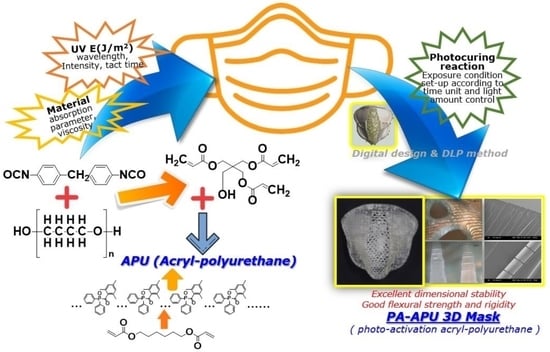
Highly Flexible and Photo-Activating Acryl-Polyurethane for 3D Steric Architectures
Abstract
:1. Introduction
2. Material and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kadry, H.; Wadnap, S.; Xu, C.; Ahsan, F. Digital light processing (DLP) 3D-printing technology and photoreac-tive polymers in fabrication of modified-release tablets. Eur. J. Pharm. Sci. 2019, 135, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, Y.; Lin, X.; Yang, Q.; Yang, G. Printability of External and Internal Structures Based on Digital Light Processing 3D Printing Technique. Pharmaceutics 2020, 12, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metral, B.; Bischoff, A.; Ley, C.; Ibrahim, A.; Allonas, X. Photochemical Study of a Three-Component Photocyclic Initiating System for Free Radical Photopolymerization: Implementing a Model for Digital Light Processing 3D Printing. ChemPhotoChem 2019, 3, 1109–1118. [Google Scholar] [CrossRef]
- Xiang, H.; Wang, X.; Ou, Z.; Lin, G.; Yin, J.; Liu, Z.; Zhang, L.; Liu, X. UV-curable, 3D printable and biocompatible silicone elastomers. Prog. Org. Coatings 2019, 137, 105372. [Google Scholar] [CrossRef]
- Li, J.; Cui, Y.; Qin, K.; Yu, J.; Guo, C.; Yang, J.; Zhang, C.; Jiang, D.; Wang, X. Synthesis and properties of a low-viscosity UV-curable oligomer for three-dimensional printing. Polym. Bull. 2015, 73, 571–585. [Google Scholar] [CrossRef]
- Gurr, M.; Hofmann, D.; Ehm, M.; Thomann, Y.; Kübler, R.; Mülhaupt, R. Acrylic Nanocomposite Resins for Use in Stereolithography and Structural Light Modulation Based Rapid Prototyping and Rapid Manufacturing Tech-nologies. Adv. Funct. Mater. 2008, 18, 2390–2397. [Google Scholar] [CrossRef]
- Cortés, A.; Sánchez-Romate, X.F.; Jiménez-Suárez, A.; Campo, M.; Ureña, A.; Prolongo, S.G. Mechanical and Strain-Sensing Capabilities of Carbon Nanotube Reinforced Composites by Digital Light Processing 3D Printing Technology. Pharmaceutics 2020, 12, 975. [Google Scholar] [CrossRef]
- Joo, H.; Cho, S. Comparative Studies on Polyurethane Composites Filled with Polyaniline and Graphene for DLP-Type 3D Printing. Pharmaceutics 2020, 12, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, J.; Yin, H.; Yu, X.; Xie, C.; Jiang, H.; Jin, Y.; Sheng, F. Combination of 3D printing technologies and compressed tablets for preparation of riboflavin floating tablet-in-device (TiD) systems. Int. J. Pharm. 2018, 549, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Kuebler, S.M.; Braun, K.L.; Zhou, W.; Cammack, J.; Yu, T.; Ober, C.K.; Marder, S.R.; Perry, J.W. Design and application of high-sensitivity two-photon initiators for three-dimensional microfabrication. J. Photochem. Photobiol. A Chem. 2003, 158, 163–170. [Google Scholar] [CrossRef]
- Schemidt, K.A.; Doan, V.A.; Xu, P.; Stockwell, J.S.; Holden, S.K. (3D Systems, Inc.) Ultra-Violet Light Curable Hot Melt Composition. U.S. Patent 7378460B2, 27 May 2008. [Google Scholar]
- Khosravani, M.R.; Reinicke, T. 3D-printed sensors: Current progress and future challenges. Sens. Actuator A-Phys. 2020, 305, 111916. [Google Scholar] [CrossRef]
- Jiménez, M.; Romero, L.; Domínguez, I.A.; Espinosa, M.D.M.; Domínguez, M. Additive Manufacturing Technologies: An Overview about 3D Printing Methods and Future Prospects. Complexity 2019, 2019, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A Review of 3D Printing Technology for Medical Ap-plications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Taormina, G.; Sciancalepore, C.; Messori, M.; Bondioli, F. 3D printing processes for photocurable polymeric materials: Technologies, materials, and future trends. J. Appl. Biomater. Funct. Mater. 2018, 16, 151–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stampfl, J.; Wöß, A.; Seidler, S.; Fouad, H.; Pisaipan, A.; Schwager, F.; Liska, R. Water Soluble, Photocurable Resins for Rapid Prototyping Applications. Macromol. Symp. 2004, 217, 99–108. [Google Scholar] [CrossRef]
- Choi, S.-J.; Kim, H.N.; Bae, W.G.; Suh, K.-Y. Modulus- and surface energy-tunable ultraviolet-curable polyure-thane acrylate: Properties and applications. J. Mater. Chem. 2011, 21, 14325–14335. [Google Scholar] [CrossRef]
- Paweł, F.; Joanna, O. A New Approach to Micromachining: High-Precision and Innovative Additive Manufac-turing Solutions Based on Photopolymerization Technology. Materials 2020, 13, 2951. [Google Scholar]
- Cristian, M.F.; Juliana, O.; Ikerne, E.; José Luis, V.V.; Senentxu, L.M. State-of-the-Art and Future Challenges of UV Curable Polymer-Based Smart Materials for Printing Technologies. Adv. Mater. Technol. 2009, 4, 1800618. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, J.-H.; Won, J.C.; Lim, W.B.; Lee, J.H.; Min, J.G.; Kim, S.W.; Kim, J.-H.; Huh, P. Highly Flexible and Photo-Activating Acryl-Polyurethane for 3D Steric Architectures. Polymers 2021, 13, 844. https://doi.org/10.3390/polym13060844
Bae J-H, Won JC, Lim WB, Lee JH, Min JG, Kim SW, Kim J-H, Huh P. Highly Flexible and Photo-Activating Acryl-Polyurethane for 3D Steric Architectures. Polymers. 2021; 13(6):844. https://doi.org/10.3390/polym13060844
Chicago/Turabian StyleBae, Ji-Hong, Jong Chan Won, Won Bin Lim, Ju Hong Lee, Jin Gyu Min, Si Woo Kim, Ji-Hyo Kim, and PilHo Huh. 2021. "Highly Flexible and Photo-Activating Acryl-Polyurethane for 3D Steric Architectures" Polymers 13, no. 6: 844. https://doi.org/10.3390/polym13060844
APA StyleBae, J. -H., Won, J. C., Lim, W. B., Lee, J. H., Min, J. G., Kim, S. W., Kim, J. -H., & Huh, P. (2021). Highly Flexible and Photo-Activating Acryl-Polyurethane for 3D Steric Architectures. Polymers, 13(6), 844. https://doi.org/10.3390/polym13060844