Food Packaging
5. Conclusions and Future Insights
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, X.S. Overview of Plant Polymers: Resources, Demands, and Sustainability. In Handbook of Biopolymers and Biodegradable Plastics Properties, Processing and Applications, 1st ed.; Ebnesajjad, S., Ed.; Elsevier: Waltham, MA, USA, 2013. [Google Scholar]
- Ashter, S.A. Introduction to Bioplastics Engineering, 1st ed.; Elsevier: Cambridge, MA, USA, 2016. [Google Scholar] [CrossRef]
- B.F.R. Center. Soybean Car. 1997. Available online: https://www.thehenryford.org/collections-and-research/digital-resources/popular-topics/soy-bean-car/ (accessed on 10 July 2020).
- Mohanty, A.K.; Manjusri, M.; Lawrence, T.D. Natural Fibers, Biopolymers, and Biocomposites, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Gross, R.A.; Kalra, B. Biodegradable polymers for the environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, A.K.; Misra, M.; Hinrichsen, G. Biofibres, biodegradable polymers and biocomposites: An overview. Macromol. Mater. Eng. 2000, 276–277, 1–24. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Sustainable Bio-Composites from renewable resources: Opportunities and challenges in the green materials world. J. Polym. Environ. 2002, 10, 19–26. [Google Scholar] [CrossRef]
- Netravali, A.N.; Chabba, S. Composites get greener. Mater. Today 2003, 6, 22–29. [Google Scholar] [CrossRef]
- Rudnik, E. Biodegradability Testing of Compostable Polymer Materials. In Handbook of Biopolymers and Biodegradable Plastics Properties, Processing and Applications; Ebnesajjad, S., Ed.; Elsevier: Waltham, MA, USA, 2013. [Google Scholar]
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic acid technology. Adv. Mater. 2000, 12, 1841–1846. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Wibowo, A.; Misra, M.; Drzal, L.T. Effect of process engineering on the performance of natural fiber reinforced cellulose acetate biocomposites. Compos. Part A Appl. Sci. Manuf. 2004, 35, 363–370. [Google Scholar] [CrossRef]
- Mehta, G.; Mohanty, A.K.; Misra, M.; Drzal, L.T. Biobased resin as a toughening agent for biocomposites. Green Chem. 2004, 6, 254–258. [Google Scholar] [CrossRef]
- Canada One-Step Closer to Zero Plastic Waste by 2030. 2020. Available online: https://www.canada.ca/en/environment-climate-change/news/2020/10/canada-one-step-closer-to-zero-plastic-waste-by-2030.html (accessed on 10 April 2021).
- Clark, J.H.; Deswarte, F.E.I. The Biorefinery Concept-An Integrated Approach. In Introduction to Chemicals from Biomass, 1st ed.; John Wiley & Sons Ltd.: Chichester, UK, 2008. [Google Scholar]
- Niaounakis, M. Biopolymers: Applications and Trends, 1st ed.; Elsevier: Waltham, MA, USA, 2015. [Google Scholar]
- Kricheldorf, H.; Dunsing, R. Polylactones, 8. Mechanism of the cationic polymerization of L,L-dilactide. Die Makromol. Chemie. 1986, 187, 1611–1625. [Google Scholar] [CrossRef]
- Maharana, T.; Mohanty, B.; Negi, Y.S. Melt-solid polycondensation of lactic acid and its biodegradability. Prog. Polym. Sci. 2009, 34, 99–124. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, S.Y.; Yang, M.; Yu, T.; Ren, T.B.; Ren, J. Synthesis and characterization of biodegradable lactic acid-based polymers by chain extension. Polym. Int. 2008, 57, 982–986. [Google Scholar] [CrossRef]
- Kun, E.; Marossy, K. Effect of crystallinity on PLA’s microbiological behaviour. Mater. Sci. Forum 2013, 752, 241–247. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Crystallization from the melt of poly(lactide)s with different optical purities and their blends. Macromol. Chem. Phys. 1996, 197, 3483–3499. [Google Scholar] [CrossRef]
- Garlotta, D. A literature review of poly(lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Standau, T.; Zhao, C.; Castellón, S.M.; Bonten, C.; Altstädt, V. Chemical modification and foam processing of polylactide (PLA). Polymers 2019, 11, 306. [Google Scholar] [CrossRef] [Green Version]
- Tuominen, J.; Kylma, J.; Kapanen, A.; Venelampi, O.; Itävaara, M.; Seppälä, J. Biodegradation of lactic acid based polymers under controlled composting conditions and evaluation of the ecotoxicological impact. Biomacromolecules 2002, 3, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Massardier-Nageotte, V.; Pestre, C.; Cruard-Pradet, T.; Bayard, R. Aerobic and anaerobic biodegradability of polymer films and physico-chemical characterization. Polym. Degrad. Stab. 2006, 91, 620–627. [Google Scholar] [CrossRef]
- Su, S.; Kopitzky, R.; Tolga, S.; Kabasci, S. Polylactide (PLA) and its blends with poly(butylene succinate) (PBS): A brief review. Polymers 2019, 11, 1193. [Google Scholar] [CrossRef] [Green Version]
- Total Corbion, Our Luminy® PLA Portfolio. 2021. Available online: https://www.total-corbion.com/luminy-pla-portfolio/ (accessed on 11 September 2021).
- NatureWorks LLC. Products. 2021. Available online: https://www.natureworksllc.com/Products (accessed on 11 September 2021).
- Loos, K. Biocatalysis in Polymer Chemistry, 1st ed.; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar] [CrossRef]
- Griffin, G. Chemistry and Technology of Biodegradable Polymers, 1st ed.; Springer: Dordrecht, The Netherlands; London, UK, 1994. [Google Scholar]
- Steinbüchel, A.; Valentin, H.E. Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol. Lett. 1995, 128, 219–228. [Google Scholar] [CrossRef]
- Witholt, B.; Kessler, B. Perspectives of medium chain length poly(hydroxyalkanoates), a versatile set of bacterial bioplastics. Curr. Opin. Biotechnol. 1999, 10, 279–285. [Google Scholar] [CrossRef]
- Steinbüchel, A.; Lütke-Eversloh, T. Metabolic engineering and pathway construction for biotechnological production of relevant polyhydroxyalkanoates in microorganisms. Biochem. Eng. J. 2003, 16, 81–96. [Google Scholar] [CrossRef]
- Madison, L.L.; Huisman, G.W. Metabolic Engineering of Poly(3-Hydroxyalkanoates): From DNA to Plastic. Microbiol. Mol. Biol. Rev. 1999, 63, 21–53. [Google Scholar] [CrossRef] [Green Version]
- Shang, L.; Jiang, M.; Chang, H.N. Poly(3-hydroxybutyrate) synthesis in fed-batch culture of Ralstonia eutropha with phosphate limitation under different glucose concentrations. Biotechnol. Lett. 2003, 25, 1415–1419. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.F.; Peoples, O.P. Biodegradable plastics from plants. Chem Tech. 1996, 26, 38–44. [Google Scholar]
- Girdhar, A.; Bhatia, M.; Nagpal, S.; Kanampalliwar, A.; Tiwari, A. Process Parameters for Influencing Polyhydroxyalkanoate Producing Bacterial Factories: An Overview. J. Pet. Environ. Biotechnol. 2013, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y. Plastic bacteria? Progress and prospects for polyhydroxyalkanoate production in bacteria. Trends Biotechnol. 1996, 14, 431–438. [Google Scholar] [CrossRef]
- Chen, G.Q.; Wu, Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 2005, 26, 6565–6578. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, J.; Loh, X.J. Polyhydroxyalkanoates: Opening doors for a sustainable future. NPG Asia Mater. 2016, 8, 1–20. [Google Scholar] [CrossRef]
- Zinn, M.; Witholt, B.; Egli, T. Occurrence, synthesis and medical application of bacterial polyhydroxyalkanoate. Adv. Drug Deliv. Rev. 2001, 53, 5–21. [Google Scholar] [CrossRef]
- Zhang, M.; Thomas, N.L. Blending polylactic acid with polyhydroxybutyrate: The effect on thermal, mechanical, and biodegradation properties. Adv. Polym. Technol. 2011, 30, 67–79. [Google Scholar] [CrossRef]
- Steinbüchel, A. Perspectives for Biotechnological Production and Utilization of Biopolymers: Metabolic Engineering of Polyhydroxyalkanoate Biosynthesis Pathways as a Successful Example. Macromol. Biosci. 2001, 1, 1–24. [Google Scholar] [CrossRef]
- De Koning, G.J.M.; Lemstra, P.J. Crystallization phenomena in bacterial poly[(R)-3-hydroxybutyrate]: 2. Embrittlement and rejuvenation. Polymer 1993, 34, 4089–4094. [Google Scholar] [CrossRef] [Green Version]
- Rivera-Briso, A.L.; Serrano-Aroca, Á. Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate): Enhancement strategies for advanced applications. Polymers 2018, 10, 732. [Google Scholar] [CrossRef] [Green Version]
- Fei, B.; Chen, C.; Wu, H.; Peng, S.; Wang, X.; Dong, L.; Xin, J.H. Modified poly(3-hydroxybutyrate-co-3-hydroxyvalerate) using hydrogen bonding monomers. Polymer 2004, 45, 6275–6284. [Google Scholar] [CrossRef]
- Rudnik, E. Compostable polymer materials—Definitions, structures and methods of preparation. In Compostable Polymer Materials; Elsevier: Oxford, UK, 2019. [Google Scholar] [CrossRef]
- Pilla, S. Handbook of Bioplastics and Biocomposites Engineering Applications, 1st ed.; Scrivener Publishing: Beverly, MA, USA, 2011. [Google Scholar] [CrossRef]
- Saito, Y.; Doi, Y. Microbial synthesis and properties of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) in Comamonas acidovorans. Int. J. Biol. Macromol. 1994, 16, 99–104. [Google Scholar] [CrossRef]
- Goldstein, N. Demystifying Biopolymers And Compostable Packaging. 2020. Available online: https://www.biocycle.net/demystifying-biopolymers-and-compostable-packaging/ (accessed on 13 June 2021).
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–372. [Google Scholar] [CrossRef] [Green Version]
- NatureWorks LLC. IngeoTM Biopolymer 2003D, 3052D, and 3801 X Technical Data Sheets; NatureWorks LLC: Minnetonka, MN, USA, 2018. [Google Scholar]
- Naser, A.Z.; Deiab, I.; Darras, B.M. Poly(lactic acid) (PLA) and polyhydroxyalkanoates (PHAs), green alternatives to petroleum-based plastics: A review. RSC Adv. 2021, 28, 17151–17196. [Google Scholar] [CrossRef]
- Daniels, A.U.; Chang, M.K.; Andriano, K.P. Mechanical properties of biodegradable polymers and composites proposed for internal fixation of bone. J. Appl. Biomater. 1990, 1, 57–78. [Google Scholar] [CrossRef]
- Anderson, K.S.; Schreck, K.M.; Hillmyer, M.A. Toughening polylactide. Polym. Rev. 2008, 48, 85–108. [Google Scholar] [CrossRef]
- Mascia, L.; Xanthos, M. An overview of additives and modifiers for polymer blends: Facts, deductions, and uncertainties. Adv. Polym. Technol. 1992, 11, 237–248. [Google Scholar] [CrossRef]
- Sinclair, R.G. The case for polylactic acid as a commodity packaging plastic. J. Macromol. Sci. Part A Pure Appl. Chem. 1996, 33, 585–597. [Google Scholar] [CrossRef]
- Jacobsen, S.; Fritz, H.G. Plasticizing polylactide—The effect of different plasticizers on the mechanical properties. Polym. Eng. Sci. 1999, 39, 1303–1310. [Google Scholar] [CrossRef]
- Martin, O.; Averous, L. Poly(lactic acid): Plasticization and properties of biodegradable multiphase systems. Polymer 2001, 42, 6209–6219. [Google Scholar] [CrossRef]
- Baiardo, M.; Frisoni, G.; Scandola, M.; Rimelen, M.; Lips, D.; Ruffieux, K.; Wintermantel, E. Thermal and mechanical properties of plasticized poly(L-lactic acid). J. Appl. Polym. Sci. 2003, 90, 1731–1738. [Google Scholar] [CrossRef]
- Pillin, I.; Montrelay, N.; Grohens, Y. Thermo-mechanical characterization of plasticized PLA: Is the miscibility the only significant factor? Polymer 2006, 47, 4676–4682. [Google Scholar] [CrossRef]
- Kulinski, Z.; Piorkowska, E.; Gadzinowska, K.; Stasiak, M. Plasticization of poly(L-lactide) with poly(propylene glycol). Biomacromolecules 2006, 7, 2128–2135. [Google Scholar] [CrossRef]
- Nijenhuis, A.J.; Colstee, E.; Grijpma, D.W.; Pennings, A.J. High molecular weight poly(L-lactide) and poly(ethylene oxide) blends: Thermal characterization and physical properties. Polymer 1996, 37, 5849–5857. [Google Scholar] [CrossRef]
- Labrecque, L.V.; Kumar, R.A.; Davé, V.; Gross, R.A.; Mccarthy, S.P. Citrate esters as plasticizers for poly(lactic acid). J. Appl. Polym. Sci. 1997, 66, 1507–1513. [Google Scholar] [CrossRef]
- Yoon, J.S.; Oh, S.H.; Kim, M.N.; Chin, I.J.; Kim, Y.H. Thermal and mechanical properties of poly(L-lactic acid)-poly (ethylene co-vinyl acetate) blends. Polymer 1999, 40, 2303–2312. [Google Scholar] [CrossRef]
- Ren, Z.; Dong, L.; Yang, Y. Dynamic mechanical and thermal properties of plasticized poly(lactic acid). J. Appl. Polym. Sci. 2006, 101, 1583–1590. [Google Scholar] [CrossRef]
- Brüster, B.; Adjoua, Y.O.; Dieden, R.; Grysan, P.; Federico, C.E.; Berthé, V.; Addiego, F. Plasticization of polylactide with myrcene and limonene as bio-based plasticizers: Conventional vs. reactive extrusion. Polymers 2019, 11, 1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaochanchaikul, K.; Pongmuksuwan, P. Influence of Ozonized Soybean Oil as a Biobased Plasticizer on the Toughness of Polylactic Acid. J. Polym. Environ. 2021, 1–11. [Google Scholar] [CrossRef]
- Dominguez-candela, I.; Ferri, J.M.; Cardona, S.C.; Lora, J.; Fombuena, V. Dual plasticizer/thermal stabilizer effect of epoxidized chia seed oil (Salvia hispanica l.) to improve ductility and thermal properties of poly(lactic acid). Polymers 2021, 13, 283. [Google Scholar] [CrossRef]
- Llanes, L.C.; Clasen, S.H.; Pires, A.T.N.; Gross, I.P. Mechanical and thermal properties of poly(lactic acid) plasticized with dibutyl maleate and fumarate isomers: Promising alternatives as biodegradable plasticizers. Eur. Polym. J. 2021, 142, 1–11. [Google Scholar] [CrossRef]
- Notta-Cuvier, D.; Odent, J.; Delille, R.; Murariu, M.; Lauro, F.; Raquez, J.M.; Bennani, B.; Dubois, P. Tailoring polylactide (PLA) properties for automotive applications: Effect of addition of designed additives on main mechanical properties. Polym. Test. 2014, 36, 1–9. [Google Scholar] [CrossRef]
- Taib, R.M.; Ghaleb, Z.A.; Mohd Ishak, Z.A. Thermal, mechanical, and morphological properties of polylactic acid toughened with an impact modifier. J. Appl. Polym. Sci. 2012, 123, 2715–2725. [Google Scholar] [CrossRef]
- Barletta, M.; Pizzi, E.; Puopolo, M.; Vesco, S. Design and manufacture of degradable polymers: Biocomposites of micro-lamellar talc and poly(lactic acid). Mater. Chem. Phys. 2017, 196, 62–74. [Google Scholar] [CrossRef]
- Diaz, C.; Pao, H.Y.; Kim, S. Film Performance of Poly(lactic acid) Blends for Packaging Applications. J. Appl. Packag. Res. 2016, 8, 4. [Google Scholar]
- Choochottiros, C.; Chin, I.J. Potential transparent PLA impact modifiers based on PMMA copolymers. Eur. Polym. J. 2013, 49, 957–966. [Google Scholar] [CrossRef]
- Wang, L.; Ma, W.; Gross, R.A.; McCarthy, S.P. Reactive compatibilization of biodegradable blends of poly(lactic acid) and poly(ε-caprolactone). Polym. Degrad. Stab. 1998, 59, 161–168. [Google Scholar] [CrossRef]
- Broz, M.E.; VanderHart, D.L.; Washburn, N.R. Structure and mechanical properties of poly(D,L-lactic acid)/poly(ε-caprolactone) blends. Biomaterials 2003, 24, 4181–4190. [Google Scholar] [CrossRef]
- Odent, J.; Raquez, J.M.; Duquesne, E.; Dubois, P. Random aliphatic copolyesters as new biodegradable impact modifiers for polylactide materials. Eur. Polym. J. 2012, 48, 331–340. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J. Research progress in toughening modification of poly(lactic acid). J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1051–1083. [Google Scholar] [CrossRef]
- Scaffaro, R.; Morreale, M.; Mirabella, F.; La Mantia, F.P. Preparation and recycling of plasticized PLA. Macromol. Mater. Eng. 2011, 296, 141–150. [Google Scholar] [CrossRef]
- Murariu, M.; Da Silva Ferreira, A.; Duquesne, E.; Bonnaud, L.; Dubois, P. Polylactide (PLA) and highly filled PLA—Calcium sulfate composites with improved impact properties. Macromol. Symp. 2008, 272, 1–12. [Google Scholar] [CrossRef]
- Afrifah, K.A.; Matuana, L.M. Impact modification of polylactide with a biodegradable ethylene/acrylate copolymer. Macromol. Mater. Eng. 2010, 295, 802–811. [Google Scholar] [CrossRef]
- PARALOIDTM BPM-520 Impact Modifier. 2021. Available online: https://www.dow.com/en-us/pdp.paraloid-bpm-520-impact-modifier.185484z.html (accessed on 29 June 2021).
- Niaounakis, M. Biopolymers: Processing and Products, 1st ed.; Elsevier: Oxford, UK, 2015. [Google Scholar]
- Qin, Y. Applications of advanced technologies in the development of functional medical textile materials. In Medical Textile Materials, 1st ed.; Woodhead Publishing: London, UK, 2016. [Google Scholar] [CrossRef]
- Vogel, C.; Wessel, E.; Siesler, H.W. FT-IR spectroscopic imaging of anisotropic poly(3-hydroxybutyrate)/poly(lactic acid) blends with polarized radiation. Macromolecules 2008, 41, 2975–2977. [Google Scholar] [CrossRef]
- Furukawa, T.; Sato, H.; Murakami, R.; Zhang, J.; Duan, Y.X.; Noda, I.; Ochiai, S.; Ozaki, Y. Structure, dispersibility, and crystallinity of poly(hydroxybutyrate)/poly(L-lactic acid) blends studied by FT-IR microspectroscopy and differential scanning calorimetry. Macromolecules 2005, 38, 6445–6454. [Google Scholar] [CrossRef]
- Ohkoshi, I.; Abe, H.; Doi, Y. Miscibility and solid-state structures for blends of poly[(S)-lactide] with atactic poly[(R,S)-3-hydroxybutyrate]. Polymer 2000, 41, 5985–5992. [Google Scholar] [CrossRef]
- Blümm, E.; Owen, A.J. Miscibility, crystallization and melting of poly(3-hydroxybutyrate)/poly(l-lactide) blends. Polymer 1995, 36, 4077–4081. [Google Scholar] [CrossRef]
- Iannace, S.; Ambrosio, L.; Huang, S.J.; Nicolais, L. Poly(3-hydroxybutyrate)-co-(3-hydroxyvalerate)/Poly-L-lactide blends: Thermal and mechanical properties. J. Appl. Polym. Sci. 1994, 54, 1525–1536. [Google Scholar] [CrossRef]
- Ferreira, B.M.P.; Zavaglia, C.A.C.; Duek, E.A.R. Films of PLLA/PHBV: Thermal, morphological, and mechanical characterization. J. Appl. Polym. Sci. 2002, 86, 2898–2906. [Google Scholar] [CrossRef]
- Yoon, J.S.; Lee, W.S.; Kim, K.S.; Chin, I.J.; Kim, M.N.; Kim, C. Effect of poly(ethylene glycol)-block-poly(L-lactide) on the poly[(R)-3-hydroxybutyrate]/poly(L-lactide) blends. Eur. Polym. J. 2000, 36, 435–442. [Google Scholar] [CrossRef]
- Takagi, Y.; Yasuda, R.; Yamaoka, M.; Yamane, T. Morphologies and mechanical properties of polylactide blends with medium chain length poly(3-hydroxyalkanoate) and chemically modified poly(3-hydroxyalkanoate). J. Appl. Polym. Sci. 2004, 93, 2363–2369. [Google Scholar] [CrossRef]
- Noda, I.; Satkowski, M.M.; Dowrey, A.E.; Marcott, C. Polymer alloys of nodax copolymers and poly(lactic acid). Macromol. Biosci. 2004, 4, 269–275. [Google Scholar] [CrossRef]
- Schreck, K.M.; Hillmyer, M.A. Block copolymers and melt blends of polylactide with NodaxTM microbial polyesters: Preparation and mechanical properties. J. Biotechnol. 2007, 132, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Bartczak, Z.; Galeski, A.; Kowalczuk, M.; Sobota, M.; Malinowski, R. Tough blends of poly(lactide) and amorphous poly([R,S]-3-hydroxy butyrate)—Morphology and properties. Eur. Polym. J. 2013, 49, 3630–3641. [Google Scholar] [CrossRef]
- Nanda, M.R.; Misra, M.; Mohanty, A.K. The effects of process engineering on the performance of PLA and PHBV blends. Macromol. Mater. Eng. 2011, 273, 719–728. [Google Scholar] [CrossRef]
- Noda, I.; Green, P.R.; Satkowski, M.M.; Schechtman, L.A. Preparation and properties of a novel class of polyhydroxyalkanoate copolymers. Biomacromolecules 2005, 6, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, S.; Kong, M.; Geng, W.; Li, R.K.Y.; Song, C.; Kong, D. Phase morphology, physical properties, and biodegradation behavior of novel PLA/PHBHHx blends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 23–31. [Google Scholar] [CrossRef]
- Rasal, R.M.; Hirt, D.E. Toughness decrease of PLA-PHBHHx blend films upon surface-confined photopolymerization. J. Biomed. Mater. Res. Part A 2009, 88, 1079–1086. [Google Scholar] [CrossRef]
- Lim, J.S.; Park, K.I.; Chung, G.S.; Kim, J.H. Effect of composition ratio on the thermal and physical properties of semicrystalline PLA/PHB-HHx composites. Mater. Sci. Eng. C 2013, 33, 2131–2137. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; López, J.; Kenny, J.M. Bionanocomposite films based on plasticized PLA-PHB/cellulose nanocrystal blends. Carbohydr. Polym. 2015, 121, 265–275. [Google Scholar] [CrossRef] [Green Version]
- El-Hadi, A.M. Development of novel biopolymer blends based on poly(L-lactic acid), poly((R)-3-hydroxybutyrate), and plasticizer. Polym. Eng. Sci. 2014, 54, 1394–1402. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Rayón, E.; Jiménez, A. Disintegrability under composting conditions of plasticized PLA–PHB blends. Polym. Degrad. Stab. 2014, 108, 307–318. [Google Scholar] [CrossRef] [Green Version]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J.; Kenny, J.M. Multifunctional PLA-PHB/cellulose nanocrystal films: Processing, structural and thermal properties. Carbohydr. Polym. 2014, 107, 16–24. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Hernández, A.; Rayón, E. Ternary PLA-PHB-Limonene blends intended for biodegradable food packaging applications. Eur. Polym. J. 2014, 50, 255–270. [Google Scholar] [CrossRef]
- Abdelwahab, M.A.; Flynn, A.; Chiou, B.S.; Imam, S.; Orts, W.; Chiellini, E. Thermal, mechanical and morphological characterization of plasticized PLA-PHB blends. Polym. Degrad. Stab. 2012, 97, 1822–1828. [Google Scholar] [CrossRef]
- Guessasma, S.; Belhabib, S.; Nouri, H. Thermal cycling, microstructure and tensile performance of PLA-PHA polymer printed using fused deposition modelling technique. Rapid Prototyp. J. 2020, 26, 122–133. [Google Scholar] [CrossRef]
- Olejnik, O.; Masek, A.; Zawadziłło, J. Processability and mechanical properties of thermoplastic polylactide/polyhydroxybutyrate (PLA/PHB) bioblends. Materials 2021, 14, 898. [Google Scholar] [CrossRef] [PubMed]
- Hiljanen-Vainio, M.; Varpomaa, P.; Seppälä, J.; Törmälä, P. Modification of poly(L-lactides) by blending: Mechanical and hydrolytic behavior. Macromol. Chem. Phys. 1996, 197, 1503–1523. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Blends of aliphatic polyesters. I. Physical properties and morphologies of solution-cast blends from poly(DL-lactide) and poly(ε-caprolactone). J. Appl. Polym. Sci. 1996, 60, 2367–2375. [Google Scholar] [CrossRef]
- Maglio, G.; Migliozzi, A.; Palumbo, R.; Immirzi, B.; Volpe, M.G. Compatibilized poly(ε-caprolactone)/poly(L-lactide) blends for biomedical uses. Macromol. Rapid Commun. 1999, 20, 236–238. [Google Scholar] [CrossRef]
- Tsuji, H.; Yamada, T.; Suzuki, M.; Itsuno, S. Blends of aliphatic polyesters. Part 7. Effects of poly(L-lactide-co-ε-caprolactone) on morphology, structure, crystallization, and physical properties of blends of poly(L-lactide) and poly(ε-caprolactone). Polym. Int. 2003, 52, 269–275. [Google Scholar] [CrossRef]
- Semba, T.; Kitagawa, K.; Ishiaku, U.S.; Hamada, H. The effect of crosslinking on the mechanical properties of polylactic acid/polycaprolactone blends. J. Appl. Polym. Sci. 2006, 101, 1816–1825. [Google Scholar] [CrossRef]
- Grijpma, D.W.; Van Hofslot, R.D.A.; Supèr, H.; Nijenhuis, A.J.; Pennings, A.J. Rubber toughening of poly(lactide) by blending and block copolymerization. Polym. Eng. Sci. 1994, 34, 1674–1684. [Google Scholar] [CrossRef]
- Joziasse, C.A.P.; Topp, M.D.C.; Pennings, D.W.G.J. Rubber toughened linear and star-shaped. Polymer 1998, 39, 467–474. [Google Scholar] [CrossRef]
- Chen, C.C.; Chueh, J.Y.; Tseng, H.; Huang, H.M.; Lee, S.Y. Preparation and characterization of biodegradable PLA polymeric blends. Biomaterials 2003, 24, 1167–1173. [Google Scholar] [CrossRef]
- Hasook, A.; Tanoue, S.; Lemoto, Y.; Unryu, T. Characterization and mechanical properties of poly(lactic acid)/poly(ε-caprolactone)/organoclay nanocomposites prepared by melt compounding. Polym. Eng. Sci. 2006, 46, 1001–1007. [Google Scholar] [CrossRef]
- Jeantet, L.; Regazzi, A.; Taguet, A.; Pucci, M.F.; Caro, A.S.; Quantin, J.C. Biopolymer blends for mechanical property gradient 3D printed parts. Express Polym. Lett. 2021, 15, 137–152. [Google Scholar] [CrossRef]
- Doganci, M.D. Effects of star-shaped PCL having different numbers of arms on the mechanical, morphological, and thermal properties of PLA/PCL blends. J. Polym. Res. 2021, 28, 1–13. [Google Scholar] [CrossRef]
- Ebrahimifar, M.; Taherimehr, M. Evaluation of in-vitro drug release of polyvinylcyclohexane carbonate as a CO2-derived degradable polymer blended with PLA and PCL as drug carriers. J. Drug Deliv. Sci. Technol. 2021, 63, 1–13. [Google Scholar] [CrossRef]
- Yang, Z.T.; Yang, J.X.; Fan, J.H.; Feng, Y.H.; Huang, Z.X. Preparation of super-toughened Poly(L-lactide) composites under elongational flow: A strategy for balancing stiffness and ductility. Compos. Sci. Technol. 2021, 208, 1–8. [Google Scholar] [CrossRef]
- Pezzin, A.P.T.; Alberda van Ekenstein, G.O.R.; Zavaglia, C.A.C.; Ten Brinke, G.; Duek, E.A.R. Poly(para-dioxanone) and poly(1-lactic acid) blends: Thermal, mechanical, and morphological properties. J. Appl. Polym. Sci. 2003, 88, 2744–2755. [Google Scholar] [CrossRef]
- Ma, X.; Jiugao, Y.; Wang, N. Compatibility characterization of poly(lactic acid)/poly(propylene carbonate) blends. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 94–101. [Google Scholar] [CrossRef]
- Liu, T.Y.; Lin, W.C.; Yang, M.C.; Chen, S.Y. Miscibility, thermal characterization and crystallization of poly(l-lactide) and poly(tetramethylene adipate-co-terephthalate) blend membranes. Polymer 2005, 46, 12586–12594. [Google Scholar] [CrossRef]
- Jiang, L.; Wolcott, M.P.; Zhang, J. Study of biodegradable polylactide/poly(butylene adipate-co-terephthalate) blends. Biomacromolecules 2006, 7, 199–207. [Google Scholar] [CrossRef]
- Liu, X.; Dever, M.; Fair, N.; Benson, R.S. Thermal and mechanical properties of poly(lactic acid) and poly(ethylene/butylene succinate) blends. J. Environ. Polym. Degrad. 1997, 5, 225–235. [Google Scholar] [CrossRef]
- Shibata, M.; Inoue, Y.; Miyoshi, M. Mechanical properties, morphology, and crystallization behavior of blends of poly(l-lactide) with poly(butylene succinate-co-l-lactate) and poly(butylene succinate). Polymer 2006, 47, 3557–3564. [Google Scholar] [CrossRef]
- Chen, G.X.; Kim, H.S.; Kim, E.S.; Yoon, J.S. Compatibilization-like effect of reactive organoclay on the poly(l-lactide)/poly(butylene succinate) blends. Polymer 2005, 46, 11829–11836. [Google Scholar] [CrossRef]
- Chen, C.X.; Yoon, J.S. Morphology and thermal properties of poly(L -lactide)/poly (butylene succinate-co-butylene adipate) compounded with twice functionalized clay. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 478–487. [Google Scholar] [CrossRef]
- Shibata, M.; Teramoto, N.; Inoue, Y. Mechanical properties, morphologies, and crystallization behavior of plasticized poly(l-lactide)/poly(butylene succinate-co-l-lactate) blends. Polymer 2007, 48, 2768–2777. [Google Scholar] [CrossRef]
- Zhang, L.; Xiong, C.; Deng, X. Miscibility, crystallization and morphology of poly(β-hydroxybutyrate)/poly(d,l-lactide) blends. Polymer 1996, 37, 235–241. [Google Scholar] [CrossRef]
- Jandas, P.J.; Mohanty, S.; Nayak, S.K. Sustainability, compostability, and specific microbial activity on agricultural mulch films prepared from poly(lactic acid). Ind. Eng. Chem. Res. 2013, 52, 17714–17724. [Google Scholar] [CrossRef]
- Burgos, N.; Armentano, I.; Fortunati, E.; Dominici, F.; Luzi, F.; Fiori, S.; Cristofaro, F.; Visai, L.; Jiménez, A.; Kenny, J.M. Functional Properties of Plasticized Bio-Based Poly(Lactic Acid)_Poly(Hydroxybutyrate) (PLA_PHB) Films for Active Food Packaging. Food Bioprocess Technol. 2017, 10, 770–780. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Xu, H.; Niu, B.; Ji, X.; Chen, J.; Li, Z.M.; Hsiao, B.S.; Zhong, G.J. Unprecedented access to strong and ductile poly(lactic acid) by introducing in situ nanofibrillar poly(butylene succinate) for green packaging. Biomacromolecules 2014, 15, 4054–4064. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, E.; Puglia, D.; Iannoni, A.; Terenzi, A.; Kenny, J.M.; Torre, L. Processing conditions, thermal and mechanical responses of stretchable poly (lactic acid)/poly (butylene succinate) films. Materials 2017, 10, 809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostafinska, A.; Fortelny, I.; Nevoralova, M.; Hodan, J.; Kredatusova, J.; Slouf, M. Synergistic effects in mechanical properties of PLA/PCL blends with optimized composition, processing, and morphology. RSC Adv. 2015, 120, 98971–98982. [Google Scholar] [CrossRef]
- Jost, V.; Kopitzky, R. Blending of polyhydroxybutyrate-co-valerate with polylactic acid for packaging applications—Reflections on miscibility and effects on the mechanical and barrier properties. Chem. Biochem. Eng. Q. 2015, 29, 221–246. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Jiménez, A.; Puglia, D.; Pezzolla, D.; Gigliotti, G.; Kenny, J.M.; Chiralt, A.; Torre, L. Production and characterization of PLA_PBS biodegradable blends reinforced with cellulose nanocrystals extracted from hemp fibres. Ind. Crop. Prod. 2016, 93, 276–289. [Google Scholar] [CrossRef]
- Songtipya, L.; Limchu, T.; Phuttharak, S.; Songtipya, P.; Kalkornsurapranee, E. Poly(lactic acid)-based Composites Incorporated with Spent Coffee Ground and Tea Leave for Food Packaging Application: A Waste to Wealth. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019. [Google Scholar] [CrossRef]
- Ma, Y.; Li, L.; Wang, Y. Development of PLA-PHB-based biodegradable active packaging and its application to salmon. Packag. Technol. Sci. 2018, 31, 739–746. [Google Scholar] [CrossRef]
- Hongsriphan, N.; Sanga, S. Antibacterial food packaging sheets prepared by coating chitosan on corona-treated extruded poly(lactic acid)/poly(butylene succinate) blends. J. Plast. Film Sheeting 2018, 34, 160–178. [Google Scholar] [CrossRef]
- Zhang, K.; Mohanty, A.K.; Misra, M. Fully biodegradable and biorenewable ternary blends from polylactide, poly(3-hydroxybutyrate-co-hydroxyvalerate) and poly(butylene succinate) with balanced properties. ACS Appl. Mater. Interfaces 2012, 4, 3091–3101. [Google Scholar] [CrossRef]
- Balakrishnan, H.; Hassan, A.; Wahit, M.U.; Yussuf, A.A.; Razak, S.B.A. Novel toughened polylactic acid nanocomposite: Mechanical, thermal and morphological properties. Mater. Des. 2010, 31, 3289–3298. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, T.G.; Park, H.S.; Lee, D.S.; Lee, Y.K.; Yoon, S.C.; Nam, J.D. Thermal and mechanical characteristics of poly(L-lactic acid) nanocomposite scaffold. Biomaterials 2003, 24, 2773–2778. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, J.; Wolcott, M.P. Comparison of polylactide/nano-sized calcium carbonate and polylactide/montmorillonite composites: Reinforcing effects and toughening mechanisms. Polymer 2007, 48, 7632–7644. [Google Scholar] [CrossRef]
- Harris, A.M.; Lee, E.C. Improving mechanical performance of injection molded PLA by controlling crystallinity. J. Appl. Polym. Sci. 2008, 107, 2246–2255. [Google Scholar] [CrossRef]
- Yu, F.; Liu, T.; Zhao, X.; Yu, X.; Lu, A.; Wang, J. Effects of talc on the mechanical and thermal properties of polylactide. J. Appl. Polym. Sci. 2012, 125, E99–E109. [Google Scholar] [CrossRef]
- Ouchiar, S.; Stoclet, G.; Cabaret, C.; Georges, E.; Smith, A.; Martias, C.; Addad, A.; Gloaguen, V. Comparison of the influence of talc and kaolinite as inorganic fillers on morphology, structure and thermomechanical properties of polylactide based composites. Appl. Clay Sci. 2015, 116–117, 231–240. [Google Scholar] [CrossRef]
- Zhou, Y.; Lei, L.; Yang, B.; Li, J.; Ren, J. Preparation and characterization of polylactic acid (PLA) carbon nanotube nanocomposites. Polym. Test. 2018, 68, 34–38. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, J.; Sakai, E.; Wei, X. The relationship between microstructure and mechanical properties of carbon nanotubes/polylactic acid nanocomposites prepared by twin-screw extrusion. Compos. Part A Appl. Sci. Manuf. 2016, 89, 18–25. [Google Scholar] [CrossRef]
- Silva, A.P.B.; Montagna, L.S.; Passador, F.R.; Rezende, M.C.; Lemes, A.P. Biodegradable nanocomposites based on PLA/PHBV blend reinforced with carbon nanotubes with potential for electrical and electromagnetic applications. Express Polym. Lett. 2021, 15, 987–1003. [Google Scholar] [CrossRef]
- Chen, P.Y.; Lian, H.Y.; Shih, Y.F.; Chen-Wei, S.M.; Jeng, R.J. Preparation, characterization and crystallization kinetics of Kenaf fiber/multi-walled carbon nanotube/polylactic acid (PLA) green composites. Mater. Chem. Phys. 2017, 196, 249–255. [Google Scholar] [CrossRef]
- Quan, H.; Li, Z.M.; Yang, M.B.; Huang, R. On transcrystallinity in semi-crystalline polymer composites. Compos. Sci. Technol. 2005, 65, 999–1021. [Google Scholar] [CrossRef]
- Reverte, J.M.; Caminero, M.Á.; Chacón, J.M.; García-Plaza, E.; Núñez, P.J.; Becar, J.P. Mechanical and geometric performance of PLA-based polymer composites processed by the fused filament fabrication additive manufacturing technique. Matererials 2020, 13, 1924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wootthikanokkhan, J.; Cheachun, T.; Sombatsompop, N.; Thumsorn, S.; Kaabbuathong, N.; Wongta, N.; Wong-On, J.; Na Ayutthaya, S.I.; Kositchaiyong, A. Crystallization and thermomechanical properties of PLA composites: Effects of additive types and heat treatment. J. Appl. Polym. Sci. 2013, 129, 215–223. [Google Scholar] [CrossRef]
- Ahmed, W.; Siraj, S.; Al-Marzouqi, A.H. 3d printing pla waste to produce ceramic based particulate reinforced composite using abundant silica-sand: Mechanical properties characterization. Polymers 2020, 12, 2579. [Google Scholar] [CrossRef] [PubMed]
- Swaroop, C.; Shukla, M. Nano-magnesium oxide reinforced polylactic acid biofilms for food packaging applications. Int. J. Biol. Macromol. 2018, 113, 729–736. [Google Scholar] [CrossRef] [Green Version]
- John, M.J.; Anandjiwala, R.D. Recent developments in chemical modification and characterization of natural fiber-reinforced composites. Polym. Compos. 2008, 29, 187–207. [Google Scholar] [CrossRef]
- Foruzanmehr, M.; Vuillaume, P.Y.; Elkoun, S.; Robert, M. Physical and mechanical properties of PLA composites reinforced by TiO2 grafted flax fibers. Mater. Des. 2016, 106, 295–304. [Google Scholar] [CrossRef]
- Lee, B.H.; Kim, H.S.; Lee, S.; Kim, H.J.; Dorgan, J.R. Bio-composites of kenaf fibers in polylactide: Role of improved interfacial adhesion in the carding process. Compos. Sci. Technol. 2009, 69, 15–16. [Google Scholar] [CrossRef]
- Kumar, R.; Yakabu, M.K.; Anandjiwala, R.D. Effect of montmorillonite clay on flax fabric reinforced poly lactic acid composites with amphiphilic additives. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1620–1627. [Google Scholar] [CrossRef]
- Kanakannavar, S.; Pitchaimani, J. Fracture toughness of flax braided yarn woven PLA composites. Int. J. Polym. Anal. Charact. 2021, 26, 364–379. [Google Scholar] [CrossRef]
- Wang, A.; Qi, R.; Xiong, C.; Huang, M. Effects of coupling agent and interfacial modifiers on mechanical properties of poly(lactic acid) and wood flour biocomposites. Iran. Polym. J. Engl. Ed. 2011, 20, 281–294. [Google Scholar]
- Da Silva, W.A.; Luna, C.B.B.; de Melo, J.B.d.C.A.; Araújo, E.M.; Filho, E.A.d.S.; Duarte, R.N.C. Feasibility of Manufacturing Disposable Cups using PLA/PCL Composites Reinforced with Wood Powder. J. Polym. Environ. 2021, 29, 2932–2951. [Google Scholar] [CrossRef]
- Wasti, S.; Triggs, E.; Farag, R.; Auad, M.; Adhikari, S.; Bajwa, D.; Li, M.; Ragauskas, A.J. Influence of plasticizers on thermal and mechanical properties of biocomposite filaments made from lignin and polylactic acid for 3D printing. Compos. Part B Eng. 2021, 205, 1–8. [Google Scholar] [CrossRef]
- Paul, U.C.; Fragouli, D.; Bayer, I.S.; Zych, A.; Athanassiou, A. Effect of Green Plasticizer on the Performance of Microcrystalline Cellulose/Polylactic Acid Biocomposites. ACS Appl. Polym. Mater. 2021, 3, 3071–3081. [Google Scholar] [CrossRef]
- Rasheed, M.; Jawaid, M.; Parveez, B. Bamboo fiber based cellulose nanocrystals/poly(Lactic acid)/poly(butylene succinate) nanocomposites: Morphological, mechanical and thermal properties. Polymers 2021, 13, 1076. [Google Scholar] [CrossRef]
- Frone, A.N.; Batalu, D.; Chiulan, I.; Oprea, M.; Gabor, A.R.; Nicolae, C.A.; Raditoiu, V.; Trusca, R.; Panaitescu, D.M. Morpho-structural, thermal and mechanical properties of PLA/PHB/Cellulose biodegradable nanocomposites obtained by compression molding, extrusion, and 3d printing. Nanomaterials 2020, 10, 51. [Google Scholar] [CrossRef] [Green Version]
- Alam, F.; Verma, P.; Mohammad, W.; Teo, J.; Varadarajan, K.M.; Kumar, S. Architected poly(lactic acid)/poly(ε-caprolactone)/halloysite nanotube composite scaffolds enabled by 3D printing for biomedical applications. J. Mater. Sci. 2021, 56, 14070–14083. [Google Scholar] [CrossRef]
- Komal, U.K.; Lila, M.K.; Singh, I. Processing of PLA/pineapple fiber based next generation composites. Mater. Manuf. Process. 2021, 36, 1–16. [Google Scholar] [CrossRef]
- Mohamad, S.N.K.; Ramli, I.; Abdullah, L.C.; Mohamed, N.H.; Islam, M.S.; Ibrahim, N.A.; Ishak, N.S. Evaluation on structural properties and performances of graphene oxide incorporated into chitosan/poly-lactic acid composites: Cs/pla versus cs/pla-go. Polymers 2021, 13, 1839. [Google Scholar] [CrossRef]
- McChalicher, C.W.J.; Srienc, F. Investigating the structure-property relationship of bacterial PHA block copolymers. J. Biotechnol. 2007, 123, 296–302. [Google Scholar] [CrossRef]
- Sudesh, K.; Iwata, T. Sustainability of biobased and biodegradable plastics. Clean Soil Air Water 2008, 36, 433–442. [Google Scholar] [CrossRef]
- Philip, S.; Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Biodegradable polymers with a range of applications. J. Chem. Technol. Biotechnol. 2007, 82, 233–247. [Google Scholar] [CrossRef]
- Khanna, S.; Srivastava, A.K. Recent advances in microbial polyhydroxyalkanoates. Process Biochem. 2005, 40, 607–619. [Google Scholar] [CrossRef]
- Rai, R.; Keshavarz, T.; Roether, J.A.; Boccaccini, A.R.; Roy, I. Medium chain length polyhydroxyalkanoates, promising new biomedical materials for the future. Mater. Sci. Eng. R Rep. 2011, 72, 29–47. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef] [Green Version]
- Kushwah, B.S.; Kushwah, A.V.S.; Singh, V. Towards understanding polyhydroxyalkanoates and their use. J. Polym. Res. 2016, 23, 1–14. [Google Scholar] [CrossRef]
- Bucci, D.Z.; Tavares, L.B.B.; Sell, I. PHB packaging for the storage of food products. Polym. Test. 2005, 24, 564–571. [Google Scholar] [CrossRef]
- Aldor, I.S.; Kim, S.W.; Jones Prather, K.L.; Keasling, J.D. Metabolic engineering of a novel propionate-independent pathway for the production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in recombinant Salmonella enterica serovar typhimurium. Appl. Environ. Microbiol. 2002, 68, 3848–3854. [Google Scholar] [CrossRef] [Green Version]
- Ha, C.S.; Cho, W.J. Miscibility, properties, and biodegradability of microbial polyester containing blends. Prog. Polym. Sci. 2002, 27, 759–809. [Google Scholar] [CrossRef]
- Ohashi, E.; Drumond, W.S.; Zane, N.P.; Barros, P.W.D.F.; Lachtermacher, M.G.; Wiebeck, H.; Wang, S.H. Biodegradable poly(3-hydroxybutyrate) nanocomposite. Macromol. Symp. 2009, 279, 138–144. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- La Cara, F.; Immirzi, B.; Ionata, E.; Mazzella, A.; Portofino, S.; Orsello, G.; De Prisco, P.P. Biodegradation of poly-ε-caprolactone/poly-β-hydroxybutyrate blend. Polym. Degrad. Stab. 2003, 79, 37–43. [Google Scholar] [CrossRef]
- Lovera, D.; Márquez, L.; Balsamo, V.; Taddei, A.; Castelli, C.; Müller, A.J. Crystallization, morphology, and enzymatic degradation of polyhydroxybutyrate/polycaprolactone (PHB/PCL) blends. Macromol. Chem. Phys. 2007, 208, 924–937. [Google Scholar] [CrossRef]
- Gonçalves, S.P.C.; Martins Franchetti, S.M. Respirometric evaluation of the biodegradability of films of PE/PHBV blends. Int. J. Mater. Sci. 2013, 11, 54–60. [Google Scholar]
- Masood, F.; Yasin, T.; Hameed, A. Comparative oxo-biodegradation study of poly-3-hydroxybutyrate-co-3-hydroxyvalerate/polypropylene blend in controlled environments. Int. Biodeterior. Biodegrad. 2014, 87, 1–8. [Google Scholar] [CrossRef]
- Gonçalves, S.P.C.; Martins-Franchetti, S.M.; Chinaglia, D.L. Biodegradation of the films of PP, PHBV and its blend in soil. J. Polym. Environ. 2009, 17, 280–285. [Google Scholar] [CrossRef]
- Burlein, G.A.D.; Rocha, M.C.G. Mechanical and morphological properties of LDPE/PHB blends filled with castor oil pressed cake. Mater. Res. 2014, 17, 97–105. [Google Scholar] [CrossRef]
- Quental, A.C.; de Carvalho, F.P.; Rezende, M.L.; Rosa, D.S.; Felisberti, M.I. Aromatic/Aliphatic Polyester Blends. J. Polym. Environ. 2010, 18, 308–317. [Google Scholar] [CrossRef]
- Ma, P.; Hristova-Bogaerds, D.G.; Lemstra, P.J.; Zhang, Y.; Wang, S. Toughening of PHBV/PBS and PHB/PBS blends via in situ compatibilization using dicumyl peroxide as a free-radical grafting initiator. Macromol. Mater. Eng. 2012, 297, 402–410. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Cui, Z.; Wang, X.; Turng, L.S.; Peng, X. Processing and characterization of solid and microcellular poly(lactic acid)/polyhydroxybutyrate-valerate (PLA/PHBV) blends and PLA/PHBV/Clay nanocomposites. Compos. Part B Eng. 2013, 51, 79–91. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Ferri, J.M.; Boronat, T.; Lopez-Martinez, J.; Balart, R. Processing and characterization of binary poly(hydroxybutyrate) (PHB) and poly(caprolactone) (PCL) blends with improved impact properties. Polym. Bull. 2016, 73, 3333–3350. [Google Scholar] [CrossRef] [Green Version]
- Chee, M.J.K.; Ismail, J.; Kammer, H.W.; Kummerloöwe, C. Study on miscibility of PEO and PCL in blends with PHB by solution viscometry. Polymer 2001, 43, 1235–1239. [Google Scholar] [CrossRef]
- Nishida, M.; Tanaka, T.; Hayakawa, Y.; Ogura, T.; Ito, Y.; Nishida, M. Multi-scale instrumental analyses of plasticized polyhydroxyalkanoates (PHA) blended with polycaprolactone (PCL) and the effects of crosslinkers and graft copolymers. RSC Adv. 2019, 9, 1551–1561. [Google Scholar] [CrossRef] [Green Version]
- Mendibil, X.; González-Pérez, F.; Bazan, X.; Díez-Ahedo, R.; Quintana, I.; Rodríguez, F.J.; Basnett, P.; Nigmatullin, R.; Lukasiewicz, B.; Roy, I.; et al. Bioresorbable and Mechanically Optimized Nerve Guidance Conduit Based on a Naturally Derived Medium Chain Length Polyhydroxyalkanoate and Poly(ϵ-Caprolactone) Blend. ACS Biomater. Sci. Eng. 2021, 7, 672–689. [Google Scholar] [CrossRef]
- Qiu, Z.; Ikehara, T.; Nishi, T. Poly(hydroxybutyrate)/poly(butylene succinate) blends: Miscibility and nonisothermal crystallization. Polymes 2003, 44, 2503–2508. [Google Scholar] [CrossRef]
- Qiu, Z.; Ikehara, T.; Nishi, T. Miscibility and crystallization behaviour of biodegradable blends of two aliphatic polyesters. Poly(3-hydroxybutyrate-co-hydroxyvalerate) and poly(butylene succinate) blends. Polymer 2003, 44, 7519–7527. [Google Scholar] [CrossRef]
- Meléndez-Rodríguez, B.; Torres-Giner, S.; Reis, M.A.M.; Silva, F.; Matos, M.; Cabedo, L.; Lagarón, J.M. Blends of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with fruit pulp biowaste derived poly(3-hydroxybutyrate-co-3- hydroxyvalerate-co-3-hydroxyhexanoate) for organic recycling food packaging. Polymers 2021, 13, 1155. [Google Scholar] [CrossRef]
- Lim, S.T.; Hyun, Y.H.; Lee, C.H.; Choi, H.J. Preparation and characterization of microbial biodegradable poly(3-hydroxybutyrate)/organoclay nanocomposite. J. Mater. Sci. Lett. 2003, 22, 299–302. [Google Scholar] [CrossRef]
- Shan, G.F.; Gong, X.; Chen, W.P.; Chen, L.; Zhu, M.F. Effect of multi-walled carbon nanotubes on crystallization behavior of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Colloid Polym. Sci. 2011, 289, 1005–1014. [Google Scholar] [CrossRef]
- Lai, M.; Li, J.; Yang, J.; Liu, J.; Tong, X.; Cheng, H. The morphology and thermal properties of multi-walled carbon nanotube and poly(hydroxybutyrate-co-hydroxyvalerate) composite. Polym. Int. 2004, 53, 1479–1484. [Google Scholar] [CrossRef]
- Maiti, P.; Batt, C.A.; Giannelis, E.P. New biodegradable polyhydroxybutyrate/layered silicate nanocomposites. Biomacromolecules 2007, 8, 3393–3400. [Google Scholar] [CrossRef]
- Wang, S.; Song, C.; Chen, G.; Guo, T.; Liu, J.; Zhang, B.; Takeuchi, S. Characteristics and biodegradation properties of poly(3-hydroxybutyrate-co- 3-hydroxyvalerate)/organophilic montmorillonite (PHBV/OMMT) nanocomposite. Polym. Degrad. Stab. 2005, 87, 69–76. [Google Scholar] [CrossRef]
- Chen, G.X.; Hao, G.J.; Guo, T.Y.; Song, M.D.; Zhang, B.H. Crystallization kinetics of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/clay nanocomposites. J. Appl. Polym. Sci. 2004, 93, 655–661. [Google Scholar] [CrossRef]
- Botana, A.; Mollo, M.; Eisenberg, P.; Torres Sanchez, R.M. Effect of modified montmorillonite on biodegradable PHB nanocomposites. Appl. Clay Sci. 2010, 47, 263–270. [Google Scholar] [CrossRef]
- Carli, L.N.; Crespo, J.S.; Mauler, R.S. PHBV nanocomposites based on organomodified montmorillonite and halloysite: The effect of clay type on the morphology and thermal and mechanical properties. Compos. Part A Appl. Sci. Manuf. 2011, 42, 1601–1608. [Google Scholar] [CrossRef]
- Misra, M.; Desai, S.M.; Mohanty, A.K.; Drzal, L.T. Novel solvent-free method for functionalization of polyhydroxyalkanoates: Synthesis and chracterizations. In Proceedings of the Annual Technical Conference—ANTEC, Chicago, IL, USA, 16–20 May 2004. [Google Scholar]
- Martínez-Sanz, M.; Villano, M.; Oliveira, C.; Albuquerque, M.G.E.; Majone, M.; Reis, M.; Lopez-Rubio, A.; Lagaron, J.M. Characterization of polyhydroxyalkanoates synthesized from microbial mixed cultures and of their nanobiocomposites with bacterial cellulose nanowhiskers. N. Biotechnol. 2014, 31, 364–376. [Google Scholar] [CrossRef]
- Ten, E.; Turtle, J.; Bahr, D.; Jiang, L.; Wolcott, M. Thermal and mechanical properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/cellulose nanowhiskers composites. Polymer 2010, 51, 2652–2660. [Google Scholar] [CrossRef]
- Jiang, L.; Morelius, E.; Zhang, J.; Wolcott, M.; Holbery, J. Study of the poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/cellulose nanowhisker composites prepared by solution casting and melt processing. J. Compos. Mater. 2008, 42, 2629–2645. [Google Scholar] [CrossRef]
- Xie, Y.; Kohls, D.; Noda, I.; Schaefer, D.W.; Akpalu, Y.A. Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) nanocomposites with optimal mechanical properties. Polymer 2009, 50, 4656–4670. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.D.; Lagaron, J.M.; Hoa, S.V. Effect of addition of carbon nanofibers and carbon nanotubes on properties of thermoplastic biopolymers. Compos. Sci. Technol. 2010, 70, 1095–1105. [Google Scholar] [CrossRef]
- Jo, J.; Kim, H.; Jeong, S.Y.; Park, C.; Hwang, H.S.; Koo, B. Changes in mechanical properties of polyhydroxyalkanoate with double silanized cellulose nanocrystals using different organosiloxanes. Nanomaterials 2021, 11, 1542. [Google Scholar] [CrossRef]
- Liao, H.T.; Wu, C.S. Poly(3-hydroxybutyrate)/multi-walled carbon nanotubes nanocomposites: Preparation and characterizations. Des. Monomers Polym. 2013, 16, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Fan, Y.; Xue, Y.; Wu, L.; Lu, Y.; Chen, J.; Wang, X.; Dong, D.; Meng, F.; Lu, Y.; et al. Electrospun CF-PHA nanocomposites: Effect of surface modifications of carbon fibers. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 262–267. [Google Scholar] [CrossRef]
- Gumel, A.M.; Annuar, M.S.M.; Ishak, K.A.; Ahmad, N. Carbon nanofibers-poly-3-hydroxyalkanoates nanocomposite: Ultrasound-assisted dispersion and thermostructural properties. J. Nanomater. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Cataldi, P.; Steiner, P.; Raine, T.; Lin, K.; Kocabas, C.; Young, R.J.; Bissett, M.; Kinloch, I.A.; Papageorgiou, D.G. Multifunctional Biocomposites Based on Polyhydroxyalkanoate and Graphene/Carbon Nanofiber Hybrids for Electrical and Thermal Applications. ACS Appl. Polym. Mater. 2020, 2, 3525–3534. [Google Scholar] [CrossRef]
- Kotnis, M.A.; O’Brien, G.S.; Willett, J.L. Processing and mechanical properties of biodegradable Poly(hydroxybutyrate-co-valerate)-starch compositions. J. Environ. Polym. Degrad. 1995, 3, 97–105. [Google Scholar] [CrossRef]
- Shogren, R.L. Poly(ethylene oxide)-coated granular starch-poly(hydroxybutyrate-co-hydroxyvalerate) composite materials. J. Environ. Polym. Degrad. 1995, 3, 75–80. [Google Scholar] [CrossRef]
- Koller, I.; Owen, A.J. Starch-filled PHB and PHB/HV copolymer. Polym. Int. 1996, 39, 175–181. [Google Scholar] [CrossRef]
- Rosa, D.D.S.; Rodrigues, T.C.; Das Graças Fassina Guedes, C.; Calil, M.R. Effect of thermal aging on the biodegradation of PCL, PHB-V, and their blends with starch in soil compost. J. Appl. Polym. Sci. 2003, 89, 3539–3546. [Google Scholar] [CrossRef]
- Ramsay, B.A.; Langlade, V.; Carreau, P.J.; Ramsay, J.A. Biodegradability and mechanical properties of poly-(β-hydroxybutyrate- co-β-hydroxyvalerate)-starch blends. Appl. Environ. Microbiol. 1993, 59, 1242–1246. [Google Scholar] [CrossRef] [Green Version]
- Avella, M.; Errico, M.E.; Rimedio, R.; Sadocco, P. Preparation of biodegradable polyesters/high-amylose-starch composites by reactive blending and their characterization. J. Appl. Polym. Sci. 2002, 83, 1432–1442. [Google Scholar] [CrossRef]
- Innocentini-Mei, L.H.; Bartoli, J.R.; Baltieri, R.C. Mechanical and thermal properties of poly(3-hydroxybutyrate) blends with starch and starch derivatives. In Macromolecular Symposia; Wiley: Weinheim, Germany, 2003. [Google Scholar]
- Willett, J.L.; Kotnis, M.A.; O’Brien, G.S.; Fanta, G.F.; Gordon, S.H. Properties of starch-graft-poly(glycidyl methacrylate)-PHBV composites. J. Appl. Polym. Sci. 1998, 70, 1121–1127. [Google Scholar] [CrossRef]
- Liao, H.T.; Wu, C.S. Performance of an acrylic-acid-grafted poly(3-hydroxybutyric acid)/starch bio-blend: Characterization and physical properties. Des. Monomers Polym. 2007, 10, 1–18. [Google Scholar] [CrossRef]
- Koenig, M.F.; Huang, S.J. Biodegradable blends and composites of polycaprolactone and starch derivatives. Polymer 1995, 36, 1877–1882. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, X.; Zhao, S.; Huang, Z. Biodegradable polymer blends of poly(3-hydroxybutyrate) and starch acetate. Polym. Int. 1997, 44, 104–110. [Google Scholar] [CrossRef]
- Seves, A.; Beltrame, P.L.; Selli, E.; Bergamasco, L. Morphology and thermal behaviour of poly(3-hydroxybutyrate-co-hydroxyvalerate)/starch valerate blends. Angew. Makromol. Chem. 1998, 260, 65–70. [Google Scholar] [CrossRef]
- Godbole, S.; Gote, S.; Latkar, M.; Chakrabarti, T. Preparation and characterization of biodegradable poly-3-hydroxybutyrate-starch blend films. Bioresour. Technol. 2003, 86, 33–37. [Google Scholar] [CrossRef]
- Reis, K.C.; Pereira, J.; Smith, A.C.; Carvalho, C.W.P.; Wellner, N.; Yakimets, I. Characterization of polyhydroxybutyrate-hydroxyvalerate (PHB-HV)/maize starch blend films. J. Food Eng. 2008, 89, 361–369. [Google Scholar] [CrossRef]
- Zhang, M.; Thomas, N.L. Preparation and properties of polyhydroxybutyrate blended with different types of starch. J. Appl. Polym. Sci. 2009, 116, 688–694. [Google Scholar] [CrossRef]
- Parulekar, Y.; Mohanty, A.K. Extruded biodegradable cast films from polyhydroxyalkanoate and thermoplastic starch blends: Fabrication and characterization. Macromol. Mater. Eng. 2007, 292, 1218–1228. [Google Scholar] [CrossRef]
- Martin, O.; Schwach, E.; Avérous, L.; Couturier, Y. Properties of biodegradable multilayer films based on plasticized wheat starch. Starch/Staerke 2001, 53, 372–380. [Google Scholar] [CrossRef]
- Wang, L.; Shogren, R.L.; Carriere, C. Preparation and properties of thermoplastic starch-polyester laminate sheets by coextrusion. Polym. Eng. Sci. 2000, 40, 499–506. [Google Scholar] [CrossRef]
- Lawton, J.W. Biodegradable Coatings for Thermoplastic Starch. In Cereals: Novel Uses and Processes, 1st ed.; Springer: New York, NY, USA, 1997. [Google Scholar]
- Willett, J.L.; Shogren, R.L. Processing and properties of extruded starch/polymer foams. Polymer 2002, 43, 5935–5947. [Google Scholar] [CrossRef]
- Gordon, S.H.; Imam, S.H.; Shogren, R.L.; Govind, N.S.; Greene, R.V. Semiempirical model for predicting biodegradation profiles of individual polymers in starch-poly(β-hydroxybutyrate-co-β-hydroxyvalerate) bioplastic. J. Appl. Polym. Sci. 2000, 76, 1767–1776. [Google Scholar] [CrossRef]
- Allen, A.L.; Mayer, J.; Stote, R.; Kaplan, D.L. Simulated marine respirometry of biodegradable polymers. J. Environ. Polym. Degrad. 1994, 2, 237–244. [Google Scholar] [CrossRef]
- Vikman, M.; Itävaara, M.; Poutanen, K. Measurement of the biodegradation of starch-based materials by enzymatic methods and composting. J. Environ. Polym. Degrad. 1995, 3, 23–29. [Google Scholar] [CrossRef]
- Yasin, M.; Holland, S.J.; Jolly, A.M.; Tighe, B.J. Polymers for biodegradable medical devices. VI. Hydroxybutyrate-hydroxyvalerate copolymers: Accelerated degradation of blends with polysaccharides. Biomaterials 1989, 10, 400–412. [Google Scholar] [CrossRef]
- Tanna, S.T.; Gross, R.; McCarthy, S.P. Biodegradation of blends of bacterial polyester and starch in a compost environment. Polym. Mater. Sci. Eng. Proc. ACS Div. Polym. Mater. Sci. Eng. 1992. [Google Scholar]
- Lauzier, C.; Monasterios, C.; Saracovan, I.; Marchessault, R.; Ramsay, B. Film formation and paper coating with poly(β-hydroxyalkanoate), a biodegradable latex. Tappi J. 1993, 76. [Google Scholar]
- Yasin, M.; Tighe, B.J. Strategies for the design of biodegradable polymer systems: Manipulation of polyhydroxybutyrate-based materials. Plast. Rubber Compos. Process. Appl. 1993, 19, 15–27. [Google Scholar]
- Imam, S.H.; Gordon, S.H.; Shogren, R.L.; Greene, R.V. Biodegradation of starch-poly(β-hydroxybutyrate-co-valerate) composites in municipal activated sludge. J. Environ. Polym. Degrad. 1995, 3, 205–213. [Google Scholar] [CrossRef]
- Imam, S.H.; Chen, L.; Gorden, S.H.; Shogren, R.L.; Weisleder, D.; Greene, R.V. Biodegradation of injection molded starch-poly(3-hydroxybutyrate-co-3-hydroxyvalerate) blends in a natural compost environment. J. Environ. Polym. Degrad. 1998, 6, 91–98. [Google Scholar] [CrossRef]
- Imam, S.H.; Gordon, S.H.; Shogren, R.L.; Tosteson, T.R.; Govind, N.S.; Greene, R.V. Degradation of starch-poly([β-hydroxybutyrate-Co-β-hydroxyvalerate) bioplastic in tropical coastal waters. Appl. Environ. Microbiol. 1999, 65, 431–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaihara, S.; Osanai, Y.; Nishikawa, K.; Toshima, K.; Doi, Y.; Matsumura, S. Enzymatic transformation of bacterial polyhydroxyalkanoates into repolymerizable oligomers directed towards chemical recycling. Macromol. Biosci. 2005, 5, 644–652. [Google Scholar] [CrossRef]
- Reddy, C.S.K.; Ghai, R.; Kalia, V.C. Polyhydroxyalkanoates: An overview. Bioresour. Technol. 2003, 87, 137–146. [Google Scholar] [CrossRef]
- Patel, M.; Narayan, R. How sustainable are biopolymers and biobased products? The hope, the doubts, and the reality. In Natural Fibers, Biopolymers, and Biocomposites, 1st ed.; Taylor & Francis: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- Bruzaud, S.; Bourmaud, A. Thermal degradation and (nano)mechanical behavior of layered silicate reinforced poly(3-hydroxybutyrate-co-3-hydroxyvalerate) nanocomposites. Polym. Test. 2007, 26, 652–659. [Google Scholar] [CrossRef]
- Hsu, S.F.; Wu, T.M.; Liao, C.S. Nonisothermal crystallization behavior and crystalline structure of poly(3-hydroxybutyrate)/layered double hydroxide nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 995–1002. [Google Scholar] [CrossRef]
- Bruno, M.; Tavares, M.I.B.; Motta, L.M.; Miguez, E.; Preto, M.; Fernandez, A.O.R. Evaluation of PHB/clay nanocomposite by spin-lattice relaxation time. Mater. Res. 2008, 11, 483–485. [Google Scholar] [CrossRef]
- Erceg, M.; Kovačić, T.; Klarić, I. Poly(3-hydroxybutyrate) nanocomposites: Isothermal degradation and kinetic analysis. Thermochim. Acta 2009, 485, 26–32. [Google Scholar] [CrossRef]
- Erceg, M.; Kovačić, T.; Sanja, P. Isothermal degradation of poly(3-hydroxybutyrate)/organically modified montmorillonite nanocomposites. Polym. Compos. 2010, 31, 272–278. [Google Scholar] [CrossRef]
- Parulekar, Y.; Mohanty, A.K.; Imam, S.H. Biodegradable nanocomposites from toughened polyhydroxybutyrate and titanate-modified montmorillonite clay. J. Nanosci. Nanotechnol. 2007, 7, 3580–3589. [Google Scholar] [CrossRef]
- Hablot, E.; Bordes, P.; Pollet, E.; Avérous, L. Thermal and thermo-mechanical degradation of poly(3-hydroxybutyrate)-based multiphase systems. Polym. Degrad. Stab. 2008, 93, 413–421. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.D.; Gimenez, E.; Lagaron, J.M. Morphology and barrier properties of nanobiocomposites of poly(3-hydroxybutyrate) and layered silicates. J. Appl. Polym. Sci. 2008, 108, 2787–2801. [Google Scholar] [CrossRef]
- Maiti, P.; Prakash Yadav, J.P. Renewable plastics: Synthesis and properties of PHB nanocomposites. Polym. Mater. Sci. Eng. 2003, 88, 58–59. [Google Scholar]
- Pavlidou, S.; Papaspyrides, C.D. A review on polymer-layered silicate nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- Choi, W.M.; Kim, T.W.; Park, O.O.; Chang, Y.K.; Lee, J.W. Preparation and characterization of poly(hydroxybutyrate-co-hydroxyvalerate)-organoclay nanocomposites. J. Appl. Polym. Sci. 2003, 90, 525–529. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, G.; Abou-Hussein, R.; Hassan, M.K.; Noda, I.; Mark, J.E. Some novel layered-silicate nanocomposites based on a biodegradable hydroxybutyrate copolymer. Eur. Polym. J. 2007, 43, 3128–3135. [Google Scholar] [CrossRef]
- Wu, T.M.; Hs, S.F.; Shih, Y.F.; Liao, C.S. Thermal degradation kinetics of biodegradable poly(3-hydroxybutyrate)/layered double hydroxide nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 1207–1213. [Google Scholar] [CrossRef]
- Dagnon, K.L.; Chen, H.H.; Innocentini-Mei, L.H.; D’Souza, N.A. Poly[(3-hydroxybutyrate)-co-(3-hydroxyvalerate)]/layered double hydroxide nanocomposites. Polym. Int. 2009, 58, 133–141. [Google Scholar] [CrossRef]
- Dufresne, A.; Kellerhals, M.B.; Witholt, B. Transcrystallization in Mcl-PHAs/cellulose whiskers composites. Macromolecules 1999, 32, 7396–7401. [Google Scholar] [CrossRef]
- Chen, G.X.; Hao, G.J.; Guo, T.Y.; Song, M.D.; Zhang, B.H. Structure and mechanical properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV)/clay nanocomposites. J. Mater. Sci. Lett. 2002, 21, 1587–1589. [Google Scholar] [CrossRef]
- Thellen, C.; Coyne, M.; Froio, D.; Auerbach, M.; Wirsen, C.; Ratto, J.A. A processing, characterization and marine biodegradation study of melt-extruded polyhydroxyalkanoate (PHA) films. J. Polym. Environ. 2008, 16, 1–11. [Google Scholar] [CrossRef]
- Gregorova, A.; Wimmer, R.; Hrabalova, M.; Koller, M.; Ters, T.; Mundigler, N. Effect of surface modification of beech wood flour on mechanical and thermal properties of poly (3-hydroxybutyrate)/wood flour composites. Holzforschung 2009, 63, 565–570. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Gigante, V.; Seggiani, M.; Cinelli, P.; Signori, F.; Vania, A.; Navarini, L.; Amato, G.; Lazzeri, A. Utilization of coffee silverskin in the production of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) biopolymer-based thermoplastic biocomposites for food contact applications. Compos. Part A Appl. Sci. Manuf. 2021, 140, 1–10. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, R.; Wu, Y.; Xue, P. Additive manufacturing of wood flour/polyhydroxyalkanoates (PHA) fully bio-based composites based on micro-screw extrusion system. Mater. Des. 2021, 199, 1–14. [Google Scholar] [CrossRef]
- Wu, C.S.; Wu, D.Y.; Wang, S.S. Biodegradable Composite Nanofiber Containing Fish-Scale Extracts. ACS Appl. Bio Mater. 2021, 4, 462–469. [Google Scholar] [CrossRef]
- de Almeida Neto, G.R.; Barcelos, M.V.; Ribeiro, M.E.A.; Folly, M.M.; Rodríguez, R.J.S. Formulation and characterization of a novel PHBV nanocomposite for bone defect filling and infection treatment. Mater. Sci. Eng. C 2019, 104, 1–13. [Google Scholar] [CrossRef]
- Shahi, S.; Karbasi, S.; Ahmadi, T.; Naeimi, F.; Goodarzi, V.; Ebrahimi-Barough, S. Evaluation of physical, mechanical and biological properties of β-tri-calcium phosphate/Poly-3-hydroxybutyrate nano composite scaffold for bone tissue engineering application. Mater. Technol. 2021, 36, 237–249. [Google Scholar] [CrossRef]
- Koyama, R.; Kuboki, T.; Ding, W.D.; Adhikary, K.B.; Chen, N.; Park, C.B. Extrusion foaming of cellulose fiber reinforced polylactic acid biocomposites. In Proceedings of the Annual Technical Conference—ANTEC, Boston, MA, USA, 1–5 May 2011. [Google Scholar]
- Cho, S.Y.; Park, H.H.; Yun, Y.S.; Jin, H.J. Influence of cellulose nanofibers on the morphology and physical properties of poly(lactic acid) foaming by supercritical carbon dioxide. Macromol. Res. 2013, 21, 529–533. [Google Scholar] [CrossRef]
- Oluwabunmi, K.; D’Souza, N.A.; Zhao, W.; Choi, T.Y.; Theyson, T. Compostable, fully biobased foams using PLA and micro cellulose for zero energy buildings. Sci. Rep. 2020, 10, 1–20. [Google Scholar] [CrossRef]
- Matuana, L.M.; Faruk, O. Effect of gas saturation conditions on the expansion ratio of microcellular poly (lactic acid)/wood-flour composites. Express Polym. Lett. 2010, 4, 621–631. [Google Scholar] [CrossRef]
- Rizvi, R.; Cochrane, B.; Naguib, H.; Lee, P.C. Fabrication and characterization of melt-blended polylactide-chitin composites and their foams. J. Cell. Plast. 2011, 47, 283–300. [Google Scholar] [CrossRef]
- Ding, W.D.; Kuo, P.Y.; Kuboki, T.; Park, C.B.; Sain, M. Foaming of cellulose fiber reinforced polylactic acid composites: The effect of cellulose fiber type. In Proceedings of the Annual Technical Conference—ANTEC, Cincinnati, OH, USA, 22–24 April 2013. [Google Scholar]
- Neagu, R.C.; Cuénoud, M.; Berthold, F.; Bourban, P.E.; Gamstedt, E.K.; Lindström, M.; Månson, J.A.E. The potential of wood fibers as reinforcement in cellular biopolymers. J. Cell. Plast. 2012, 48, 71–103. [Google Scholar] [CrossRef]
- Matuana, L.M.; Diaz, C.A. Strategy to produce microcellular foamed poly(lactic acid)/wood-flour composites in a continuous extrusion process. Ind. Eng. Chem. Res. 2013, 52, 12032–12040. [Google Scholar] [CrossRef]
- Bergeret, A.; Benezet, J.C. Natural fibre-reinforced biofoams. Int. J. Polym. Sci. 2011, 2011, 1–14. [Google Scholar] [CrossRef]
- Pilla, S.; Kramschuster, A.; Lee, J.; Auer, G.K.; Gong, S.; Turng, L.S. Microcellular and solid polylactide-flax fiber composites. Compos. Interfaces 2009, 16, 869–890. [Google Scholar] [CrossRef]
- Zafar, M.T.; Zarrinbakhsh, N.; Mohanty, A.K.; Misra, M.; Ghosh, A.K. Biocomposites based on poly(Lactic acid)/willow-fiber and their injection moulded microcellular foams. Express Polym. Lett. 2016, 10, 176–186. [Google Scholar] [CrossRef]
- Noorani, R. 3D Printing Technology, Applications, and Selection, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Horvath, J. Mastering 3D Printing, 1st ed.; Apress: New York, NY, USA, 2014. [Google Scholar]
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A Review of 3D Printing Technology for Medical Applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Kikuchi, M.; Suetsugu, Y.; Tanaka, J.; Akao, M. Preparation and mechanical properties of calcium phosphate/copoly-L-lactide composites. J. Mater. Sci. Mater. Med. 1997, 8, 361–364. [Google Scholar] [CrossRef]
- Niaza, K.V.; Senatov, F.S.; Kaloshkin, S.D.; Maksimkin, A.V.; Chukov, D.I. 3D-printed scaffolds based on PLA/HA nanocomposites for trabecular bone reconstruction. In Proceedings of the Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2016; pp. 1–5. [Google Scholar]
- Gregor, A.; Filová, E.; Novák, M.; Kronek, J.; Chlup, H.; Buzgo, M.; Blahnová, V.; Lukášová, V.; Bartoš, M.; Nečas, A.; et al. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 2017, 11, 1–21. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, Q.; Li, J.; Tao, K.; Xue, L.; Yan, Q. Synergistic effect between expandable graphite and ammonium polyphosphate on flame retarded polylactide. Polym. Degrad. Stab. 2011, 96, 183–189. [Google Scholar] [CrossRef]
- Ke, C.H.; Li, J.; Fang, K.Y.; Zhu, Q.L.; Zhu, J.; Yan, Q.; Wang, Y.Z. Synergistic effect between a novel hyperbranched charring agent and ammonium polyphosphate on the flame retardant and anti-dripping properties of polylactide. Polym. Degrad. Stab. 2010, 95, 763–770. [Google Scholar] [CrossRef]
- Tang, G.; Zhang, R.; Wang, X.; Wang, B.; Song, L.; Hu, Y.; Gong, X. Enhancement of flame retardant performance of bio-based polylactic acid composites with the incorporation of aluminum hypophosphite and expanded graphite. J. Macromol. Sci. Part A Pure Appl. Chem. 2013, 50, 255–269. [Google Scholar] [CrossRef]
- Wang, K.; Wang, J.; Zhao, D.; Zhai, W. Preparation of microcellular poly(lactic acid) composites foams with improved flame retardancy. J. Cell. Plast. 2017, 53, 45–63. [Google Scholar] [CrossRef]
- Wang, J.; Ren, Q.; Zheng, W.; Zhai, W. Improved flame-retardant properties of poly(lactic acid) foams using starch as a natural charring agent. Ind. Eng. Chem. Res. 2014, 53, 1422–1430. [Google Scholar] [CrossRef]
- Vadas, D.; Igricz, T.; Sarazin, J.; Bourbigot, S.; Marosi, G.; Bocz, K. Flame retardancy of microcellular poly(lactic acid) foams prepared by supercritical CO2-assisted extrusion. Polym. Degrad. Stab. 2018, 153, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Wu, N.; Yu, J.; Lang, W.; Ma, X.; Yang, Y. Flame retardancy and toughness of poly(lactic acid)/GNR/SiAHP composites. Polymers 2019, 11, 1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Zhang, L.; Chai, W.; Yin, N.; Semple, K.; Li, L.; Zhang, W.; Dai, C. Enhancement of flame retardancy and mechanical properties of polylactic acid with a biodegradable fire-retardant filler system based on bamboo charcoal. Polymers 2021, 13, 2167. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hany, R.; Böhlen, C.; Geiger, T.; Schmid, M.; Zinn, M. Toward non-toxic antifouling: Synthesis of hydroxy-cinnamic acid-, sulfate-, and zosteric acid-labeled poly[3-hydroxyalkanoates]. Biomacromolecules 2004, 5, 1452–1456. [Google Scholar] [CrossRef]
- Gonta, S.; Savenkova, L.; Krallish, I.; Kirilova, E. Antimicrobial Activity of PHB Based Polymeric Compositions. Environ. Eng. Manag. J. 2012, 11, 99–104. [Google Scholar]
- Kwiecień, I.; Adamus, G.; Bartkowiak, A.; Kowalczuk, M. Synthesis and structural characterization at the molecular level of oligo(3-hydroxybutyrate) conjugates with antimicrobial agents designed for food packaging materials. Des. Monomers Polym. 2014, 17, 311–321. [Google Scholar] [CrossRef] [Green Version]
- Xavier, J.R.; Babusha, S.T.; George, J.; Ramana, K.V. Material Properties and Antimicrobial Activity of Polyhydroxybutyrate (PHB) Films Incorporated with Vanillin. Appl. Biochem. Biotechnol. 2015, 176, 1498–1510. [Google Scholar] [CrossRef] [PubMed]


















| Bioplastic | Company | Country | Commercial Name | Applications/Notes |
|---|---|---|---|---|
| PLA | NatureWorks LLC | USA | -Ingeo™ 8000 series, 8052D. -Ingeo™ 7000 series, 7001D TDS and 7032D TDS. -Ingeo™ 6000 series, 6060D TDS, 6201D TDS, 6202D TDS, 6204D TDS, 6251D TDS, 6252D TDS,6302D TDS, 6400D TDS, 6751D TDS, 6752D TDS. -Ingeo™ 4000 series, 4032D TDS, 4043D TDS, 4060D TDS. -Ingeo™ 3000 series, 3001D SDS, 3052D SDS, 3251D SDS, 3801X SDS. -Ingeo™ 2000 series, 2003D TDS. -Ingeo™ 3D850. | -Foams. -Bottles (7001D TDS and 7032D TDS). -Nonwovens (6060D TDS, 6202D TDS, 6251D TDS, 6252D TDS, 6302D TDS, 6751D TDS, 6752D TDS). -Apparel (6201D TDS, 6204D TDS). -Home textiles (knitted and woven) (6201D TDS, 6202D TDS, 6204D TDS, 6400D TDS). -Cards, folded cartons and films (4032D TDS, 4043D TDS, 4060D TDS). -3D printing (3D850, 4043D). -Durable goods (3001D SDS, 3052D SDS, 3251D SDS, 3801X SDS). -Service war (2003D TDS, 3001D TDS, 3052D TDS, 3251D TDS). -Food packaging (2003D TDS). |
| PLA, PDLA | Total Corbion PLA | The Netherlands | -Luminy® PLA (L175, L130, L105, LX575, LX530, LX175, LX975, LX930, D120, D070) | -High heat PLA for demanding applications (L175, L130, L105, LX575, LX530). -Standard PLA for general purpose applications (LX175). -Low heat PLA for usage in seal layers (LX975, LX930). -PDLA utilized to produce full stereocomplex compounds or used as a nucleating agent (D120, D070). |
| PLLA | Purac | The Netherlands | -Purasorb® (PL 18, PL 24, PL 32, PL 38, PL 49, PL 65, PL 10). | -Medical equipment. |
| PLLA, scPLA | Tejin | Japan | -Biofront® (HL L201 (PLLA), J20 (scPLA), J201 (scPLA)). | -Eyeglass frames, sheets, films, fibers, injection molding, medical care, automobiles, electronics, construction and packages. |
| Amorphous PLA | Toyobo | Japan | -Vyloecol series (BE-400, BE-600, BE-910, HYD-306, BE-450, BE-410, HYD-006). | -Adhesive, paint, printing ink. -BE-400 in the form of pellet, used as agent for different coating and is a general-purpose resin. -BE-600 in the form of sheet, used as anchor coating for printing ink and vapor deposition films. |
| PDLA | Purac | The Netherlands | -Purasorb® -PD 24 -PD 38 -Purapol® | -Medical equipment (Purasorb®). -Nucleating agents for PLLA (Purapol®). |
| PDLLA | Evonik | Germany | -R 202 H -R 203 H -R 202 S -R 203 S -R 205 S -R 207 S | -Medical equipment (R 207 S) and drug delivery. |
| PLDLLA | Purac | The Netherlands | -Purasorb® (PLDL 8038, PLDL 8058, PLDL 7028, PLDL 7038, PLDL 7060). | -Medical equipment. |
| PLDA | Purac | The Netherlands | -Purasorb® (PLD 9620, PLD 9655). | -Medical equipment. |
| PLA (Nature- Works)/ copolyester blend | FKuR Kunststoff GmbH | Germany | -Bio-flex® (Bio-flex® A4100 CL, Bio-flex® F 1110, Bio-flex® F 1130, Bio-flex® F 1137, Bio-flex® F 2110, Bio-flex® F 2201 CL, Bio-flex® F 6510, Bio-flex® F 6513, Bio-flex® F 6611, Bio-flex® S 5630, Bio-flex® S 6540, Bio-flex® S 9533). | -Flower wrapping, blown film extrusion and packaging (Bio-flex® A4100 CL). -Waste bag, air pillow and carrier bag (Bio-flex® F 1130). -Shopping bags (Bio-flex® F 1137). -Waste bag, netting and deep freeze packaging (Bio-flex® F 2110). -Film (Bio-flex® F 2201 CL). -Multi-layer films (Bio-flex® A4100 CL and Bio-flex® F 2201 CL). -Straws, mugs and ball pen (Bio-flex® F 6510). -Thermoforming (Bio-flex® F 6611). -Injection molding (Bio-flex® F 6513). -Thermoformed inlay (Bio-flex® S 5630). -Cosmetic jars (Bio-flex® S 6540 and Bio-flex® S 9533). |
| PLA/polyether copolymer | Toray Industries | Japan | -Ecodear® (V554R10, V554X51, V554X52, V751X52, V751X53, V911X51). | -Bags, films, fibers, packaging, personal care, accessories, office supplies and electronics. |
| Bioplastic | Company | Country | Commercial Name | Applications/Notes |
|---|---|---|---|---|
| PHB | Mitsubishi Gas Chemical Company Inc. | Japan | -Biogreen® | -Cast films and natural latex gloves. |
| PHB | PHB Industrial S/A | Brazil | -Biocycle™ (B1000, B18BC-1, B189C-1, B189D-1) | -Medical devices, films and disposables). |
| PHB and PHBV | Biomer Inc. | Germany | -Biomer®300 (P300E, P300F) | -Extrusion (P300E) -Extrusion and food contact (P300F). |
| PHBV and PHBV/PLA | Tianan Biologic, Ningbo | China | -Enmat™ (Y1000, Y1010, Y1000P, Y3000, Y3000P, F9000P). | -Thermoforming, nonwovens and fiber, injection molding, extrusion and water treatment. |
| P4HB | Tepha, Inc. | USA | -TephaFLEX® | -Surgical absorbable films and sutures. |
| PHBHHx | Kaneka Co. | Japan | -Kaneka PHBH -Aonilex® | -Foams, fibers, interior automotive materials, electrical equipment, sheets and injection molding. -Containers, bottles, interior automotive materials and electrical equipment. |
| PHBHHx | Danimer Scientific | USA | -Nodax™ | -Coating, laminates, non-woven Fibers and packaging. |
| P3HB4HB | Tianjin Green Bio- Science Co./DSM | China/The netherlands | -GreenBio® | -Films for wrapping, laminating film, fresh film, heat shrinkable film, garbage bags, food packaging, shopping and gift bags. |
| Several PHAs | CJ CheilJedang Corporation | South Korea | -CJ PHA® | -Rigid packaging, 3D printing, paper coating, agriculture and flexible packaging. |
| Several PHAs | Alterra Holdings | USA | -TerraBio® | -Paper coating, packaging, utensils, straws and disposals. |
| Properties/Applications | Ingeo™ 2003D | Ingeo™ 3052D | Ingeo™ 3801X | ASTM Method |
|---|---|---|---|---|
| Specific Gravity | 1.24 | 1.24 | 1.25 | D792 |
| Melt Flow Rate, g/10 min (210 °C, 2.16 Kg) | 6 | 14 | 8 | D1238 |
| Relative viscosity | NP | 3.3 | 3.1 | - |
| Clarity | Transparent | Transparent | Opaque | - |
| Tensile strength at break, psi (MPa) | 7700 (53) | NP | NP | D882 |
| Tensile yield strength, psi (MPa) | 8700 (60) | 9000 (62) | 3750 (25.9) | D882 |
| Tensile modulus, Kpsi (GPa) | 500 (3.5) | NP | 432 (2.98) | D882 |
| Flexural Strength, psi (MPa) | NP | 15,700 (108) | 6400 (44) | D790 |
| Flexural Modulus, psi (MPa) | NP | 515,000 (3600) | 413,000 (2850) | D790 |
| Tensile elongation, % | 6.0 | 3.5 | 8.1 | D882 |
| Notched Izod impact, ft-lb/in (J/m) | 0.3 (16) | 0.3 (16) | 2.7 (144) | D256 |
| Heat distortion temperature (°C) | 55 | 55 | 65 (at 66 psi) 140 (at 16.5 psi) | E2092 |
| Melt temperature (°C) | 210 | 200 | 188 | - |
| Crystallinity melt temperature (°C) | NP | 145–160 | 155–170 | D3418 |
| Glass transition temperature (°C) | NP | 55–60 | 45 | D3418 |
| Applications | -Designed for fresh food packaging and food service ware applications such as: dairy containers, food service ware, transparent food containers, hinged ware and cold drink cups. | -Designed for injection molding applications that require clarity with heat deflection temperatures lower than 49 °C. -Applications include: cutlery, cups, plates and saucers as well as outdoor novelties. | -Designed for non-food contact injection molding applications that require opaque molded parts with heat deflection temperatures between 65 °C and 140 °C. | - |
| Polymer | Tensile Strength (MPa) | Tensile Modulus (GPa) | Percentage Elongation | Notched Izod (J/m) |
|---|---|---|---|---|
| PLLA | 59 | 3.8 | 4–7 | 26 |
| PS | 45 | 3.2 | 3 | 21 |
| PET | 57 | 2.8–4.1 | 300 | 59 |
| Plasticizer | Plasticizer’s Concentration (wt.%) | PLA’s Type and Reference | Tensile Strength (MPa) | Young’s Modulus (MPa) | Percentage Elongation | Charpy Impact, (MJ/mm2) | Application | Comments |
|---|---|---|---|---|---|---|---|---|
| Lactide | - 25.5% - 19.2% - 17.3% - 1.3% | PLA, in the form of films [58]. | - 16.8 - 29.2 - 15.8 - 51.7 | - 232 - 658 - 820 - 1993 | - 546% - 536% - 288% - 3.00% | - | General Packaging. | Degradation increased with increasing the content of plasticizer. |
| PEG | - 0% - PEG 1500 (2.5%) - PEG 1500 (5%) - PEG 1500 (10%) | PLA (92% L-lactide and 8% meso-lactide) [59]. | - 58 - 50 - 44 - 28 | - 3800 - 3200 - 2500 - 1200 | - 3% - 4% - 7% - 40% | - 32 b - 29 - 31 - 80 | Applications demanding higher impact resistance and flexibility. | - |
| - 0% - m-PEG (10%) - m-PEG (20%) - PEG 400 (10%) - PEG 400 (20%) | PLA (92% L-lactide and 8% meso-lactide) [60]. | - | - 2050 - 1571 - 1124 - 1488 - 976 | - 9% - 18% - 142% - 26% - 160% | - | - | Biocompatible plasticizers. | |
| - 0% - PEG 400 (5%) - PEG 400 (10%) - PEG 400 (12.5%) - PEG 400 (15%) - PEG 400 (20%) - PEG 1500 (5%) - PEG 1500 (10%) - PEG 1500 (12.5%) - PEG 1500 (15%) - PEG 1500 (20%) - PEG 10,000 (5%) - PEG 10,000 (10%) - PEG 10,000 (15%) - PEG 10,000 (20%) | PLA [61]. | - 66.0 - 41.6 - 32.5 - 18.7 - 19.1 - 15.6 - 52.3 - 46.6 - 18.5 - 23.6 - 21.8 - 53.9 - 48.5 - 42.3 - 22.1 | - 3300 - 2500 - 1200 - 500 - 600 - 500 - 2900 - 2800 - 700 - 800 - 600 - 2800 - 2800 - 2500 - 700 | - 1.8% - 1.6% - 140% - 115% - 88% - 71% - 3.5% - 5.0% - 194% - 216% - 235% - 2.4% - 2.8% - 3.5% - 130% | - | Medical, personal care and food packaging applications. | - | |
| - 0% - PEG 200 (10%) - PEG 400 (10%) - PEG 400 (20%) - PEG 1000 (10%) - PEG 1000 (20%) - PEG 1000 (30%) | PLA (92% L-lactide and 8% D-lactide) [62]. | - 64.0 - 30.0 - 39.0 - 16.0 - 39.6 - 21.6 - 4.70 | - 2840 - 1700 - 1920 - 630 - 1970 - 290 - 420 | - 3.0% - 2.0% - 2.40% - 21.2% - 2.7% - 200% - 1.50% | - | Food packaging Applications. | The plasticizers used are food packaging approved. | |
| - 0% -PEG 600 (5.0%) - PEG 600 (7.50%) - PEG 600 (10.0%) - PEG 600 (12.50%) | PLA [63]. | - 25.5 - 19.3 - 17.5 - 18.5 - 19.7 | - | - 64% - 67.0% - 360% - 427% - 622% | - | - | PPGs increased the ability of PLA to plastically deform in a more efficient way than PEG. | |
| Glucose monoesters | - 0% - 2.5% - 5% - 10% | PLA (92% L-lactide and 8% meso-lactide) [59]. | - 58 - 52 - 47 - 39 | - 3800 - 3200 - 3000 - 2550 | - 3% - 5% - 6% - 12% | - 32 b - 23 b - 24 b - 18 b | Applications requiring higher impact resistance and flexibility. | - |
| Partial fatty acid esters | - 0% - 2.5% - 5% - 10% | - 58 - 52 - 48 - 44 | - 3800 - 3450 - 3100 - 3000 | - 3% - 14% - 7% - 8% | - 32 b - 25 b - 28 b - 22 b | |||
| Oligomeric lactic acid | - 0% - 10% - 20% | PLA (92% L-lactide and 8% meso-lactide) [60]. | - | - 2050 - 1256 - 744 | - 9% - 32% - 200% | - | - | Biocompatible Plasticizers. |
| ATBC | - 0% - 5% - 10% - 12.5% - 15% - 20% | PLA [61]. | - 66.0 - 53.4 - 50.1 - 17.7 - 21.3 - 23.1 | - 3300 - 3200 - 2900 - 100 - 100 - 100 | - 1.8% - 5.1% - 7.0% - 218% - 299% - 298% | - | Medical, personal care and food packaging applications. | ATBC is derived from naturally occurring citric acid. It is also non-toxic and has been approved for use in personal careand medical applications. |
| PBOH | - 0% - 10% - 20% - 30% | PLA (92% L-lactide and 8% D-lactide) [62]. | - 64.0 - 56.3 - 30.2 - 25.2 | - 2840 - 2350 - 350 - 300 | - 3.0% - 3.00% - 302.5% - 390% | - | Food packaging applications. | The plasticizers used are food packaging approved. |
| AGM | - 0% - 10% - 20% - 30% | - 64.0 - 52.1 - 27.1 - 17.9 | - 2840 - 2240 - 35.0 - 107.0 | - 3.0% - 32.0% - 335.0% - 320.0% | - | |||
| DBS | - 0% - 10% - 20% - 30% | - 64.0 - 39.2 - 23.1 - 18.3 | - 2840 - 2000 - 430.0 - 370.0 | - 3.0% - 2.30% - 269.0% - 333.0% | - | |||
| PPG | - 0% - PPG 425 (5.0%) - PPG 425(7.5%) - PPG 425 (10.0%) - PPG 425 (12.5%) - PPG 1000 (5.0%) - PPG 1000 (7.5%) - PPG 1000 (10.0%) - PPG 1000 (12.50%) | PLA [63]. | - 25.5 - 20.7 - 17.7 - 21.0 - 21.0 - 22.2 - 22.6 - 22.8 - 21.6 | - | - 64% - 19.0% - 107% - 524% - 702% - 44% - 329% - 473% - 496% | - | - | PPGs increased the ability of the used PLA to plastically deform in a more efficient way than PEG. |
| PEO | - 0% - 5% - 10% - 15% - 20% | PLLA [64]. | - 58 - 54.5 - 54 - 35 - 24 | - | - 7% - 7% - 11% - 50% - >500% | - | Nerve guides, barriers to tissue adhesion and orbital floor reconstruction. | The initial degradation of PLLA/PEO was more rapid than the neat PLLA and degradation rate increased with increasing the PEO content. |
| Triethyl citrate c | - 0% - 10% - 20% - 30% | PLA, in the form of films [65]. | - 51.7 - 28.1 - 12.6 - 7.2 | - | - 7% - 21.3% - 382% - 610% | - | - | Citrates with low molecular weight has increased the rate of eczematic degradation while the degradation rate has decreased when high molecular weight citrates were used. |
| Tributyl citrate c | - 0% - 10% - 20% | - 51.7 - 22.4 - 7.1 | - | - 7% - 6.2% - 350% | - | - | ||
| Acetyl triethyl citrate c | - 0% - 10% - 20% - 30% | - 51.7 - 34.5 - 9.6 - 7.6 | - | - 7% - 10% - 320% - 228% | - | - | ||
| Acetyl tributyl citrate c | - 0% - 10% - 20% | - 51.7 - 17.7 - 9.2 | - | - 7% - 2.3% - 420% | - | - | ||
| EVA | - 0% - 10% - 30% - 50% - 70% - 90% | PLLA [66]. | - 55.89 - 45.11 - 32.36 - 16.67 - 16.67 - 13.73 | - 2853.73 - 1804.42 - 1314.09 - 1274.86 - 1284.67 - 627.62 | - 4.5% - 4.7% - 6.9% - 10.2% - 9.0% - 208.9% | - | - | - |
| Limonene | - 0% - 20% - 20% with 1% L101 as a free radical initiator. | PLA [68]. | - 60.60 - 15.80 - 17.20 | - 2300 - 1000 - 1200 | - 7.40% - 117.50% - 120.20% | - 2.70 - 5.50 - 5.80 | Transparent packaging applications. | Biobased plasticizers |
| Myrcene | - 20% - 20% with 1% L101. | - 18.70 - 24.80 | - 1900 - 1700 | - 62.70% - 45.00% | - 12.50 - 4.90 | Opaque packaging applications. | ||
| Ozonized soybean oil | - 0% - 5% - 10% - 15% | PLA [69]. | - 54.00 - 46.00 - 41.00 - 34.00 | - 1500 - 1520 - 1400 - 1450 | - 5.50% - 6.50% - 10.50% - 8.50% | - 2.00 - 2.10 - 2.20 - 2.50 | Applications requiring flexibility and toughness. | Biobased plasticizer |
| ECO | - 0% - 2.50% - 5.00% - 7.50% - 10.00% | PLA [70]. | - 44.00 - 42.00 - 39.00 - 36.00 - 34.00 | - 3120 - 3050 - 3070 - 2950 - 2930 | - 8.50% - 17.00% - 33.50% - 58.00% - 64.00% | - | Packaging applications. | Biobased plasticizer |
| DBM | - 0% - 7.00% - 12.00% | PLA [71]. | - 19.00 - 45.00 - 15.00 | - 1672 - 2245 - 1533 | - 1.30% - 2.80% - 3.00% | - | Green alternatives for the production of PLAbased flexible films. | Biodegradable plasticizers. |
| DBF | - 7.00% - 12.00% | - 30.00 - 10.00 | - 584 - 279 | - 111.90% - 210.00% | - |
| Impact Modifier and Reference/s | Company | Application | Features | Comments |
|---|---|---|---|---|
| -Sukano® PLA im S550 [80]. | -Sukano Co. | -Transparent applications such as packaging. | -Highly cost effective. -At a 4% concentration, the impact resistance of PLA can be enhanced by a factor of 10. | -Compostable and can be used with FDA approved, biodegradable PLA. |
| -OnCap™BIO Impact T [80,81]. | -PolyOne. | -Transparent applications such as packaging. | -Improves the impact resistance of PLA while maintaining its transparency. -Improves tear resistance. | -Designed to improve the applicability of biodegradable and bio-derived polymers. -If used at prescribed loadings, it does not limit the biodegradability or food contact use of the PLA compound. |
| -Biomax® Strong 100 and 120 [80,82,83]. | -DuPont Co. | -Packaging including food packaging and industrial applications. | -Enhance PLA’s toughness and impact strength with minimal effect on transparency. -At a 2% concentration, the impact resistance of PLA can be substantially enhanced. | -Biomax® Strong 100 is designed for non-food applications, while Biomax® Strong 120 is designed for food packaging applications. |
| -Paraloid™ BPM-500, 515 and 520 [80,84]. | -Dow Chem. Co. | -Packaging, electronics, medical, injection molding and automobile applications. | -Improve the mechanical properties of PLA while maintaining its transparency. -Improvement in flexibility, slitting and cutting. -The addition of only 3% concertation can lead to an improvement in the impact Properties of PLA. -Paraloid™ BPM-520 significantly enhances the toughness of PLA and PLA blends with minimal effect on stiffness and heat distortion temperature. Moreover, it features excellent room temperature impact performance. It also provides excellent surface finish and exceptional combinations of color ability and impact strength in opaque PLA and PLA blends applications. | -Paraloid™ BPM-515 is more efficient and FDA approved for up to 5% in food contact resins. |
| -Biostrength™ 130, 150, 280 and 200 [80]. | -Arkema. | -Packaging, injection molding, transparent and opaque applications. | -Biostrength™ 130 and 200 are intended to improve PLA’s toughness while maintaining its transparency. -Biostrength™ 150 is used in opaque and durable injection molding applications. -Biostrength™ 280 is utilized in applications that require high transparency and toughness. | - |
| Blend | Concentration (wt.%) | Tensile Strength (MPa) | Young’s Modulus (MPa) | Percentage Elongation | Charpy Break Energy/ (Notched Izod Break Energy) | Toughness | Application and/or Reference |
|---|---|---|---|---|---|---|---|
| PLLA/PHBV | - 100/0 - 80/20 - 60/40 - 40/60 - 20/80 | - 71.00 - 54.00 - 39.00 - 29.00 - 24.00 | - 2415 - 2083 - 1552 - 1258 - 1076 | - 5.60% - 6.20% - 6.70% - 4.10% - 6.90% | - | - | Biomedical applications [91]. |
| - 100/0 - 80/20 - 60/40 - 40/60 - 20/80 | - 29.70 - 27.80 - 22.20 - 25.10 - 24.90 | - 2031 - 1761 - 1580 - 1301 - 1631 | - | - | - | Biomedical applications (surgical implants, sutures and drug delivery) [92]. | |
| - 100/0 - 0/100 - 50/50 - 40/60 - 30/70 | - 62.00 - 22.00 - 39.00 - 38.00 - 33.00 | - 2700 - 900 - 1800 - 1700 - 1300 | - 8.00% - 13.00% - 7.90% - 7.70% - 7.60% | - (29.00) J/m - (49.00) J/m - (28.00) J/m - (27.00) J/m - (27.00) J/m | - | [98] | |
| - 100/0 - 100/0 with 5% Lapol as a plasticizer - 100/0 with 7% Lapol - 75/25 - 75/25 with 5% Lapol - 75/25 with 7% Lapol | - 42.00 - 14.00 - 16.00 - 16.00 - 13.00 - 15.00 | - 1400 - 1450 - 1200 - 1270 - 1150 - 1120 | - 7.20% - 14.40% - 13.70% - 7.10% - 15.50% - 15.10% | - | - | Single use applications such as food packaging [108]. | |
| - 50/50 - 50/50 with 2% PLLA-PEG-PLLA triblock - 50/50 with 5% PLLA-PEG-PLLA triblock - 50/50 with 2% PEG-PLLA diblock - 50/50 with 5% PEG-PLLA diblock - 50/50 with 2% PVAc - 50/50 with 5% PVAc | - 49.60 - 69.80 - 38.50 - 65.50 - 32.70 - 41.50 - 43.40 | - 2737 - 2291 - 1921 - 2581 - 2155 - 1850 - 2087 | - 4.40% - 5.10% - 5.10% - 4.40% - 5.90% - 4.80% - 4.90% | - | - 5.90 N.mm - 9.20 N.mm - 7.90 N.mm - 6.50 N.mm - 8.30 N.mm - 8.40 N.mm - 6.60 N.mm | [93] | |
| PLA/PHA | - 100/0 - 90/10 - 80/20 - 70/30 | - 55.00 - 50.00 - 37.00 - 35.00 | - | - | - 0.052 J - 0.081 J - 0.137 J - 0.161 J | - | Biodegradable blends for applications that require improved impact toughness (impact toughness similar to that of PS and ABS) [94]. |
| PLA/ePHA | - 90/10 - 80/20 - 70/30 | - 53.00 - 48.00 - 37.00 | - | - | - 0.089 J - 0.169 J - 0.260 J | - | |
| PLA/Nodax™ | - 100/0 - 90/10 - 80/20 - 60/40 - 40/60 | - | - | - | - | - 0.30 N.m - 1.90 N.m - 1.40 N.m - 0.30 N.m - 0.20 N.m | Ductile and tough plastics applications [95]. |
| - 100/0 - 90/10 - 85/15 - 80/20 - 75/25 - 81/14 with 5% oligoNodax™-b-PLLA diblock copolymer (81/14/5 wt.%) | - | - | - | - (22.00) J/m - (27.00) J/m - (44.00) J/m - (43.00) J/m - (35.00) J/m - (44.00) J/m | - | Ductile and tough plastics applications [96]. | |
| PLA/PHB | - 100/0 - 25/75 - 50/50 - 75/25 | - 26.00 - 2.50 - 8.00 - 32.50 | - | - 16.00% - 6.00% - 11.00% - 17.50% | - | - | Applications that require high biodegradation rate [43]. |
| - 100/0 - 83/17 - 57/43 - 50/50 - 43/57 - 29/71 - 17/83 - 0/100 | - 50.00 - 47.00 - 40.00 - 37.50 - 35.50 - 34.50 - 27.00 - 26.50 - 26.00 | - 7.25% - 3.50% - 3.85% - 3.40% - 3.20% - 3.20% - 3.00% - 2.70% - 4.35% | - | - | Environmentally friendly packaging [110]. | ||
| - 100/0 - 75/25 - 63.75/21.25 with 15% ATBC - 60/20 with 5% CNCs and 15% ATBC - 60/20 with 5% CNCs-m and 15% ATBC | - 46.90 - 38.20 - 40.20 - 27.30 - 28.20 | - 1240 - 1810 - 550 - 570 - 490 | - 41.10% - 13.00% - 90.10% - 27.40% 147.70% | - | - | Biodegradable packaging [103]. | |
| PLA/aPHB | - 100/0 - 98/2 - 95/5 - 90/10 - 85/15 - 80/20 | - 49.30 - NP a - 46.00 - 43.50 - 38.30 - 30.50 | - 3500 - NP - 3380 - 3240 - 2910 - 2750 | - 6.00% - NP - 6.00% - 7.00% - 9.00% - 21.00% | - 50.00 b KJ/m2 - 60.00 b KJ/m2 - 60.00 b KJ/m2 - 61.00 b KJ/m2 - 103.00 b KJ/m2 - 118.00 b KJ/m2 | - | Packaging, especially for food [97]. |
| PLA/PHBHHx | - 100/0 - 80/20 - 60/40 - 50/50 - 40/60 - 20/80 - 0/100 | - 36.40 - 29.50 - 33.50 - 22.10 - 27.70 - 23.60 - 17.60 | - 1390 - 1320 - 1240 - 910 - 1250 - 590 - 370 | - 13.80% - 99.60% - 7.68% - 7.26% - 11.50% - 83.50% - 19.30% | - | - | Biomedical applications, such as artificial vascular graft [100]. |
| - 100/0 - 90/10 - 80/20 - 60/40 - 0/100 | - 62.20 - 54.10 - 45.30 - 40.10 - 21.60 | - 1603.00 - 1416.00 - 1265.00 - 1093.00 - 309.00 | - 3.60% - 7.60% - 113.10% - 37.60% - 524.80% | - | - 3.20 MPa - 4.00 MPa - 68.70 MPa - 20.40 MPa - 160.60 MPa | Food packaging and flexible films [102]. |
| Blend | Concentration (wt.%) | Tensile Strength (MPa) | Young’s Modulus (MPa) | Percentage Elongation | Charpy Impact/ (Izod Impact) | Application and/or Reference |
|---|---|---|---|---|---|---|
| PLA/PCL | - 100/0 - 80/20 - 60/40 - 40/60 - 20/80 - 80/20 with 2% TPP as a coupling agent - 60/40 with 2% TPP - 40/60 with 2% TPP - 20/80 with 2% TPP | - 48.26 - 44.19 - 19.37 - 18.61 - 20.13 - 33.09 - 23.58 - 11.44 - 17.23 | - 2275.26 - 584.67 - 751.52 - 164.09 - 111.69 - 1013.52 - 710.16 - 344.04 - 197.87 | - 3.0% - 28.0% - 5.00% - 23.0% - 440% - 127% - 7.00% - 3.00% - 560% | - | [77] |
| - 100/0 - 70/30 - 70/30 with 0.1 phr a Dicumyl peroxide - 70/30 with 0.2 phr Dicumyl peroxide - 70/30 with 0.3 phr Dicumyl peroxide | - 70 - 55 - 52 - 49 - 48 | - 1500 - 1300 - 1200 - 1150 - 1100 | - 11.0% - 20% - 35% - 160% - 149% | - (2.00) KJ/m2 - (3.80) KJ/m2 - (3.90) KJ/m2 - (4.00) KJ/m2 - (4.90) KJ/m2 | High performance applications [115]. | |
| - 100/0 - 95/5.0 b - 95/5.0 c - 95/5.0 d | - 45.13 - 58.62 - 52.21 - 44.49 | - 3729 - 3631 - 3422 - 3661 | - 2.06% - 3.12% - 2.90% - 2.32% | - | [119] | |
| - 0/100 - 20/80 - 30/70 - 40/60 - 60/40 - 70/30 - 80/20 - 100/0 | - | - 460 - 700 - 1220 - 1350 - 2030 - 2500 - 2940 - 3910 | - | - | Biomedical applications [120]. | |
| - 0/100 - 0/100 with 1% PDI as a compatibilizer - 90/10, linear PCL - 90/10, linear PCL with 1% PDI - 90/10, three-armed star shaped PCL - 90/10, three-armed star shaped PCL with 1% PDI - 90/10, four-armed star shaped PCL - 90/10, four-armed star shaped PCL with 1% PDI - 90/10, six-armed star shaped PCL - 90/10, six-armed star shaped PCL with 1% PDI | - | - 4600 - 4200 - 3400 - 3600 - 2100 - 2400 - 2250 - 2800 - 3450 - 3500 | - 4.00% - 2.30% - 6.40% - 6.43% - 8.20% - 8.00% - 8.40% - 8.37% - 8.60% - 8.65% | - (8.00) KJ/m2 - (11.50) KJ/m2 - (20.00) KJ/m2 - (17.00) KJ/m2 - (20.50) KJ/m2 - (17.50) KJ/m2 - (23.00) KJ/m2 - (21.00) KJ/m2 - (24.50) KJ/m2 - (21.00) KJ/m2 | Different daily and industrial applications such as, disposable products, biomedical products and food packaging [121]. | |
| - 100/0 - 80/20 - 70/30 - 60/40 | - 66.05 - 53.60 - 50.20 - 41.30 | - 1311 - 1233 - 1223 - 884 | - 8.21% - 476.70% - 514.60% - 664.70% | - | Applications requiring very high toughness properties [123]. | |
| PLLA/PCL | - 100/0 - 80/20 - 80/20 with 10% poly(L-lactide-co-ε-caprolactone) | - 60 - 30 - 40 | - 1300 - 1100 - 1100 | - 5.0% - 175.0% - 300% | - | [114] |
| - 100/0 - 80/20 - 64/16 with 20% poly(e-caprolactone/L-lactide) | - 35 - 31.0 - 11.0 | - 2530 - 2080 - 660 | - 1.6% - 9.60% - >100% | - 41.4 KJ/m2 at (−)20 °C and 49.2 KJ/m2 at 23 °C. -NP e - 5.3 KJ/m2 at (−)20 °C and 10.1 KJ/m2 at 23 °C. | Biomedical applications [111] | |
| - 70/30 - 70/30 with 4% triblock PLLA -PCL-PLLA as compatibilizing agent | - | - 1400 - 1400 | - 2.00% - 53.0% | - 1.1 KJ/m2 - 3.7 KJ/m2 | Biomedical applications [113]. | |
| - 100/0 - 80/20 - 60/40 - 50/50 - 80/20 with copolymer of ethylene oxide and propylene oxide surfactant - 60/40 with copolymer of ethylene oxide and propylene oxide surfactant - 50/50 with copolymer of ethylene oxide and propylene oxide surfactant | - 34.10 - 41.20 - 19.30 - 16.90 - 20.10 - 12.90 - 10.40 | - 19.8 - 20.7 - 10.7 - 8.10 9.50 - 4.70 - 6.60 | - 56.30% - 129.50% - 152.10% - 139.60% - 129.00% - 130.00% - 123.70% | - | Orthopedic and dental applications [118]. | |
| Triblock copolymer of PLA (85% L-lactide and 15% D-lactide), ε-CL and TMC | - 100/0 - 80/20 | - 56.8 - 36.0 | - | - | - (41.0) J/m - (293–520) J/m | Load bearing devices in biomedical applications [116]. |
| Blend | Concentration (wt.%) | Tensile Strength (MPa) | Yield Strength (MPa) | Young’s Modulus (MPa) | Percentage Elongation | Break Energy/ (Izod Break Energy) | Application and/or Reference |
|---|---|---|---|---|---|---|---|
| PLA/PPD | - 100/0 - 80/20 - 50/50 - 20/80 | - 25.30 - 15.60 - 5.30 - 5.00 | - | - 1400 - 1550 - 900 - 650 | - 14.5% - 55.0% - 3.00% - 4.00% | NP a | Medical applications [124]. |
| PLA/PPC | - 100/0 - 85/15 - 70/30 - 60/40 - 50/50 - 40/60 - 30/70 - 15/85 | - 59.0 - 45.0 - 42.0 - 28.0 - 26.0 - 25.0 - 21.0 - 14.0 | - 59.0 - 49.0 - 46.0 - 41.0 - 37.0 - 36.0 - 32.0 - 24.0 | - 3150 - 2450 - 2150 - 2050 - 1750 - 1400 - 1050 - 800 | - | - 2.00 b J/cm2 - 5.00 b J/cm2 - 14.00 b J/cm2 - 55.00 b J/cm2 - 84.00 b J/cm2 - 75.00 b J/cm2 - 72.00 b J/cm2 - 69.00 b J/cm2 | [125] |
| PLA/PBAT | - 100/0 - 95/5 - 90/10 - 85/15 - 80/20 | - 63.00 - 58.00 - 55.00 - 51.00 - 47.00 | - | - 3200 - 2900 - 2850 - 2700 - 2600 | - | - (2.70) KJ/m2 - (2.75) KJ/m2 - (2.90) KJ/m2 - (3.50) KJ/m2 - (4.40) KJ/m2 | Applications requiring increased toughness while maintaining degradability [127]. |
| PLA/Bionolle (B1001) c | - 100/0 - 95/5 - 90/10 - 80/20 - 70/30 - 60/40 - 50/50 | - 36.00 - 32.00 - 36.00 - 24.00 - 28.00 - 26.00 - 24.00 | - | - 2481 - 2471 - 2158 - 1766 - 1704 - 1468 - 1268 | - 2.00% - 1.70% - 2.40% - 5.00% - 4.00% - 5.00% - 4.20% | - | Biomedical and food applications [128]. |
| PLA/Bionolle (B3010) c | - 95/5 - 90/10 - 80/20 - 70/30 - 60/40 - 50/50 | - 27.00 - 31.00 - 26.00 - 24.00 - 22.00 - 19.00 | - | - 2389 - 2292 - 1836 - 1620 - 1359 - 1071 | - 1.50% - 1.80% - 2.20% - 2.40% - 8.20% - 3.30% | - | |
| PLLA/PTAT | - 100/0 - 75/25 - 50/50 - 25/75 | - 28.12 - 24.62 - 7.11 - 11.11 | - | - | - 19.33% - 97.00% - 34.00% - 285.33% | - | Medical applications, tissue engineering and drug delivery [126]. |
| PLLA/PBSL | - 100/0 - 99/1 - 95/5 - 90/10 - 80/20 - 99/1 with 10% RKM as a plasticizer - 95/5 with 10% RKM - 90/10 with 10% RKM - 80/20 with 10% RKM - 99/1 with 20% RKM - 95/5 with 20% RKM - 90/10 with 20% RKM - 80/20 with 20% RKM | - 63.00 - 61.00 - 62.00 - 55.00 - 51.50 - 35.00 - 29.00 - 28.00 - 30.00 - 22.00 - 21.00 - 21.00 - 21.00 | - | - 2900 - 2800 - 2650 - 2450 - 2350 - 1900 - 1150 - 1000 - 1250 - 600 - 650 - 700 - 700 | - 2.00% - 3.00% - 55.00% - 160% - 120% - 220% - 245% - 240% - 235% - 195% - 200% - 195% - 170% | - | Packaging applications [132]. |
| PLLA/PBSA | - 75/25 | - | - 36.70 | - 1160.90 | - 153.60% | - | Biodegradable sealing envelope for food packaging [131]. |
| PLLA/PBS | - 100/0 - 0/100 - 75/25 | - | - 64.60 - 32.10 - 44.70 | - 2214.70 - 326.30 - 1075.20 | - 6.90% - 320.60% - 71.80% | - | [130] |
| Blend | Concentration (wt.%) | Features | Applications and/or Reference |
|---|---|---|---|
| PLA/PHB | - 80/20 and 60/40 | -Improvement in the percentage elongation at break. | -Biomedical applications [133]. |
| - 75/25 | -Higher elongation at break with the use of 5% Lapol | -Single-use applications such as fast-food packaging [108]. | |
| - | -Improved biodegradation rate, flexibility and impact properties. | -Food packaging [134]. | |
| - 75/25 | -Improved barrier and mechanical properties. | -Food packaging [107]. | |
| - 75/25 | -Biodegradable blend. | -Biodegradable food packaging [105]. | |
| - 85/15 | -Good barrier to water vapor and improved oxygen barrier properties | -Active food packaging [135]. | |
| - | -Enhanced mechanical and active properties | -Biodegradable active packaging for chilled salmon [142]. | |
| PLA/PHBV | - 75/25 and 50/50 | -Improved permeability. | -Food packaging [139]. |
| PLA/PBS | - 90/10, 80/20 and 70/30 | -Exceptional combination of ductility, modulus and strength. | -Green packaging [136]. |
| - 90/10, 80/20 and 70/30 | -Enhancement in PLA’s water vapor and oxygen permeability. -The levels of migration were maintained below the European legislative limits. | -Biodegradable food packaging [140]. | |
| - 80/20 | -Improved elongation at break. | -Food packaging [137]. | |
| - 90/10 | -Higher antibacterial activity. -Transparent sheets. -Mechanical properties allowed thermoforming for applications of food packaging. | -Antibacterial food packaging sheets [143]. | |
| PLA/PCL | - 90/10, 85/15, 80/20, 75/25, 70/30, 60/40 and 50/50 | -Well balanced combination of toughness and stiffness. | -Packaging, biomedical and agricultural applications [138]. |
| PLA/PBAT | - 70/30 | -Migration levels were below the limit specified by Food contact materials EU NO. 10/2011; therefore, the blend is safe for food contact packaging applications. | -Food contact materials for containers and packaging [141]. |
| PLA/PHBV/PBS | - 60/30/10 and 60/10/30 | -Entirely biodegradable. -An enhancement in the PLA’s crystallization, flexibility and toughness was observed in the resulting ternary complex. -Optimum performance with excellent balanced thermal resistance and stiffness-toughness. | [144] |
| Composite/ Nano- Composite | Concent- Ration (wt.%) | Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation at Break | Charpy Break Energy/ (Notched Izod Break Energy)/ Toughness | Flexural Strength (MPa) | Flexural Modulus (MPa) | Applications and/or Reference |
|---|---|---|---|---|---|---|---|---|
| PLA/MMT | - 100/0 - 100/2 phr a - 100/4 phr - 90/2 phr with 10% LLDPE - 90/4 phr with 10% LLDPE | - 58.0 - 55.0 - 53.0 - 41.50 - 42.50 | - 3000 - 3400 - 3500 - 2650 - 2850 | - | - | - 109 - 84 - 83 - 75 - 66 | - 3300 - 3500 - 3850 - 3000 - 3150 | Structural applications [145]. |
| PLA/talc | - 100/0 - 98/2 - 95.5/4.5 - 90.9/9.1 - 87.2/12.8 - 81.9/18.1 - 75.7/24.3 | - 54.0 - 58.5 - 58.4 - 58.5 - 58.2 - 59 - 60 | - | - 2.4% - 2.5% - 2.65% - 3.20% - 4.1% - 5.1% - 2.4% | - | - 66.0 - 90.5 - 91.0 - 93.0 - 94.0 - 98.0 - 103.5 | - 3300 - 3450 - 4000 - 4300 - 4900 - 6100 - 6750 | Packaging applications [149]. |
| - 100/0 - 95/5 - 90/10 - 80/20 - 70/30 | - 47.0 - 47.0 - 48.0 - 46.0 - 48.0 | - 2400 - 2560 - 3050 - 3650 - 4550 | - 6.70% - 3.0% - 3.0% - 2.50% - 1.70% | - | - | - | Packaging applications [150]. | |
| PLA/kaolinite | - 100/0 - 95/5 - 90/10 - 80/20 - 70/30 | - 47.0 - 48.0 - 42.0 - 42.0 - 46.0 | - 2400 - 2550 - 2700 - 3100 - 3350 | - 6.70% - 2.4% - 1.90% - 1.50% - 1.40% | - | - | - | Packaging applications [150]. |
| PLA/CNT | - 100/0 b - 99/0.1 b - 99.5/0.5 b - 99/1 b - 98/2 b | - 39.50 - 40.50 - 42.80 - 40.60 - 39.60 | - | - 22.50% - 28.30% - 33.60% - 26.70% - 20.50% | - (15.50) KJ/m2 - (22.60) KJ/m2 - (27.70) KJ/m2 - (20.50 KJ/m2) - (8.80) KJ/m2 | - | - | Industrial applications [151]. |
| - 100/0 - 99/1 - 97/3 - 95/5 - 90/10 | - 60.5 c - 65.0 c - 68.0 c - 65.0 c - 63.0 c - 51.0 d - 58.0 d - 67.5 d - 64.5 d - 59.5 d | - | - 5.00% c - 5.60% c - 5.70% c - 5.70% c - 5.90% c - 5.00% d - 5.00% d - 7.00% d - 6.00% d - 4.80% d | - | - | - | Applications requiring good electrical and mechanical properties [152]. | |
| - 100/0 - 80/0 with 20% PHBV - 80/0.5 with 20% PHBV - 80/1 with 20% PHBV | - | - | - | - (2.14) KJ/m2 - (4.10) KJ/m2 - (2.33) KJ/m2 - (2.46) KJ/m2 | - 58.07 - 51.60 - 58.66 - 61.01 | - 2940 - 3100 - 3300 - 3250 | Electronic devices and military applications [153]. | |
| PLA/KF | - 100/0 - 89/10 e with 1% MWCNTs - 79/20 e with 1% MWCNTs - 69/30 e with 1% MWCNTs - 59/40 e with 1% MWCNTs - 100/0 f - 89/10 f with 1% MWCNTs - 79/20 f with 1% MWCNTs - 69/30 f with 1% MWCNTs - 59/40 f with 1% MWCNTs - 70/30 g | - 49.70 - 61.60 - 62.80 - 78.50 - 47.70 - 46.80 - 61.60 - 70.40 - 91.50 - 53.60 | - | - | - (17.50) J/m - (30.80) J/m - (36.80) J/m - (37.40) J/m - (43.80) J/m - (30.90) J/m - (30.30) J/m - (35.50) J/m - (35.40) J/m - (44.90) J/m | - | - | Antistatic applications [154]. |
| - 100/0 - 100/0 f - PLA/20 pph h - PLA/20 pph f | - 63.20 - 65.30 - 39.50 - 32.70 | - 1410 - 1634 - 1618 - 1742 | - 6.60% - 5.30% - 3.50% - 2.50% | - | - | - | Packaging applications such as hot boiling water containers [157]. | |
| - 90/10 - 70/30 - 50/50 - 30/70 - 90/10 with 1% GPS as a coupling agent - 70/30 with 1% GPS - 50/50 with 1% GPS - 30/70 with 1% GPS - 90/10 with 3% GPS - 70/30 with 3% GPS - 50/50 with 3% GPS - 30/70 with 3% GPS - 90/10 with 5% GPS - 70/30 with 5% GPS - 50/50 with 5% GPS - 30/70 with 5% GPS | - | - | - | - | - 22.0 - 40.0 - 49.0 - 50.0 - 38.0 - 43.0 - 60.0 - 63.0 - 30.0 - 49.0 - 64.0 - 62.0 - 37.0 - 48.0 - 63.0 - 62.0 | - 2100 - 4000 - 4700 - 5900 - 4700 - 4000 - 5700 - 6800 - 4200 - 4500 - 5800 - 6800 - 4200 - 4650 - 5700 - 6800 | Prototypes of automobile headliners [162]. | |
| PLA/nano clay or organoclay | - 100/0 - 100/0 f - PLA with 5 pph Cloisite 30B® - PLA with 5 pph Cloisite 30B® f | - 63.20 - 65.30 - 51.20 - 51.60 | - 1410 - 1634 - 1599 - 1893 | - 6.60% - 5.30% - 5.20% - 3.50% | - | - | - | Packaging applications such as hot boiling water containers [157]. |
| - PLA (100/0) -PLA/PCL i/Organoclay 9S-Ben W (90.48/4.76/4.76 wt.%) -PLA/PCL j/Organoclay 9S-Ben W (90.48/4.76/4.76 wt.%) -PLA/PCL k/Organoclay 9S-Ben W (90.48/4.76/4.76 wt.%) | - 45.13 - 47.26 - 53.91 - 39.94 | - 3729 - 4371 - 4069 - 4237 | - 2.06% - 2.24% - 3.18% - 2.00% | - | - | - | [119] | |
| - PLLA/PBS (100/0) - PLLA/PBS (0/100) - PLLA/PBS (75/25) - PLLA/PBS 75/25 with 2% Cloisite 25 A® - PLLA/PBS 75/25 with 5% Cloisite 25 A® -PLLA/PBS 75/25 with 10% Cloisite 25 A® - PLLA/PBS 75/25 with 2% TFC - PLLA/PBS 75/25 with 5% TFC -PLLA/PBS 75/25 with 10% TFC | - | - 2214.70 - 326.30 - 1075.20 - 1364.60 - 1616.60 - 1940.10 - 1407.90 - 1624.60 - 1990.30 | - 6.90% - 320.60% - 71.80% - 4.40% - 4.10% - 3.60% - 75.50% - 100.60% - 118.10% | - | - | - | [130] | |
| - PLLA/PBSA 75/25 with 2% Cloisite 25 A® - PLLA/PBSA 75/25 with 5% Cloisite 25 A® - PLLA/PBSA 75/25 with 10% Cloisite 25 A® - PLLA/PBSA 75/25 with 2% TFC - PLLA/PBSA 75/25 with 5% TFC - PLLA/PBSA 75/25 with 10% TFC | - | - 1394.10 - 1585.00 - 1748.40 - 1445.60 - 1698.30 - 1780.70 | - 11.30% - 10.60% - 5.25% - 69.50% - 43.10% - 45.70% | - | - | - | Biodegradable sealing envelope for food packaging [131]. | |
| 3D-printed PLA wastes/SiO2 | - 100/0 - 95/5 - 90/10 - 85/15 | - 62.80 - 76.50 - 121.00 - 53.90 | - 839.60 - 895.10 - 1020.70 - 793.20 | - 11.10% - 12.60% - 15.30% - 11.40% | - 3.60 MPa - 4.60 MPa - 5.60 MPa - 3.10 MPa | - | - | Recycled PLA filaments for 3D printing [158]. |
| PLA/MgO | - 100/0 - 99/1 - 98/2 - 97/3 - 96/4 | - 29.10 - 34.00 - 37.50 - 26.60 - 26.20 | - 1891 - 2418 - 2470 - 2101 - 1961 | - 4.40% - 3.30% - 3.90% - 2.30% - 2.40% | - | - | - | Food packaging applications that are transparent and require superior antibacterial efficiency [159]. |
| PLA/flax fibers | - 100/0 - PLA/modified non- cellulose oxidizedTiO2 grafted flax fibers -PLA/modified cellulose oxidized TiO2 grafted flax fibers | - NP l - 172.00 - 211.00 | - 11,000 - 9000 - 105,000 | - 3.40% - 3.80% - 4.50% | - (5.00) KJ/m2 - (16.10) KJ/m2 - (15.70) KJ/m2 | - | - | [161] |
| PLA/flax fiber braided yarn plain woven fabric | - 100/0 - 82/18 - 100/74 - 100/65 | - 47.00 - 65.00 - 73.00 - 80.00 | - 820 - 1090 - 1190 - 1310 | - 6.50% - 9.00% - 9.00% - 9.45% | - | - | Housing and automobile interiors such as seat back, door trim and telephone stand [164]. | |
| PLA/wood flour | - 100/0 - 100/10 phr - 100/20 phr - 100/30 phr - 100/26 phr with 0.52 phr epoxy silane as a coupling agent and EMAGMA/13 as a compatibilizer - 100/26 phr with 0.52 phr epoxy silane as a coupling agent and EMAGMA/26 as a compatibilizer - 100/26 phr with 0.52 phr epoxy silane as a coupling agent and EMAGMA/52 as a compatibilizer (100%/26 phr/0.52 phr) | - 54.90 - 37.40 - 34.00 - 27.60 - 31.30 - 27.40 - 21.00 | - | - 2.50% - 2.60% - 1.80% - 1.30% - 6.90% - 11.70% - 24.40% | - (2.30) KJ/m2 - (3.00) KJ/m2 - (2.60) KJ/m2 - (2.40) KJ/m2 - (3.40) KJ/m2 - (3.80) KJ/m2 - (4.10) KJ/m2 | - | - | Blow molding applications [165]. |
| PLA/wood powder | - 100/0 - 60/10 with 30% PCL - 53.34/20 with 26.66% PCL - 46.66/30 with 23.34% PCL | - 62.00 - 37.00 - 35.00 - 33.00 | - 1300 - 890 - 1000 - 1085 | - 12.20% - 12.45% - 11.00% - 10.80% | - 30.00 J/mm - 60.00 J/mm - 57.00 J/mm - 43.00 J/mm | - | - | Disposable cups [166]. |
| PLA/cellulose nanocrystals | - 80/0 with 20% PBS - 79.5/0.5 with 20% PBS - 79.25/0.75 with 20% PBS - 79/1 with 20% PBS - 78.5/1.5 with 20% PBS | - 75.6 - 74.6 - 85.1 - 92.6 - 64.6 | - 3200 - 3975 - 6925 - 755 - 3275 | - 17.50% - 16.35% - 15.25% - 12.90% - 12.45% | - | - | - | Green packaging [169]. |
| PLA/Lignin | - 100/0 - 80/20 - 78/20 with 2% PEG 2000 as a plasticizer - 75/20 with 5% PEG 2000 - 79.5/20 with 0.5% TR451 as a plasticizer - 79/20 with 1% TR451 | - 56.00 - 43.50 - 52.00 - 44.50 - 45.50 - 45.00 | - 1800 - 2300 - 2150 - 1600 - 1700 - 2150 | - 4.20% - 2.90% - 4.00% - 3.90% - 3.70% - 3.20% | - | - | - | 3D printing applications [167]. |
| PLA/MCC | - 100/0 - 50/50 - 95/0 with 5% TEC as a plasticizer - 47.5/47.5 with 5% TEC - 90/0 with 10% TEC - 45/45 with 10% TEC - 85/0 with 15% TEC - 42.5/42.5 with 15% TEC | - 59.00 - 42.00 - 49.00 - 36.00 - 46.00 - 28.00 - 26.00 - 13.00 | - 1556 - 2517 - 1553 - 2495 - 1366 - 563 - 161 - 22 | - 6.00% - 2.00% - 10.00% - 2.00% - 13.00% - 26.00% - 595.00% - 300.00% | - | - | - | Eco friendly food packaging applications [168]. |
| PLA/HNTs | - 100/0 - 50/1 with 50% PCL - 50/3 with 50% PCL - 50/5 with 50% PCL - 50/7 with 50% PCL | - 17.25 - 11.45 - 12.87 - 15.52 - 16.62 | - 246.56 - 184.10 - 213.53 - 267.65 - 281.19 | - 7.18% - 12.30% - 8.53% - 9.37% - 6.78% | - | - | - | Bone replacements and regeneration applications [171]. |
| PLA/PFs | - 100/0 - NP | - 54.00 - 62.00 | - 1100 - 1450 | - | - (139) J/m - (92) J/m | - 103 - 103 | - 3500 - 5450 | Complex geometries in which the uniform distribution of mechanical performance and fibers are vital [172]. |
| PLA/short carbon fibers | - 100/0 m - NP m | - 47.80 - 70.30 | - 3350 - 9210 | - | - | 55.60 - 105.50 | - 2090 - 6940 | Applications demanding dimensional stability and higher stiffness [156]. |
| Polymer a | Tensile Modulus (GPa) | Tensile Strength (MPa) | Percentage Elongation at Break (%) |
|---|---|---|---|
| PHB | 1.7–3.5 | 40 | 3.0–6.0 |
| PHBV | 0.7–2.9 | 30–38 | 20 |
| PLA | 1.2–2.7 | 28–50 | 7.0–9.0 |
| PCL | 0.4 | 16.0 | 120–800 |
| TPS | 0.5–1.0 b | 2.6 | 47.0 |
| PET | 2.2 | 56.0 | 70–100 |
| LDPE | 0.2 | 10–15 | 300–500 |
| PP | 1.7 | 35–40 | 150 |
| PS | 1.6–3.1 | 12–50 | 3.0–4.0 |
| PVC | 0.3–2.4 | 10–60 | 12–32 |
| Percentage of Starch | Biodegradation Environment | Thickness (cm) | Days | Percentage of Weight Loss | Reference |
|---|---|---|---|---|---|
| 30% | Compost | NP a | 20 | 100% | [226] |
| 30% | 0.05 | 100% | [245] | ||
| 0% | 0.05 | 60% | |||
| 50% | Marine | 0.05 | 150 | 90–100% | [250] |
| 30% | 50–90% | ||||
| 0% | 10–20% | ||||
| 50% | Soil | 0.32 | 125 | 49% | [249] |
| 30% | 25% | ||||
| 0% | 7% | ||||
| 50% | Activated sludge | 0.08 | 30 | 100% | [225] |
| 25% | 85% | ||||
| 0% | 30% |
| Blend/Composite/Nanocomposite | Concentration (wt.%) | Tensile Strength (MPa) | Young’s Modulus (MPa) | Percentage Elongation | Charpy Impact Strength/(Notched Izod Break Energy) | Applications and/or Reference |
|---|---|---|---|---|---|---|
| PHB/PCL | - 100/0 - 75/25 - 50/50 - 25/75 | - 22.20 - 21.40 - 19.80 - 17.30 | - 1939 - 1643 - 1387 - 690 | - 8.10% - 11.20% - 17.60% - >1000% | - | [195] |
| PHA a/PCL | - 70/30 - 50/50 - 30/70 | - 4.0 b - 5.0 b - 13.0 b | - | - 4.00% b - 64.00% b - 63.00% b | - | Medical applications and packaging [197]. |
| P(3HO-3HD)/PCL | - 100/0 - 75/25 - 95/5 | - 14.30 - 5.90 - 13.70 | - 8.40 - 110 - 13.70 | - 640.00% - 490.00% - 620.00% | - | Nerve re-generation [198] |
| PHB/PBS | - 100/0 - 80/20 - 80/20 with 0.5% DCP as a free-radical grafting initiator - 70/30 - 70/30 with 0.5% DCP - 50/50 - 50/50 with 0.5% DCP | - | - | - 1.00% - 2.00% - 4.00% - 2.00% - 11.00% - 4.00% - 15.00% | - (0.60) KJ/m2 - (1.50) KJ/m2 - (3.50) KJ/m2 - (3.00) KJ/m2 - (4.00) KJ/m2 - (3.00) KJ/m2 - (5.50) KJ/m2 | Injection molding applications [193]. |
| PHBV/PBS | - 80/20 - 80/20 with 0.2% DCP as a free-radical grafting initiator - 80/20 with 0.5% DCP - 80/20 with 1% DCP | - | - | - 8.00% - 200.00% - 400.00% - 350.00% | - (2.80) KJ/m2 - (3.00) KJ/m2 - (5.00) KJ/m2 - (5.50) KJ/m2 | Injection molding applications [193]. |
| PHBV/PLA/PBS | - 0/100/0 - 100/0/0 - 30/60/10 - 10/60/30 - 60/30/10 - 60/10/30 | - 70.00 - 22.00 - 54.00 - 55.00 - 34.00 - 28.00 | - 2750 - 1300 - 2300 - 2150 - 1750 - 1200 | - 5.00% - 10.00% - 20.00% - 51.00% - 62.00% - 82.00% | - (17.5) J/m - (29.00) J/m - (33.00) J/m - (36.5) J/m - (30.00) J/m - (32.5) J/m | Structural materials [144]. |
| PHB/PETG | - 100/0 - 90/10 - 80/20 - 70/30 - 60/40 | - 58.00 - 49.00 - 42.00 - 33.00 - 33.00 | - | - | - (24.00) J/m - (13.00) J/m - (22.00) J/m - (27.00) J/m - (15.00) J/m | Applications that require improved processability while miniating PHB’s biodegradability [192]. |
| PHBV/P(3HB-co-3HV-co-3HHx) | - 100/0 - 90/10 - 75/25 - 50/50 | - 34.00 - 40.00 - 30.00 - 25.00 | - 4000 - 3950 - 2750 - 2000 | - 2.00% - 1.800% - 2.60% - 3.30% | - | Organic recycling food packaging [201]. |
| PHB-gAA/MWNTs-OH | - 100/0 - 99.5/0.5 - 99/1 - 97/3 | - 16.00 - 23.50 - 33.50 - 26.50 | - | - 8.0% - 7.0% - 6.0% - 4.0% | - | Applications that require higher performance [217]. |
| PHB/starch | - 100/0 - 90/10 - 80/20 - 70/30 - 60/40 - 50/50 | - 18.00 - 14.50 - 13.50 - 12.00 - 8.50 - 7.00 | - | - | - | Applications requiring better biodegradation, thermal, mechanical properties as well as processibility [229]. |
| - 100/0 - 90/10 - 80/20 - 70/30 - 60/40 - 50/50 - 40/60 - 30/70 | - 18.29 - 17.20 - 19.70 - 19.23 - 7.70 - 10.06 - 5.24 - 4.99 | - 1708 - 1716 - 1085 - 949 - 856 - 694 - 686 - 578 | - 3.32% - 9.80% - 6.00% - 9.40% - 8.50% - 5.27% - 3.45% - 4.30% | - | Low-cost coating material on cardboard or paper for food packaging [233]. | |
| - 70/30 (starch contains 70% amylose) - 70/30 (starch contains 72% amylose) | - 12.50 - 7.30 | - | - 3.90% - 2.80% | - 0.90 KJ/m2 - 0.70 KJ/m2 | [235] | |
| PHB-gAA/starch | - 100/0 - 90/10 - 80/20 - 70/30 - 60/40 - 50/50 | - 16.00 - 17.00 - 16.00 - 15.50 - 15.00 - 14.90 | - | - | - | Applications demanding better biodegradation, thermal, mechanical properties as well as processibility [229]. |
| - Plasticized 70% amylose corn starch blended with thermoplastic partner (PCL or PBAT) followed by PHB addition. - PHB blended with thermoplastic partner (PCL or PBAT) followed by plasticized 70% amylose corn starch - Addition of PHB with thermoplastic partner (PCL or PBAT) with plasticized 70% amylose corn starch all in one step | - | - 15.00 - 18.00 - 21.00 | - 900 - 1080 - 1020 | - 47.00% - 32.00% - 114.00% | - | Flexible packaging [236]. |
| PHB/ENR/MR/TMC | - 100/0/0/0 - 60/40/0/0 - 60/30/10/0 - 58/30/10/2 - 55/30/10/5 - 53/30/10/7 | - | - | - | - (23.00) J/m - (25.00) J/m - (124.00) J/m - (93.00) J/m - (116.00) J/m - (87.00) J/m | Applications requiring high impact properties [259]. |
| PHB/ENR/MR/COC | - 55/30/10/5 | - | - | - | - (49.00) J/m | |
| PHB/MMT | - 100/0 - PHB/Cloisite® Na+ - PHB/modified Cloisite® 30B | - 29.60 - 24.90 - 27.00 | - 3060 - 3200 - 3440 | - | - | [208] |
| PHBHx/SiO2 fiber | - 100/0 c - 100/0 d - 99/1 c, e - 97/3 c, e - 95/5 c, e - 99/1 c, f - 97/3 c, f - 95/5 c, f - 99/1 d, e - 97/3 d, e - 95/5 d, e - 99/1 d, f - 97/3 d, f - 95/5 d, f | - 23.00 - 24.50 - 24.50 - 22.50 - 24.60 - 23.00 - 24.50 - 23.50 - 24.40 - 25.00 - 24.50 - 25.00 - 24.50 - 24.50 | - 1000 - 1300 - 1300 - 1400 - 1600 - 1300 - 1490 - 1400 - 1300 - 1400 - 1490 - 1400 - 1400 - 1490 | - | - | Medical applications and tissue engineering [214]. |
| PHBV/wheat starch | - 100/0 - 75/25 - 50/50 | - 17.70 - 8.60 - 7.70 | - 1525 - 2132 - 2498 | - 25.00% - 5.10% - 1.00% | - | Complete biodegradable materials with reduced cost [225]. |
| PHBV/maize starch | - 100/0 - 80/20 - 70/30 - 80/20 with 2% free radical former g - 70/30 with 2% free radical former g | - | - | - | - 1.80 KJ/m2 - 1.20 KJ/m2 - 0.90 KJ/m2 - 2.10 KJ/m2 - 1.90 KJ/m2 | Biodegradable disposable plastics with low cost and the required performance [226]. |
| - 100 - 80/20 - 70/30 - 60/40 - 50/50 | - 18.00 - 6.00 - 3.50 - 4.00 - 2.85 | - 1200 - 750 - 310 - 305 - 160 | - 2.10% - 1.00% - 1.10% - 1.10% - 1.40% | - | Packaging [234]. | |
| PHBV/corn starch | - 75/25 with 5% Acetyl tributyl citrate as plasticizer | - 17.10 | - 458.00 | - 15.60% | - | Applications that require improved mechanical properties [228]. |
| PHBV/starch-g-PGMA | - 75/25 with 5% Acetyl tributyl citrate as plasticizer | - 23.60 | - 539.00 | - 13.00% | - | |
| PHBV/granular cornstarch | - 100/0 with 10% Tiracetin as an additive - 70/30 with 10% Tiracetin - 50/50 with 10% Tiracetin - 70/30 with 10% Tiracetin and 9% PEO - 50/50 with 10% Tiracetin and 9% PEO - 50/50 with 10% Tiracetin and 5% PEO - 50/50 with 10% Tiracetin and 2% PEO | - 24.00 - 15.00 - 10.00 - 19.00 - 18.00 - 15.00 - 12.00 | - 180 - 250 - 300 - 220 - 170 - 210 - 280 | - 38.00% - 21.00% - 11.00% - 21.00% - 21.00% - 15.00% - 10.00% | - | Single use applications such as plastic knives and forks [222]. |
| PHBV/Cloisite® 30B | - 100/0 - 99/1 - 97/3 - 95/5 | - 37.50 - 40.60 - 30.70 - 30.40 | - 3500 - 5100 - 4600 - 7100 | - 3.00% - 2.40% - 0.70% - 0.60% | - (20.00) J/m - (11.00) J/m - (11.00) J/m - (10.00) J/m | [209] |
| - 100/0 - 99/1 - 98/2 - 97/3 | - 31.00 - 32.00 - 35.00 - 33.00 | - 481 - 555 - 730 - 795 | - 8.50% - 7.60% - 7.70% - 5.60% | - | Applications that require enhanced processing behaviors, crystallinity, low cost and improved mechanical properties [264]. | |
| PHBV/PLA/Cloisite® 30B | - 15/85/0 - 15/85/4 - 30/70 - 30/70/4 | - 52.00 - 49.00 - 46.00 - 44.50 | - 1600 - 2000 - 1750 - 2000 | - 8.80% - 8.00% - 5.00% - 2.50% | - | Injection molding applications with high modulus, heat deflection resistance and superior gas barrier properties [194]. |
| PHBV/Cloisite® 15A | - 100/0 - 99/1 - 97.5/2.5 - 95/5 | - 5.90 - 11.80 - 18.00 - 28.90 | - 633.00 - 1043.00 - 1311.00 - 1677.00 | - 3.30% - 2.70% - 1.80% - 1.40% | - | Applications demanding enhanced mechanical properties [254]. |
| PHBV/OMMT | - 97/3 - 90/10 | - 26.90 - 35.60 - 21.80 | - 1373 - 1412 - 1375 | - 4.10% - 3.90% - 2.10% | - | Applications that require enhanced crystallization and mechanical properties [207]. |
| PHBV/LDH-SA | - 100/0 - 99/1 - 97/3 - 95/5 - 93/7 | - 25.10 - 28.20 - 28.50 - 24.20 - 24.40 | - 1120 - 1230 - 1330 - 1420 - 1240 | - 4.03% - 3.50% - 3.25% - 2.50% - 2.70% | - | Medical applications [267]. |
| PHBV/HNT | - 100/0 - 99/1 - 97/3 - 95/5 | - 37.50 - 39.30 - 39.40 - 38.70 | - 3500 - 4000 - 3600 - 5700 | - 3.00% - 3.70% - 3.10% - 4.10% | - (20.00) J/m - (19.00) J/m - (20.00) J/m - (19.00) J/m | [209] |
| PHBV/CNW | - 100/0 - 98/2 with PEG as a compatibilizer - 95/5 with PEG | - 14.10 - 15.50 - 26.10 | - 820 - 1100 - 1760 | - 12.40% - 7.10% - 7.80% | - | Sustainable composite applications [213]. |
| PHBV/CS | - 100/0 - 100/0 with 10% ATBC as a plasticizer and 5% CaCO3 - 85/5 with 10% ATBC and 5% CaCO3 - 85/7.5 with 10% ATBC and 5% CaCO3 - 85/10 with 10% ATBC and 5% CaCO3 - 85/12.5 with 10% ATBC and 5% CaCO3 | - 34.80 - 23.00 - 20.80 - 19.70 - 18.40 - 17.30 | - 2610 - 1300 - 1730 - 1930 - 2030 - 2050 | - 2.60% - 6.20% - 4.00% - 2.90% - 2.50% - 2.30% | - 2.50 KJ/m2 - 5.80 KJ/m2 - 3.70 KJ/m2 - 3.70 KJ/m2 - 3.20 KJ/m2 - 3.80 KJ/m2 | Food contact injection molding applications such as coffee capsules [273]. |
| PHA/TFSB | - 100/0 - 99/1 - 98/2 - 97/3 - 96/4 - 95/5 | - 1.66 - 1.51 - 1.41 - 1.36 - 1.22 - 1.08 | - | - 85.30% - 78.80% - 73.60% - 67.10% - 62.50% - 57.10% | - | Biomedical applications, such as bioprotective films, wound healing and bone tissue engineering (e.g., bone screws, bone joints and tooth roots) [275]. |
| MPHA/TFSB | - 100/0 - 99/1 - 98/2 - 97/3 - 96/4 - 95/5 | - 1.60 - 1.88 - 2.13 - 2.43 - 2.36 - 2.24 | - | - 86.10% - 76.30% - 67.80% - 59.30% - 52.50% - 39.60% | - | |
| PHA/GNPs | - 100/0 - 97.5/2.5 - 95/5 - 92.5/7.5 - 90/10 - 85/15 | - 17.00 - 16.40 - 14.00 - 18.00 - 11.00 - 11.20 | - | - 14.00% - 10.00% - 11.00% - 12.00% - 3.00% - 3.00% | - | Thermal and electrical applications [220]. |
| PHA/GNPs and CNFs | - 97.5/2.5 - 95/5 - 92.5/7.5 - 90/10 - 85/15 | - 16.40 - 16.50 - 16.00 - 17.10 - 17.00 | - | - 12.00% - 9.00% - 6.00% - 5.50% - 2.00% | - | |
| Mixture of P3HB and P4HB /FD-TMCNCs | - 100/0 - 95/5 - 90/10 | - 25.84 - 19.20 - 17.02 | - 5.27 - 5.52 - 3.64 | - 101.33% - 301.00% - 231.00% | Commercial applications demanding high ductility [216]. | |
| Mixture of P3HB and P4HB /TD-TMCNCs | - 90/10 | - 15.58 | - 0.09 | - 247.67% |
| Blend/Nanocomposite | Concentration (wt.%) | Features | Applications and/or Reference |
|---|---|---|---|
| PHB/PLA | - 80/20 and 60/40 | -Improvement in the percentage elongation at break. Percentage elongation at break for the PHB/PLA 60/40 wt.% was eight time that of the neat PHB. | -Biomedical applications [133]. |
| PHB/PCL | - 30/70 | -An increase in the crystallinity of PHB and the blend PHB-phase. -Complete degradation. | -Biotechnological applications [186]. |
| - 75/25, 50/50 and 25/75 | -Increase in the blend’s flexibility and ductility. -Significant increase in the percentage elongation at break and the energy absorption in impact conditions. | [195] | |
| - 65/30 with 5% crosslinking agent | -Increase in the percentage elongation at break as well as the tensile strength in the quasi-static tensile test. | -Multi-scale instrumental analyses [197]. | |
| PHBV/PBS | - 90/10, 80/20 and70/30 | -Significant enhancement in the elongation at break of the PHBV/PBS blends due to the better interfacial adhesion between the PHBV and PBS phases. -Improvement in tensile strength. | [193] |
| PHBV/PLA/PBS | - 60/30/10 and 60/10/30 | -Entirely biodegradable. -An enhancement in the PLA’s crystallization, flexibility and toughness was observed in the resulting ternary complex. -Optimum performance with excellent balanced thermal resistance and stiffness-toughness. | [144] |
| PHB/LDPE | - | -Substantial improvement in the LDPE’s modulus of elasticity. | [191] |
| PHBV/PE | - 10/90 and 30/70 | -The rate of degradation was proportional to the quantity of PHBV in contact with PE. | [188] |
| PHB/PETG | - 80/20, 60/40, 50/50, 40/60 and 20/80 | -Substantial enhancement in the flexural modulus. -Improvement in the processability and modulus of elasticity without significant changes in the impact resistance while keeping the biodegradability of PHB intact. | [192] |
| PHBV/MWNTs | - 98/2 | -Improvement in thermal stability. | -Applications that require higher thermal stability, hardness and improved electrical conductivity [204]. |
| - 99/1, 97/3, 95/5 and 93/7 | -Enhancement in the crystallinity and crystallite sizes of PHBV. | [203] | |
| PHBV/CNTs | - 99/1, 97/3, 95/5 and 90/10 | -Improvement in water and oxygen transmission, barrier properties, conductivity and mechanical properties. | -Medicine, aerospace engineering, home appliances, public transportations as well as beverage and food packaging [215]. |
| PHBV/CNFs | |||
| P3HB-co-4HB/CNFs | - 99/1 treated with n-octanol, silane coupling agent (KH-550) and nitric acid | -Both, the crystallinity and the glass transition temperature increased. | -Biomedical and electronic applications [218]. |
| mcl-PHAs/CNFs | - | -Improvement in the crystallinity, thermomechanical properties and physical morphology. | -Smart biomaterials, such as: biosensors, organic electroconductive materials and stimuli-responsive drug delivery devices [219]. |
| PHBV/plasticized (with Glycerol) wheat starch | - | -Low cost. -Sufficient adhesion between layers. -Moisture barrier properties. -Satisfactory water resistance. -Improved mechanical properties. | -Compostable multilayers film for disposable articles and food packaging [237]. |
| PHBV/extruded starch | - 95/5, 90/10 and 80/20 | -The expansion of the foam has been reduced. | -Loose fill packaging applications [240]. |
| PHBV/TPS | - | -Cost effective. -PHA works as a water-resistant outer coating. | -Food packaging, insect dies, controlled drug release and pesticides [239]. |
| PHB/organo-modified fluoromica | - 98/2 | -Significant enhancement in mechanical and thermal properties as well as the biodegradation rate. | [205] |
| PHB/MMT | - 98.8/1.2, 97.7 and 96.4/3.6 | ||
| Nodax®™/clay 20A and clay 25A | - 99/1, 97/3, 95/5, 93/7, 90/10 and 85/15 | -Improved mechanical properties and a slight enhancement in thermal stability. | -Applications that Require improved mechanical properties [265]. |
| PHB/PMLDH | - 98/2 and 95/5 | -Significant enhancement in storage modulus. -An increase in crystallization rate. -A reduction in activation energy. | [255] |
| mcl-PHAs/hydrolyzed tunicin cellulose whiskers | - | -Substantial enhancement in mechanical properties. | [268] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naser, A.Z.; Deiab, I.; Defersha, F.; Yang, S. Expanding Poly(lactic acid) (PLA) and Polyhydroxyalkanoates (PHAs) Applications: A Review on Modifications and Effects. Polymers 2021, 13, 4271. https://doi.org/10.3390/polym13234271
Naser AZ, Deiab I, Defersha F, Yang S. Expanding Poly(lactic acid) (PLA) and Polyhydroxyalkanoates (PHAs) Applications: A Review on Modifications and Effects. Polymers. 2021; 13(23):4271. https://doi.org/10.3390/polym13234271
Chicago/Turabian StyleNaser, Ahmed Z., Ibrahim Deiab, Fantahun Defersha, and Sheng Yang. 2021. "Expanding Poly(lactic acid) (PLA) and Polyhydroxyalkanoates (PHAs) Applications: A Review on Modifications and Effects" Polymers 13, no. 23: 4271. https://doi.org/10.3390/polym13234271
APA StyleNaser, A. Z., Deiab, I., Defersha, F., & Yang, S. (2021). Expanding Poly(lactic acid) (PLA) and Polyhydroxyalkanoates (PHAs) Applications: A Review on Modifications and Effects. Polymers, 13(23), 4271. https://doi.org/10.3390/polym13234271