Incorporation of ZnO Nanoparticles into Soy Protein-Based Bioplastics to Improve Their Functional Properties
Abstract
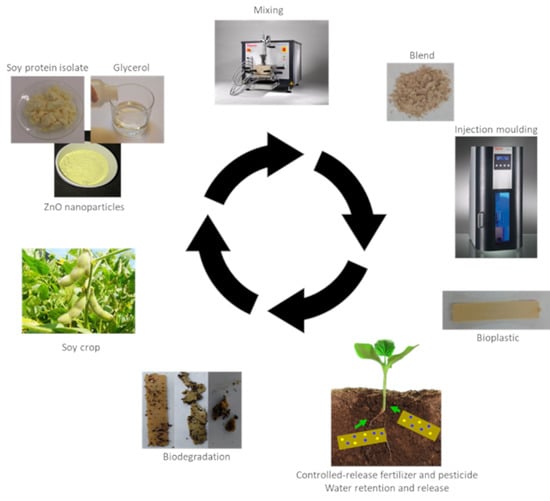
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ZnO Nanoparticles
2.3. Bioplastics Processing Method
2.4. Characterization of Bioplastics
2.4.1. Mechanical Properties
Flexural Properties
Tensile Properties
2.4.2. Morphological Properties
Scanning Electron Microscopy (SEM)
Energy Dispersive X-ray Analysis (EDXA)
2.4.3. Functional Properties
Water Uptake Capacity (WUC)
Nanoparticle Release
Biodegradability
2.5. Statistical Analysis
3. Results and Discussion
3.1. Mechanical Properties
3.1.1. Flexural Properties
3.1.2. Tensile Properties
3.2. Morphological Properties
3.3. Functional Properties
3.3.1. Water Uptake Capacity
3.3.2. Nanoparticle Release
3.3.3. Biodegradability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Easter, K.W. Irrigation Innvestment, Technology and Management Strategies for Development; Routledge: New York, NY, USA, 2019. [Google Scholar]
- Ganetri, I.; Essamlali, Y.; Amadine, O.; Danoun, K.; Aboulhrouz, S.; Zahouily, M. Controlling factors of slow or controlled-release fertilizers. In Controlled Release Fertilizers for Sustainable Agriculture; Elsevier: Amsterdam, The Netherlands, 2021; pp. 111–129. [Google Scholar] [CrossRef]
- Bahadir, M.; Pfister, G. Controlled release formulations of pesticides. In Chemistry of Plant Protection; Springer: New York, NY, USA, 1990; pp. 1–64. [Google Scholar] [CrossRef]
- Mann, M.; Kruger, J.E.; Andari, F.; McErlean, J.; Gascooke, J.R.; Smith, J.A.; Worthington, M.J.H.; McKinley, C.C.C.; Campbell, J.A.; Lewis, D.A.; et al. Sulfur polymer composites as controlled-release fertilisers. Org. Biomol. Chem. 2019, 17, 1929–1936. [Google Scholar] [CrossRef]
- Beig, B.; Niazi, M.B.K.; Jahan, Z.; Kakar, S.J.; Shah, G.A.; Shahid, M.; Zia, M.; Haq, M.U.; Rashid, M.I. Biodegradable Polymer Coated Granular Urea Slows Down N Release Kinetics and Improves Spinach Productivity. Polymers 2020, 12, 2623. [Google Scholar] [CrossRef] [PubMed]
- Mortain, L.; Dez, I.; Madec, P.-J. Development of new composites materials, carriers of active agents, from biodegradable polymers and wood. C. R. Chim. 2004, 7, 635–640. [Google Scholar] [CrossRef]
- Karan, H.; Funk, C.; Grabert, M.; Oey, M.; Hankamer, B. Green Bioplastics as Part of a Circular Bioeconomy. Trends Plant Sci. 2019, 24, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Olad, A.; Doustdar, F.; Gharekhani, H. Fabrication and characterization of a starch-based superabsorbent hydrogel composite reinforced with cellulose nanocrystals from potato peel waste. Colloids Surf. Physicochem. Eng. Asp. 2020, 601, 124962. [Google Scholar] [CrossRef]
- Michalik, R.; Wandzik, I. A Mini-Review on Chitosan-Based Hydrogels with Potential for Sustainable Agricultural Applications. Polymers 2020, 12, 2425. [Google Scholar] [CrossRef]
- Kartini, I.; Lumbantobing, E.T.; Suyanta, S.; Sutarno, S.; Adnan, R. Bioplastic Composite of Carboxymethyl Cellulose/N-P-K Fertilizer. Key Eng. Mater. 2020, 840, 156–161. [Google Scholar] [CrossRef]
- Bi, S.; Barinelli, V.; Sobkowicz, M.J. Degradable Controlled Release Fertilizer Composite Prepared via Extrusion: Fabrication, Characterization, and Release Mechanisms. Polymers 2020, 12, 301. [Google Scholar] [CrossRef] [Green Version]
- Merino, D.; Gutiérrez, T.J.; Alvarez, V.A. Potential Agricultural Mulch Films Based on Native and Phosphorylated Corn Starch With and Without Surface Functionalization with Chitosan. J. Polym. Environ. 2019, 27, 97–105. [Google Scholar] [CrossRef]
- Singh, A.; Dhiman, N.; Kar, A.K.; Singh, D.; Purohit, M.P.; Ghosh, D.; Patnaik, S. Advances in controlled release pesticide formulations: Prospects to safer integrated pest management and sustainable agriculture. J. Hazard. Mater. 2020, 385, 121525. [Google Scholar] [CrossRef]
- Girotto, F.; Alibardi, L.; Cossu, R. Food waste generation and industrial uses: A review. Waste Manag. 2015, 45, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, M.; Ramezani, F.; Gerami, M. Nanoparticles in pest incidences and plant disease control. In Nanotechnology for Agriculture: Crop Production & Protection; Springer: Singapore, 2019; pp. 233–272. [Google Scholar] [CrossRef]
- Dissanayake, N.M.; Arachchilage, J.S.; Samuels, T.A.; Obare, S.O. Highly sensitive plasmonic metal nanoparticle-based sensors for the detection of organophosphorus pesticides. Talanta 2019, 200, 218–227. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.; Naidu, R. Nanoencapsulation, Nano-guard for Pesticides: A New Window for Safe Application. J. Agric. Food Chem. 2016, 64, 1447–1483. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munné-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.J.; Yepes-Molina, L.; Martinez-Alonso, A.; Carvajal, M. Nanobiofertilization as a novel technology for highly efficient foliar application of Fe and B in almond trees. R. Soc. Open Sci. 2020, 7, 200905. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total. Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Naderi, M.H.; Danesh-Shahraki, A. Nanofertilizers and Their Roles in Sustainable Agriculture. Environ. Sci. 2013, 5, 2229–2232. [Google Scholar]
- Eichert, T.; Kurtz, A.; Steiner, U.; Goldbach, H.E. Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiol. Plant. 2008, 134, 151–160. [Google Scholar] [CrossRef]
- Martínez-Fernández, D.; Barroso, D.; Komárek, M. Root water transport of Helianthus annuus L. under iron oxide nanoparticle exposure. Environ. Sci. Pollut. Res. 2016, 23, 1732–1741. [Google Scholar] [CrossRef]
- Petkova, P.S.P.; Francesko, A.; Perelshtein, I.; Gedanken, A.; Tzanov, T. Simultaneous sonochemical-enzymatic coating of medical textiles with antibacterial ZnO nanoparticles. Ultrason. Sonochem. 2016, 29, 244–250. [Google Scholar] [CrossRef]
- Shi, L.-E.; Li, Z.-H.; Zheng, W.; Zhao, Y.-F.; Jin, Y.-F.; Tang, Z.-X. Synthesis, antibacterial activity, antibacterial mechanism and food applications of ZnO nanoparticles: A review. Food Addit. Contam. Part A 2014, 31, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Strand, R.; Kjolberg, K.L. Regulating Nanoparticles: The Problem of Uncertainty. Eur. J. Law Technol. 2011, 2, 1–10. [Google Scholar]
- Jiménez-Rosado, M.; Perez-Puyana, V.; Rubio-Valle, J.F.; Guerrero, A.; Romero, A. Processing of biodegradable and multifunctional protein-based polymer materials for the potential controlled release of zinc and water in horticulture. J. Appl. Polym. Sci. 2020, 137, 49419. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Pérez-Puyana, V.; Cordobés, F.; Romero, A.; Guerrero, A. Development of soy protein-based matrices containing zinc as micronutrient for horticulture. Ind. Crop. Prod. 2018, 121, 345–351. [Google Scholar] [CrossRef]
- Chen, C.; Liu, P.; Lu, C. Synthesis and characterization of nano-sized ZnO powders by direct precipitation method. Chem. Eng. J. 2008, 144, 509–513. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Del Toro, A.; Aguilar, J.M.; Guerrero, A.; Bengoechea, C. Optimization of a thermal process for the production of superabsorbent materials based on a soy protein isolate. Ind. Crop. Prod. 2018, 125, 573–581. [Google Scholar] [CrossRef]
- ISO 178:2019, Plastics. Determination of Flexural Properties; ISO/TC 46; ISO: Geneva, Switzerland, 2019. [Google Scholar]
- ISO 570-2:1993, Plastics. Determination of Tensile Properties: Part 2: Test Conditions for Moulding and Extrusion Plastics; ISO/TC 46; ISO: Paris, France, 1993. [Google Scholar]
- ASTM Interntional. ASTM D570-98: Standard Test Method for Water Absorption of Plastics; ASTM Interntional: West Conshohocken, PA, USA, 2005. [Google Scholar]
- Essawy, H.A.; Ghazy, M.B.; El-Hai, F.A.; Mohamed, M.F. Superabsorbent hydrogels via graft polymerization of acrylic acid from chitosan-cellulose hybrid and their potential in controlled release of soil nutrients. Int. J. Biol. Macromol. 2016, 89, 144–151. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Bouroudian, E.; Perez-Puyana, V.; Guerrero, A.; Romero, A. Evaluation of different strengthening methods in the mechanical and functional properties of soy protein-based bioplastics. J. Clean. Prod. 2020, 262, 121517. [Google Scholar] [CrossRef]
- Jerez, A.; Partal, P.; Martínez, I.; Gallegos, C.; Guerrero, A. Protein-based bioplastics: Effect of thermo-mechanical processing. Rheol. Acta 2007, 46, 711–720. [Google Scholar] [CrossRef]
- Amjadi, S.; Emaminia, S.; Davudian, S.H.; Pourmohammad, S.; Hamishehkar, H.; Roufegarinejad, L. Preparation and characterization of gelatin-based nanocomposite containing chitosan nanofiber and ZnO nanoparticles. Carbohydr. Polym. 2019, 216, 376–384. [Google Scholar] [CrossRef]
- Ramesan, M.T.; Siji, C.; Kalaprasad, G.; Bahuleyan, B.K.; Al-Maghrabi, M.A. Effect of Silver Doped Zinc Oxide as Nanofiller for the Development of Biopolymer Nanocomposites from Chitin and Cashew Gum. J. Polym. Environ. 2018, 26, 2983–2991. [Google Scholar] [CrossRef]
- Saenghirunwattana, P.; Noomhorm, A.; Rungsardthong, V. Mechanical properties of soy protein based “green” composites reinforced with surface modified cornhusk fiber. Ind. Crop. Prod. 2014, 60, 144–150. [Google Scholar] [CrossRef]
- Fu, H.; Li, X.; Gong, W.; Tian, H.; Zhou, H. Enhanced electrical and dielectric properties of plasticized soy protein bioplastics through incorporation of nanosized carbon black. Polym. Compos. 2020, 25790. [Google Scholar] [CrossRef]
- Fernández-Espada, L.; Bengoechea, C.; Cordobés, F.; Guerrero, A. Thermomechanical properties and water uptake capacity of soy protein-based bioplastics processed by injection molding. J. Appl. Polym. Sci. 2016, 133, 43524. [Google Scholar] [CrossRef]
- Yue, H.-B.; Cui, Y.-D.; Shuttleworth, P.S.; Clark, J.H. Preparation and characterisation of bioplastics made from cottonseed protein. Green Chem. 2012, 14, 2009–2016. [Google Scholar] [CrossRef]
- Perez, V.; Felix, M.; Romero, A.; Guerrero, A.; Information, P.E.K.F.C. Characterization of pea protein-based bioplastics processed by injection moulding. Food Bioprod. Process. 2016, 97, 100–108. [Google Scholar] [CrossRef]
- Gómez-Heincke, D.; Martínez, I.; Stading, M.; Gallegos, C.; Partal, P. Improvement of mechanical and water absorption properties of plant protein based bioplastics. Food Hydrocoll. 2017, 73, 21–29. [Google Scholar] [CrossRef]
- Klüver, E.; Meyer, M. Thermoplastic processing, rheology, and extrudate properties of wheat, soy, and pea proteins. Polym. Eng. Sci. 2015, 55, 1912–1919. [Google Scholar] [CrossRef]
- Su, J.-F.; Huang, Z.; Yuan, X.-Y.; Wang, X.-Y.; Li, M. Structure and properties of carboxymethyl cellulose/soy protein isolate blend edible films crosslinked by Maillard reactions. Carbohydr. Polym. 2010, 79, 145–153. [Google Scholar] [CrossRef]
- Pourrahimi, A.M.; Liu, D.; Ström, V.; Hedenqvist, M.S.; Olsson, R.T.; Gedde, U. Heat treatment of ZnO nanoparticles: New methods to achieve high-purity nanoparticles for high-voltage applications. J. Mater. Chem. A 2015, 3, 17190–17200. [Google Scholar] [CrossRef] [Green Version]
- Gamero, S.; Jiménez-Rosado, M.; Romero, A.; Bengoechea, C.; Guerrero, A. Reinforcement of Soy Protein-Based Bioplastics Through Addition of Lignocellulose and Injection Molding Processing Conditions. J. Polym. Environ. 2019, 27, 1285–1293. [Google Scholar] [CrossRef]
- Korsmeyer, R.; Peppas, N. Swelling-controlled delivery systems for pharmaceutical applications: Macromolecular and modelling considerations. In Controlled Release Delivery Systems; Marcel Dekker: New York, NY, USA, 1983; pp. 77–90. [Google Scholar]
- Perez-Puyana, V.; Felix, M.; Romero, A.; Guerrero, A. Development of eco-friendly biodegradable superabsorbent materials obtained by injection moulding. J. Clean. Prod. 2018, 198, 312–319. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Rubio-Valle, J.F.; Perez-Puyana, V.; Guerrero, A.; Romero, A. Comparison between pea and soy protein-based bioplastics obtained by injection molding. J. Appl. Polym. Sci. 2020, 50412. [Google Scholar] [CrossRef]
- Abdullah, A.H.D.; Putri, O.D.; Fikriyyah, A.K.; Nissa, R.C.; Hidayat, S.; Septiyanto, R.F.; Karina, M.; Satoto, R. Harnessing the Excellent Mechanical, Barrier and Antimicrobial Properties of Zinc Oxide (ZnO) to Improve the Performance of Starch-based Bioplastic. Polym. Technol. Mater. 2020, 59, 1259–1267. [Google Scholar] [CrossRef]





| Maximum Release Time (min) | Degradation Time (Days) | |||
|---|---|---|---|---|
| 70 °C | 90 °C | 110 °C | ||
| 0 wt% | - | 40 | 60 | 70 |
| 1.0 wt% | 240 | 40 | 60 | 70 |
| 2.0 wt% | 270 | 20 | 40 | 50 |
| 4.5 wt% | 390 | 10 | 20 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Rosado, M.; Perez-Puyana, V.; Sánchez-Cid, P.; Guerrero, A.; Romero, A. Incorporation of ZnO Nanoparticles into Soy Protein-Based Bioplastics to Improve Their Functional Properties. Polymers 2021, 13, 486. https://doi.org/10.3390/polym13040486
Jiménez-Rosado M, Perez-Puyana V, Sánchez-Cid P, Guerrero A, Romero A. Incorporation of ZnO Nanoparticles into Soy Protein-Based Bioplastics to Improve Their Functional Properties. Polymers. 2021; 13(4):486. https://doi.org/10.3390/polym13040486
Chicago/Turabian StyleJiménez-Rosado, Mercedes, Víctor Perez-Puyana, Pablo Sánchez-Cid, Antonio Guerrero, and Alberto Romero. 2021. "Incorporation of ZnO Nanoparticles into Soy Protein-Based Bioplastics to Improve Their Functional Properties" Polymers 13, no. 4: 486. https://doi.org/10.3390/polym13040486
APA StyleJiménez-Rosado, M., Perez-Puyana, V., Sánchez-Cid, P., Guerrero, A., & Romero, A. (2021). Incorporation of ZnO Nanoparticles into Soy Protein-Based Bioplastics to Improve Their Functional Properties. Polymers, 13(4), 486. https://doi.org/10.3390/polym13040486