Highly Thermal Conductive and Electrical Insulating Epoxy Composites with a Three-Dimensional Filler Network by Sintering Silver Nanowires on Aluminum Nitride Surface
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of the AgNW- Deposited AlN Filler
2.3. Preparation of the 3D Network and EP Composites
2.4. Characterization
3. Results and Discussion
3.1. Composite Preparation Process and Filler Morphology
3.2. Filler Crystalline Structure
3.3. AgNW Coalescence during Heating
3.4. Morphologies of the 3D Network and Fabricated Composites
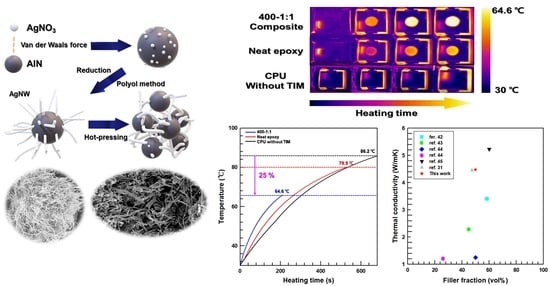
3.5. Thermal Properties of the Prepared 3D Network Composites
3.6. Electrical Properties of the Prepared 3D Network Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Worwood, D.; Kellner, Q.; Wojtala, M.; Widanage, W.D.; McGlen, R.; Greenwood, D.; Marco, J. A new approach to the internal thermal management of cylindrical battery cells for automotive applications. J. Power Sources 2017, 346, 151–166. [Google Scholar]
- Darcovich, K.; MacNeil, D.D.; Recoskie, S.; Cadic, Q.; Ilinca, F. Comparison of cooling plate configurations for automotive battery pack thermal management. Appl. Therm. Eng. 2019, 155, 185–195. [Google Scholar]
- Fu, C.; Li, Q.; Lu, J.; Mateti, S.; Cai, Q.; Zeng, X.; Du, G.; Sun, R.; Chen, Y.; Xu, J.; et al. Improving thermal conductivity of polymer composites by reducing interfacial thermal resistance between boron nitride nanotubes. Compos. Sci. Technol. 2018, 165, 322–330. [Google Scholar]
- Wang, Z.-G.; Gong, F.; Yu, W.-C.; Huang, Y.-F.; Zhu, L.; Lei, J.; Xu, J.-Z.; Li, Z.-M. Synergetic enhancement of thermal conductivity by constructing hybrid conductive network in the segregated polymer composites, Compos. Sci. Technol. 2018, 162, 7–13. [Google Scholar]
- Lule, Z.; Ju, H.; Kim, J. Effect of surface-modified Al2O3 on the thermomechanical properties of polybutylene succinate/Al2O3 composites. Ceram. Int. 2018, 44, 13530–13537. [Google Scholar]
- Oh, H.; Kim, Y.; Wie, J.; Kim, K.; Kim, J. Tailoring of Si–C–N–O ceramic-coated reduced graphene oxide by oil/water-solution process for high thermal conductive epoxy composite with electrical insulation. Compos. Sci. Technol. 2010, 197, 108257. [Google Scholar]
- Ryu, S.; Oh, T.; Kim, J. Surface modification of a BN/ETDS composite with aniline trimer for high thermal conductivity and excellent mechanical properties. RSC Adv. 2018, 8, 22846–22852. [Google Scholar]
- Kim, Y.; Oh, H.; Kim, J. Enhanced thermal conductivity of epoxy composites using boron nitride nanoplatelets prepared by Fe3O4 assisted liquid-phase exfoliation. Ceram. Int. 2019, 45, 24121–24126. [Google Scholar]
- Wu, Y.; Ye, K.; Liu, Z.; Wang, B.; Yan, C.; Wang, Z.; Lin, C.T.; Jiang, N.; Yu, J. Cotton Candy-Templated Fabrication of Three-Dimensional Ceramic Pathway within Polymer Composite for Enhanced Thermal Conductivity. ACS Appl. Mater. Interfaces 2019, 11, 44700–44707. [Google Scholar]
- Fang, H.; Zhang, X.; Zhao, Y.; Bai, S.-L. Dense graphene foam and hexagonal boron nitride filled PDMS composites with high thermal conductivity and breakdown strength. Compos. Sci. Technol. 2017, 152, 243–253. [Google Scholar]
- Lee, W.; Kim, J. Enhanced through-plane thermal conductivity of paper-like cellulose film with treated hybrid fillers comprising boron nitride and aluminum nitride. Compos. Sci. Technol. 2020, 200, 108424. [Google Scholar]
- Yang, X.; Guo, Y.; Han, Y.; Li, Y.; Ma, T.; Chen, M.; Kong, J.; Zhu, J.; Gu, J. Significant improvement of thermal conductivities for BNNS/PVA composite films via electrospinning followed by hot-pressing technology. Compos. B Eng. 2019, 175, 107070. [Google Scholar]
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal conductivity of polymer-based composites: Fundamentals and applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar]
- Kim, K.; Kim, J. Exfoliated boron nitride nanosheet/MWCNT hybrid composite for thermal conductive material via epoxy wetting. Compos. B Eng. 2018, 140, 9–15. [Google Scholar]
- Lule, Z.; Kim, J. Thermally conductive and highly rigid polylactic acid (PLA) hybrid composite filled with surface treated alumina/nano-sized aluminum nitride. Compos. Part A Appl. Sci. Manuf. 2019, 124, 105506. [Google Scholar]
- Lu, H.; Zhang, J.; Luo, J.; Gong, W.; Li, C.; Li, Q.; Zhang, K.; Hu, M.; Yao, Y. Enhanced thermal conductivity of free-standing 3D hierarchical carbon nanotube-graphene hybrid paper. Compos. Part A Appl. Sci. Manuf. 2017, 102, 1–8. [Google Scholar]
- Fu, C.; Yan, C.; Ren, L.; Zeng, X.; Du, G.; Sun, R.; Xu, J.; Wong, C.-P. Improving thermal conductivity through welding boron nitride nanosheets onto silver nanowires via silver nanoparticles. Compos. Sci. Technol. 2019, 177, 118–126. [Google Scholar]
- Barani, Z.; Mohammadzadeh, A.; Geremew, A.; Huang, C.Y.; Coleman, D.; Mangolini, L.; Kargar, F.; Balandin, A.A. Thermal Properties of the Binary-Filler Hybrid Composites with Graphene and Copper Nanoparticles. Adv. Funct. Mater. 2019, 30, 1904008. [Google Scholar]
- Slack, G.A.; Tanzilli, R.A.; Pohl, R.; Vandersande, J. The intrinsic thermal conductivity of AIN. J. Phys. Chem. Solids 1987, 48, 641–647. [Google Scholar]
- Wang, S.; Tian, Y.; Hang, C.; Wang, C. Cohesively enhanced electrical conductivity and thermal stability of silver nanowire networks by nickel ion bridge joining. Sci. Rep. 2018, 8, 5260. [Google Scholar]
- Yurko, E.O.; Gryaznova, T.V.; Kholin, K.V.; Khrizanforova, V.V.; Budnikova, Y.H. External oxidant-free cross-coupling: Electrochemically induced aromatic C-H phosphonation of azoles with dialkyl-H-phosphonates under silver catalysis. Dalton. Trans. 2017, 47, 190–196. [Google Scholar]
- Siow, K.; Lin, Y. Identifying the development state of sintered silver (Ag) as a bonding material in the microelectronic packaging via a patent landscape study. J. Electron. Packag. 2016, 138, 020804. [Google Scholar]
- Chen, C.; Xue, Y.; Li, Z.; Wen, Y.; Li, X.; Wu, F.; Li, X.; Shi, D.; Xue, Z.; Xie, X. Construction of 3D boron nitride nanosheets/silver networks in epoxy-based composites with high thermal conductivity via in-situ sintering of silver nanoparticles. Chem. Eng. J. 2019, 369, 1150–1160. [Google Scholar]
- Yu, C.; Gong, W.; Tian, W.; Zhang, Q.; Xu, Y.; Lin, Z.; Hu, M.; Fan, X.; Yao, Y. Hot-pressing induced alignment of boron nitride in polyurethane for composite films with thermal conductivity over 50 Wm−1 K−1. Compos. Sci. Technol. 2018, 160, 199–207. [Google Scholar]
- You, J.; Choi, H.-H.; Lee, Y.M.; Cho, J.; Park, M.; Lee, S.-S.; Park, J.H. Plasma-assisted mechanochemistry to produce polyamide/boron nitride nanocomposites with high thermal conductivities and mechanical properties. Compos. B Eng. 2019, 164, 710–719. [Google Scholar]
- Yu, C.; Zhang, J.; Li, Z.; Tian, W.; Wang, L.; Luo, J.; Li, Q.; Fan, X.; Yao, Y. Enhanced through-plane thermal conductivity of boron nitride/epoxy composites. Compos. Part A Appl. Sci. Manuf. 2017, 98, 25–31. [Google Scholar]
- Yao, Y.; Sun, J.; Zeng, X.; Sun, R.; Xu, J.B.; Wong, C.P. Construction of 3D Skeleton for Polymer Composites Achieving a High Thermal Conductivity. Small 2018, 14, e1704044. [Google Scholar]
- Yang, G.Y.; Chen, L.; Jiang, P.; Guo, Z.Y.; Wang, W.; Liu, Z.P. Fabrication of tunable 3D graphene mesh network with enhanced electrical and thermal properties for high-rate aluminum-ion battery application. RSC Adv. 2016, 6, 47655–47660. [Google Scholar]
- Jiang, Y.; Liu, Y.; Min, P.; Sui, G. BN@PPS core-shell structure particles and their 3D segregated architecture composites with high thermal conductivities. Compos. Sci. Technol. 2017, 144, 63–69. [Google Scholar]
- Zhou, H.; Wang, H.; Du, X.; Zhang, Y.; Zhou, H.; Yuan, H.; Liu, H.-Y.; Mai, Y.-W. Facile fabrication of large 3D graphene filler modified epoxy composites with improved thermal conduction and tribological performance. Carbon 2018, 139, 1168–1177. [Google Scholar]
- Wei, Z.; Xie, W.; Ge, B.; Zhang, Z.; Yang, W.; Xia, H.; Wang, B.; Jin, H.; Gao, N.; Shi, Z. Enhanced thermal conductivity of epoxy composites by constructing aluminum nitride honeycomb reinforcements. Compos. Sci. Technol. 2020, 199, 108304. [Google Scholar]
- Xu, X.; Hu, R.; Chen, M.; Dong, J.; Xiao, B.; Wang, Q.; Wang, H. 3D boron nitride foam filled epoxy composites with significantly enhanced thermal conductivity by a facial and scalable approach. Chem. Eng. J. 2020, 397, 125447. [Google Scholar]
- Coskun, S.; Aksoy, B.; Unalan, H.E. Polyol Synthesis of Silver Nanowires: An Extensive Parametric Study. Cryst. Growth Des. 2011, 11, 4963–4969. [Google Scholar]
- Zhan, K.; Su, R.; Bai, S.; Yu, Z.; Cheng, N.; Wang, C.; Xu, S.; Liu, W.; Guo, S.; Zhao, X.Z. One-pot stirring-free synthesis of silver nanowires with tunable lengths and diameters via a Fe(3+) & Cl(−) co-mediated polyol method and their application as transparent conductive films. Nanoscale 2016, 8, 18121–18133. [Google Scholar]
- Lee, H.; Yeo, J.; Lee, J.; Cho, H.; Kwon, J.; Han, S.; Kim, S.; Hong, S.; Ko, S.H. Selective Thermochemical Growth of Hierarchical ZnO Nanowire Branches on Silver Nanowire Backbone Percolation Network Heaters. J. Phys. Chem. C 2017, 121, 22542–22549. [Google Scholar]
- Wang, X.; Qiang, D.; Hosier, I.; Zhu, Y.; Chen, G.; Andritsch, T. Effect of water on the breakdown and dielectric response of polypropylene/nano-aluminium nitride composites. J. Mater. Sci. 2020, 55, 8900–8916. [Google Scholar]
- Song, J.; Huang, Y.; Fan, Y.; Zhao, Z.; Yu, W.; Rasco, B.A.; Lai, K. Detection of Prohibited Fish Drugs Using Silver Nanowires as Substrate for Surface-Enhanced Raman Scattering. Nanomaterials 2016, 6, 175. [Google Scholar]
- Lee, K.-M.; Weissgärber, T.; Kieback, B. Microstructural and chemical properties of AlN-Cu nanocomposite powders prepared by planetary ball milling. J. Mater. Sci. 2004, 39, 5235–5238. [Google Scholar]
- Richardson, J.; Crump, J. Crystallite size distributions of sintered nickel catalysts. J. Catal. 1979, 57, 417–425. [Google Scholar]
- Moon, K.-S.; Dong, H.; Maric, R.; Pothukuchi, S.; Hunt, A.; Li, Y.; Wong, C. Thermal behavior of silver nanoparticles for low-temperature interconnect applications. J. Electron. Mater. 2005, 34, 168–175. [Google Scholar]
- Yang, L.; Ji, W.; Zhang, Z.; Jin, X. Thermal conductivity enhancement of water by adding graphene Nano-sheets: Consideration of particle loading and temperature effects. Int. Commun. Heat Mass Transf. 2019, 109, 104353. [Google Scholar]
- Choi, S.; Kim, J. Thermal conductivity of epoxy composites with a binary-particle system of aluminum oxide and aluminum nitride fillers. Compos. B Eng. 2013, 51, 140–147. [Google Scholar]
- Rybak, A.; Gaska, K. Functional composites with core–shell fillers: I. Particle synthesis and thermal conductivity measurements. J. Mater. Sci. 2015, 50, 7779–7789. [Google Scholar]
- Teng, C.-C.; Ma, C.-C.M.; Chiou, K.-C.; Lee, T.-M. Synergetic effect of thermal conductive properties of epoxy composites containing functionalized multi-walled carbon nanotubes and aluminum nitride. Compos. B Eng. 2012, 43, 265–271. [Google Scholar]
- Dang, T.M.L.; Kim, C.-Y.; Zhang, Y.; Yang, J.-F.; Masaki, T.; Yoon, D.-H. Enhanced thermal conductivity of polymer composites via hybrid fillers of anisotropic aluminum nitride whiskers and isotropic spheres. Compos. B Eng. 2017, 114, 237–246. [Google Scholar]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.; Kim, J. Highly Thermal Conductive and Electrical Insulating Epoxy Composites with a Three-Dimensional Filler Network by Sintering Silver Nanowires on Aluminum Nitride Surface. Polymers 2021, 13, 694. https://doi.org/10.3390/polym13050694
Lee W, Kim J. Highly Thermal Conductive and Electrical Insulating Epoxy Composites with a Three-Dimensional Filler Network by Sintering Silver Nanowires on Aluminum Nitride Surface. Polymers. 2021; 13(5):694. https://doi.org/10.3390/polym13050694
Chicago/Turabian StyleLee, Wondu, and Jooheon Kim. 2021. "Highly Thermal Conductive and Electrical Insulating Epoxy Composites with a Three-Dimensional Filler Network by Sintering Silver Nanowires on Aluminum Nitride Surface" Polymers 13, no. 5: 694. https://doi.org/10.3390/polym13050694
APA StyleLee, W., & Kim, J. (2021). Highly Thermal Conductive and Electrical Insulating Epoxy Composites with a Three-Dimensional Filler Network by Sintering Silver Nanowires on Aluminum Nitride Surface. Polymers, 13(5), 694. https://doi.org/10.3390/polym13050694