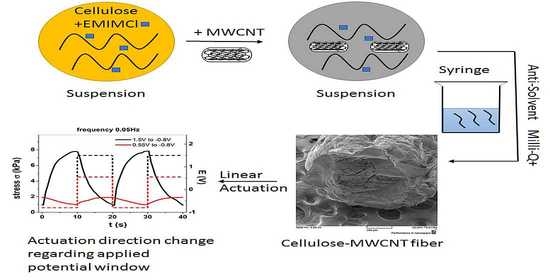
Wider Potential Windows of Cellulose Multiwall Carbon Nanotube Fibers Leading to Qualitative Multifunctional Changes in an Organic Electrolyte
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell-CNT Fiber Formation
2.3. Electromechanical Deformation
2.4. Characterization
3. Results and Discussion
3.1. Characterizations of Cell-CNT Fibers
3.2. Electromechanical Response
3.2.1. Cyclic Voltammetry
3.2.2. Square Wave Potential Steps
3.3. Energy Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jang, Y.; Kim, S.M.; Spinks, G.M.; Kim, S.J. Carbon Nanotube Yarn for Fiber-Shaped Electrical Sensors, Actuators, and Energy Storage for Smart Systems. Adv. Mater. 2019, 32, 1902670. [Google Scholar] [CrossRef]
- Amri, M.R.; Yasin, F.; Abdullah, L.C.; Al-Edrus, S.S.O.; Mohamad, S.F. Ternary Nanocomposite System Composing of Graphene Nanoplatelet, Cellulose Nanofiber and Jatropha Oil Based. Polymers 2021, 13, 3740. [Google Scholar] [CrossRef] [PubMed]
- Pinkert, A.; Marsh, K.N.; Pang, S.; Staiger, M.P. Ionic liquids and their interaction with cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar] [CrossRef]
- Elhi, F.; Aid, T.; Koel, M. Ionic liquids as solvents for making composite materials from cellulose. Proc. Est. Acad. Sci. 2016, 65, 255–266. [Google Scholar] [CrossRef]
- Mo, M.; Chen, C.; Gao, H.; Chen, M.; Li, D. Wet-spinning assembly of cellulose nanofibers reinforced graphene/polypyrrole microfibers for high performance fiber-shaped supercapacitors. Electrochim. Acta 2018, 269, 11–20. [Google Scholar] [CrossRef]
- Gupta, K.M.; Hu, Z.; Jiang, J. Molecular insight into cellulose regeneration from a cellulose/ionic liquid mixture: Effects of water concentration and temperature. RSC Adv. 2013, 3, 4425–4433. [Google Scholar] [CrossRef]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon nanotube chemical sensors. Chem. Rev. 2019, 119, 599–663. [Google Scholar] [CrossRef]
- Jyothibasu, J.P.; Wang, R.H.; Ong, K.; Ong, J.H.L.; Lee, R.H. Cellulose/carbon nanotube/MnO2 composite electrodes with high mass loadings for symmetric supercapacitors. Cellulose 2021, 28, 3549–3567. [Google Scholar] [CrossRef]
- Gui, Z.; Zhu, H.; Gillette, E.; Han, X.; Rubloff, G.W.; Hu, L.; Lee, S.B. Natural cellulose fiber as substrate for supercapacitor. ACS Nano 2013, 7, 6037–6046. [Google Scholar] [CrossRef]
- Jing, C.; Liu, W.; Hao, H.; Wang, H.; Meng, F.; Lau, D. Regenerated and rotation-induced cellulose-wrapped oriented CNT fibers for wearable multifunctional sensors. Nanoscale 2020, 12, 16305–16314. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, Y.; Ounaies, Z.; Bae, S.H.; Yun, S. Electroactive paper materials coated with carbon nanotubes and conducting polymers. Am. Soc. Mech. Eng. Aerosp. Div. AD 2005, 70, 59–63. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, L.; Zhang, D.; Song, W. High performance, flexible and renewable nano-biocomposite artificial muscle based on mesoporous cellulose/ionic liquid electrolyte membrane. Sens. Actuators B Chem. 2019, 283, 579–589. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Caruso, M.R.; Cavallaro, G.; Milioto, S.; Lazzara, G. Hydroxypropyl Cellulose Films Filled with Halloysite Nanotubes/Wax Hybrid Microspheres. Ind. Eng. Chem. Res. 2021, 60, 1656–1665. [Google Scholar] [CrossRef]
- Qi, H.; Schulz, B.; Vad, T.; Liu, J.; Mäder, E.; Seide, G.; Gries, T. Novel Carbon Nanotube/Cellulose Composite Fibers as Multifunctional Materials. ACS Appl. Mater. Interfaces 2015, 7, 22404–22412. [Google Scholar] [CrossRef]
- Qi, H.; Mäder, E.; Liu, J. Unique water sensors based on carbon nanotube-cellulose composites. Sens. Actuators B Chem. 2013, 185, 225–230. [Google Scholar] [CrossRef]
- Cao, D.; Pang, P.; Liu, H.; He, J.; Lindsay, M. Electronic sensitivity of a single-walled carbon nanotube to internal electrolyte composition. Nanotechnology 2012, 23, 085203. [Google Scholar] [CrossRef]
- Baughman, R.H.; Cui, C.; Zakhidov, A.A.; Iqbal, Z.; Barisci, J.N.; Spinks, G.M.; Wallace, G.G.; Mazzoldi, A.; De Rossi, D.; Rinzler, A.G.; et al. Carbon nanotube actuators. Science 1999, 284, 1340–1344. [Google Scholar] [CrossRef] [Green Version]
- Kosidlo, U.; Omastova, M.; Micusik, M.; Ciric-Marjanovic, G.; Randriamahazaka, H.; Wallmersperger, T.; Aabloo, A.; Kolaric, I.; Bauernhansl, T. Nanocarbon based ionic actuators-a review. Smart Mater. Struct. 2013, 22, 104022. [Google Scholar] [CrossRef]
- Pietronero, L.; Strässler, S. Bond-Length Change as a Tool to Determine Charge Transfer and Electron-Phonon Coupling in Graphite Intercalation Compounds. Phys. Rev. Lett. 1981, 47, 593. [Google Scholar] [CrossRef]
- Otero, T.F.; Martinez, J.G.; Asaka, K. Faradaic and capacitive components of the CNT electrochemical responses. Front. Mater. 2016, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Riemenschneider, J.; Temmen, H.; Monner, H.P. CNT based actuators: Experimental and theoretical investigation of the in-plain strain generation. J. Nanosci. Nanotechnol. 2007, 7, 3359–3364. [Google Scholar] [CrossRef]
- Elhi, F.; Peikolainen, A.L.; Kiefer, R.; Tamm, T. Cellulose-multiwall carbon nanotube fiber actuator behavior in aqueous and organic electrolyte. Materials 2020, 13, 3213. [Google Scholar] [CrossRef] [PubMed]
- Harjo, M.; Tamm, T.; Anbarjafari, G.; Kiefer, R. Hardware and Software Development for Isotonic Strain and Isometric Stress Measurements of Linear Ionic Actuators. Polymers 2019, 11, 1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaempgen, M.; Chan, C.K.; Ma, J.; Cui, Y.; Gruner, G. Printable thin film supercapacitors using single-walled carbon nanotubes. Nano Lett. 2009, 9, 1872–1876. [Google Scholar] [CrossRef]
- Yang, L.; Sun, Z.; Li, F.; Du, S.; Song, W. Performance enhancement of cellulose based biocomposite ionic actuator by doping with MWCNT. Appl. Phys. A 2019, 125, 547. [Google Scholar] [CrossRef]
- Zhang, K.; Feldner, A.; Fischer, S. FT Raman spectroscopic investigation of cellulose acetate. Cellulose 2011, 18, 995–1003. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Atalla, R.H. Raman Spectroscopy. In Surface Analysis of Paper; Conners, T.E., Banerjee, S., Eds.; CRC Press: Boca Raton, FL, USA, 1995; pp. 152–181. ISBN 9780429279997. [Google Scholar]
- Lucas, M.; Wagner, G.L.; Nishiyama, Y.; Hanson, L.; Samayam, I.P.; Schall, C.A.; Langan, P.; Rector, K.D. Reversible swelling of the cell wall of poplar biomass by ionic liquid at room temperature. Bioresour. Technol. 2011, 102, 4518–4523. [Google Scholar] [CrossRef] [Green Version]
- Baskaran, D.; Mays, J.W.; Bratcher, M.S.; Uni, V.; Hall, B.; Knox, V.; December, R.V.; Re, V.; Recei, M.; March, V. Noncovalent and Nonspecific Molecular Interactions of Polymers with Multiwalled Carbon Nanotubes. Chem. Mater. 2005, 80, 3389–3397. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Kumar, S. Polymer/carbon nanotube nano composite fibers-A review. ACS Appl. Mater. Interfaces 2014, 6, 6069–6087. [Google Scholar] [CrossRef] [PubMed]
- Foroughi, J.; Spinks, G. Carbon nanotube and graphene fiber artificial muscles. Nanoscale Adv. 2019, 1, 4592–4614. [Google Scholar] [CrossRef] [Green Version]
- Lyon, J.L.; Stevenson, K.J. Anomalous electrochemical dissolution and passivation of iron growth catalysts in carbon nanotubes. Langmuir 2007, 23, 11311–11318. [Google Scholar] [CrossRef] [PubMed]
- Kaasik, F.; Tamm, T.; Hantel, M.M.; Perre, E.; Aabloo, A.; Lust, E.; Bazant, M.Z.; Presser, V. Anisometric charge dependent swelling of porous carbon in an ionic liquid. Electrochem. Commun. 2013, 34, 196–199. [Google Scholar] [CrossRef] [Green Version]
- Plaado, M.; Kaasik, F.; Valner, R.; Lust, E.; Saar, R.; Saal, K.; Peikolainen, A.; Aabloo, A.; Kiefer, R. Electrochemical actuation of multiwall carbon nanotube fiber with embedded carbide-derived carbon particles. Carbon 2015, 94, 911–918. [Google Scholar] [CrossRef]
- Ikezawa, Y.; Ariga, T. In situ FTIR spectra at the Cu electrode/propylene carbonate solution interface. Electrochim. Acta 2007, 52, 2710–2715. [Google Scholar] [CrossRef]
- Chaban, V. Solvation of the fluorine containing anions and their lithium salts in propylene carbonate and dimethoxyethane. J. Mol. Model. 2015, 21, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Pushparaj, V.L.; Manikoth, S.M.; Ashavani, K.; Saravanababu, M.; Lijie, C.; Robert, V.; Linhardt, R.J.; Nalamasu, O.; Ajayan, P.M. Flexible energy storage devices based on nanocomposite paper. Proc. Natl. Acad. Sci. USA 2007, 104, 13574–13577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felhősi, I.; Keresztes, Z.; Marek, T.; Pajkossy, T. Properties of electrochemical double-layer capacitors with carbon-nanotubes-on-carbon-fiber-felt electrodes. Electrochim. Acta 2020, 334, 135548. [Google Scholar] [CrossRef] [Green Version]
- Mirfakhrai, T.; Oh, J.; Kozlov, M.; Fok, E.C.W.; Zhang, M.; Fang, S.; Baughman, R.H.; Madden, J.D.W. Electrochemical actuation of carbon nanotube yarns. Smart Mater. Struct. 2007, 16, S243–S249. [Google Scholar] [CrossRef]
- Pang, Z.; Sun, X.; Wu, X.; Nie, Y.; Liu, Z.; Yue, L. Fabrication and application of carbon nanotubes/cellulose composite paper. Vacuum 2015, 122, 135–142. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, L.; Zhao, J.; Song, W. Natural Cellulose-Full-Hydrogels Bioinspired Electroactive Artificial Muscles: Highly Conductive Ionic Transportation Channels and Ultrafast Electromechanical Response. J. Electrochem. Soc. 2020, 167, 047515. [Google Scholar] [CrossRef]






| Electrochemical Actuators | Electrolyte Applied Voltage Current Density | Specific Capacitance (F g−1) | Actuation |
|---|---|---|---|
| Regenerated Cellulose + coating with MWCNT [12] | Ionic liquids 1.0 V to 0.0 V 0.25–0.4 mA cm−2 (20 mV s−1) | 0.89 (20 mV s−1) | Trilayer, ±5 V Anion-dominated 0.7–1.5 cm displacement |
| Cellulose regenerated + MWCNT + rGO + MnO2 + PANI [42] | Ionic electrolyte membrane 1.0 V to 0.2 V 0.58 mA cm−2 (20 mV s−1) | 1.4 (1 A g−1) | Trilayer, ±5 V 1.6 cm displacement Anion-dominated |
| MWCNT fiber Dielectrophoresis [34] | TBACF3SO3-PC 0.6 V to −0.55 V 1 mA cm−2,(5 mV s−1) | 62 (0.2 A g−1) | Linear actuation Cation-dominated 0.1% strain |
| MWCNT yarn CVD, twisted [31] | TBAPF6/acetonitrile ±1.0 V 50 mV s−1 | - | Linear actuation Mixed actuation −1.0 V: 0.035% strain +1.0 V: 0.065% strain |
| This work Regenerated Cellulose + 50 wt.% MWCNT fiber | LiTFSI-PC 1.5 V to −0.8 V (10 mV s−1) ~2 mA cm−2 | 21 (0.015 A g−1) | Linear actuation Mixed actuation −0.8 V: 0.07% +1.0 V: 0.05% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiefer, R.; Elhi, F.; Peikolainen, A.-L.; Tamm, T. Wider Potential Windows of Cellulose Multiwall Carbon Nanotube Fibers Leading to Qualitative Multifunctional Changes in an Organic Electrolyte. Polymers 2021, 13, 4439. https://doi.org/10.3390/polym13244439
Kiefer R, Elhi F, Peikolainen A-L, Tamm T. Wider Potential Windows of Cellulose Multiwall Carbon Nanotube Fibers Leading to Qualitative Multifunctional Changes in an Organic Electrolyte. Polymers. 2021; 13(24):4439. https://doi.org/10.3390/polym13244439
Chicago/Turabian StyleKiefer, Rudolf, Fred Elhi, Anna-Liisa Peikolainen, and Tarmo Tamm. 2021. "Wider Potential Windows of Cellulose Multiwall Carbon Nanotube Fibers Leading to Qualitative Multifunctional Changes in an Organic Electrolyte" Polymers 13, no. 24: 4439. https://doi.org/10.3390/polym13244439
APA StyleKiefer, R., Elhi, F., Peikolainen, A. -L., & Tamm, T. (2021). Wider Potential Windows of Cellulose Multiwall Carbon Nanotube Fibers Leading to Qualitative Multifunctional Changes in an Organic Electrolyte. Polymers, 13(24), 4439. https://doi.org/10.3390/polym13244439