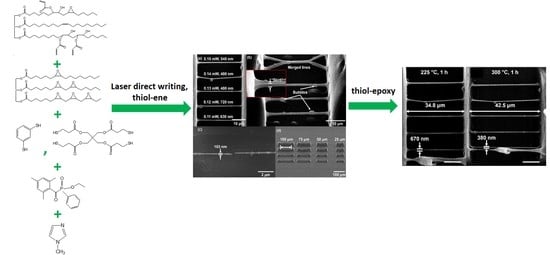
Vegetable Oil-Based Thiol-Ene/Thiol-Epoxy Resins for Laser Direct Writing 3D Micro-/Nano-Lithography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Rheometry
2.3. Preparation of Cross-Linked Polymers
2.4. Characterization Techniques
3. Results
3.1. Study of Thermal Thiol-Epoxy Curing Process
3.2. Monitoring Cross-Linking Kinetics by Rheometry
3.3. Characterization of Cross-Linked Polymer Structure
3.4. Thermal Characterization of the Materials
3.5. Mechanical Properties
3.6. Characterization of 3D Structures Produced by LDW
4. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barner-Kowollik, C.; Bastmeyer, M.; Blasco, E.; Delaittre, G.; Müller, P.; Richter, B.; Wegener, M. 3D Laser Micro- and Nanoprinting: Challenges for Chemistry. Angew. Chem. Int. Ed. 2017, 56, 15828–15845. [Google Scholar] [CrossRef]
- Skliutas, E.; Lebedevaite, M.; Kabouraki, E.; Baldacchini, T.; Ostrauskaite, J.; Vamvakaki, M.; Farsari, M.; Juodkazis, S.; Malinauskas, M. Polymerization mechanisms initiated by spatio-temporally confined light. Nanophotonics 2021, 10, 1211–1242. [Google Scholar] [CrossRef]
- Li, F.; Macdonald, N.P.; Guijt, R.M.; Breadmore, M.C. Increasing the functionalities of 3D printed microchemical devices by single material, multimaterial, and print-pause-print 3D printing. Lab Chip 2018, 19, 35–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.-L.; Qi, Y.-N.; Yin, X.-J.; Yang, X.; Chen, C.-M.; Yu, J.-Y.; Yu, J.-C.; Lin, Y.-M.; Hui, F.; Liu, P.-L.; et al. Polymer-Based Device Fabrication and Applications Using Direct Laser Writing Technology. Polymers 2019, 11, 553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telitel, S.; Morris, J.C.; Guillaneuf, Y.; Clément, J.-L.; Morlet-Savary, F.; Spangenberg, A.; Malval, J.-P.; Lalevée, J.; Gigmes, D.; Soppera, O. Laser Direct Writing of Arbitrary Complex Polymer Microstructures by Nitroxide-Mediated Photopolymerization. ACS Appl. Mater. Interfaces 2020, 12, 30779–30786. [Google Scholar] [CrossRef]
- Konuray, O.; Fernández-Francos, X.; Ramis, X.; Serra, À. State of the Art in Dual-Curing Acrylate Systems. Polymers 2018, 10, 178. [Google Scholar] [CrossRef] [Green Version]
- Ramis, X.; Fernandez-Francos, X.; de La Flor, S.; Ferrando, F.; Serra, A. Click-Based Dual-Curing Thermosets and Their Applications. In Thermosets: Structure, Properties, and Applications, 2nd ed.; Guo, Q., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 511–541. [Google Scholar] [CrossRef]
- Bauer, J.; Izard, A.G.; Zhang, Y.; Baldacchini, T.; Valdevit, L. Thermal post-curing as an efficient strategy to eliminate process parameter sensitivity in the mechanical properties of two-photon polymerized materials. Opt. Express 2020, 28, 20362–20371. [Google Scholar] [CrossRef]
- Kuang, X.; Zhao, Z.; Chen, K.; Fang, D.; Kang, G.; Qi, H.J. High-Speed 3D Printing of High-Performance Thermosetting Polymers via Two-Stage Curing. Macromol. Rapid Commun. 2018, 39, e1700809. [Google Scholar] [CrossRef] [PubMed]
- Konuray, O.; Di Donato, F.; Sangermano, M.; Bonada, J.; Tercjak, A.; Fernandez-Francos, X.; Serra, A.; Ramis, X. Dual-curable stereolithography resins for superior thermomechanical properties. Express Polym. Lett. 2020, 14, 881–894. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, M.; Ji, M.; Kuang, X.; Qi, H.J.; Wang, T. Recyclable thermosetting polymers for digital light processing 3D printing. Mater. Des. 2021, 197, 109189. [Google Scholar] [CrossRef]
- Fernández-Francos, X.; Konuray, O.; Ramis, X.; Serra, À.; de La Flor, S. Enhancement of 3D-Printable Materials by Dual-Curing Procedures. Materials 2020, 14, 107. [Google Scholar] [CrossRef]
- Konuray, O.; Fernández-Francos, X.; de La Flor, S.; Ramis, X.; Serra, À. The Use of Click-Type Reactions in the Preparation of Thermosets. Polymers 2020, 12, 1084. [Google Scholar] [CrossRef]
- Khire, V.S.; Lee, T.Y.; Bowman, C.N. Synthesis, Characterization and Cleavage of Surface-Bound Linear Polymers Formed Using Thiol—Ene Photopolymerizations. Macromolecules 2008, 41, 7440–7447. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol—Ene Click Chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef]
- Carioscia, J.A.; Schneidewind, L.; O’Brien, C.; Ely, R.; Feeser, C.; Cramer, N.; Bowman, C.N. Thiol—Norbornene materials: Approaches to develop high Tg Thiol—Ene polymers. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 5686–5696. [Google Scholar] [CrossRef]
- Liao, C.; Kannan, K. Concentrations and Profiles of Bisphenol A and Other Bisphenol Analogues in Foodstuffs from the United States and Their Implications for Human Exposure. J. Agric. Food Chem. 2013, 61, 4655–4662. [Google Scholar] [CrossRef] [PubMed]
- Michałowicz, J. Bisphenol A—Sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738–758. [Google Scholar] [CrossRef]
- Rochester, J.R.; Bolden, A.L.; Kwiatkowski, C.F. Prenatal exposure to bisphenol A and hyperactivity in children: A systematic review and meta-analysis. Environ. Int. 2018, 114, 343–356. [Google Scholar] [CrossRef]
- Onundi, Y.; Drake, B.A.; Malecky, R.T.; DeNardo, M.A.; Mills, M.R.; Kundu, S.; Ryabov, A.D.; Beach, E.S.; Horwitz, C.P.; Simonich, M.T.; et al. A multidisciplinary investigation of the technical and environmental performances of TAML/peroxide elimination of Bisphenol A compounds from water. Green Chem. 2017, 19, 4234–4262. [Google Scholar] [CrossRef] [Green Version]
- Cakmakci, E.; Şen, F.; Kahraman, M.V. Isosorbide Diallyl Based Antibacterial Thiol—Ene Photocured Coatings Containing Polymerizable Fluorous Quaternary Phosphonium Salt. ACS Sustain. Chem. Eng. 2019, 7, 10605–10615. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Q.; Cheng, J.; Zhang, J. A fully biomass based monomer from itaconic acid and eugenol to build degradable thermosets via Thiol—Ene click chemistry. Green Chem. 2020, 22, 921–932. [Google Scholar] [CrossRef]
- Dai, J.; Ma, S.; Zhu, L.; Wang, S.; Yang, L.; Song, Z.; Liu, X.; Zhu, J. UV-thermal dual cured anti-bacterial Thiol—Ene networks with superior performance from renewable resources. Polymer 2017, 108, 215–222. [Google Scholar] [CrossRef]
- Jawerth, M.; Johansson, M.; Lundmark, S.; Gioia, C.; Lawoko, M. Renewable Thiol–Ene Thermosets Based on Refined and Selectively Allylated Industrial Lignin. ACS Sustain. Chem. Eng. 2017, 5, 10918–10925. [Google Scholar] [CrossRef]
- Navaruckiene, A.; Kasetaite, S.; Ostrauskaite, J. Vanillin-based thiol-ene systems as photoresins for optical 3D printing. Rapid Prototyp. J. 2019, 26, 402–408. [Google Scholar] [CrossRef]
- Navaruckiene, A.; Bridziuviene, D.; Raudoniene, V.; Rainosalo, E.; Ostrauskaite, J. Influence of Vanillin Acrylate-Based Resin Composition on Resin Photocuring Kinetics and Antimicrobial Properties of the Resulting Polymers. Materials 2021, 14, 653. [Google Scholar] [CrossRef]
- Wang, C.; Ding, L.; He, M.; Wei, J.; Li, J.; Lu, R.; Xie, H.; Cheng, R. Facile one-step synthesis of bio-based AESO resins. Eur. J. Lipid Sci. Technol. 2016, 118, 1463–1469. [Google Scholar] [CrossRef]
- Kasetaite, S.; de La Flor, S.; Serra, À..; Ostrauskaitė, J. Effect of Selected Thiols on Cross-Linking of Acrylated Epoxidized Soybean Oil and Properties of Resulting Polymers. Polymers 2018, 10, 439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miezinyte, G.; Ostrauskaite, J.; Rainosalo, E.; Skliutas, E.; Malinauskas, M. Photoresins based on acrylated epoxidized soybean oil and benzenedithiols for optical 3D printing. Rapid Prototyp. J. 2019, 25, 378–387. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lowe, A.B.; Bowman, C.N. Thiol-click chemistry: A multifaceted toolbox for small molecule and polymer synthesis. Chem. Soc. Rev. 2010, 39, 1355–1387. [Google Scholar] [CrossRef]
- Cengiz, N.; Rao, J.; Sanyal, A.; Khan, A. Designing functionalizable hydrogels through Thiol—Epoxy coupling chemistry. Chem. Commun. 2013, 49, 11191–11193. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, A.; Guzmán, D.; Fernández-Francos, X.; de La Flor, S. Effect of the Network Structure and Programming Temperature on the Shape-Memory Response of Thiol-Epoxy “Click” Systems. Polymers 2015, 7, 2146–2164. [Google Scholar] [CrossRef]
- De, S.; Khan, A. Efficient synthesis of multifunctional polymers via Thiol—Epoxy “click” chemistry. Chem. Commun. 2012, 48, 3130–3132. [Google Scholar] [CrossRef] [PubMed]
- Konuray, A.O.; Fernández-Francos, X.; Ramis, X. Latent curing of epoxy-thiol thermosets. Polymer 2017, 116, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Francos, X.; Konuray, A.-O.; Belmonte, A.; de La Flor, S.; Serra, À.; Ramis, X. Sequential curing of off-stoichiometric Thiol—Epoxy thermosets with a custom-tailored structure. Polym. Chem. 2016, 7, 2280–2290. [Google Scholar] [CrossRef] [Green Version]
- Endo, T.; Sanda, F. Design of latent catalysts and their application to polymer synthesis. Macromol. Symp. 1996, 107, 237–242. [Google Scholar] [CrossRef]
- Miao, S.; Wang, P.; Su, Z.; Zhang, S. Vegetable-oil-based polymers as future polymeric biomaterials. Acta Biomater. 2014, 10, 1692–1704. [Google Scholar] [CrossRef]
- Nouailhas, H.; Aouf, C.; Le Guerneve, C.; Caillol, S.; Boutevin, B.; Fulcrand, H. Synthesis and properties of biobased epoxy resins. Part 1. Glycidylation of flavonoids by epichlorohydrin. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 2261–2270. [Google Scholar] [CrossRef] [Green Version]
- Aouf, C.; Benyahya, S.; Esnouf, A.; Caillol, S.; Boutevin, B.; Fulcrand, H. Tara tannins as phenolic precursors of thermosetting epoxy resins. Eur. Polym. J. 2014, 55, 186–198. [Google Scholar] [CrossRef]
- Benyahya, S.; Aouf, C.; Caillol, S.; Boutevin, B.; Pascault, J.P.; Fulcrand, H. Functionalized green tea tannins as phenolic prepolymers for bio-based epoxy resins. Ind. Crop. Prod. 2014, 53, 296–307. [Google Scholar] [CrossRef]
- A Baroncini, E.; Yadav, S.K.; Palmese, G.R.; Stanzione, J.F. Recent advances in bio-based epoxy resins and bio-based epoxy curing agents. J. Appl. Polym. Sci. 2016, 133, 44103. [Google Scholar] [CrossRef] [Green Version]
- Shibata, M.; Ohkita, T. Fully biobased epoxy resin systems composed of a vanillin-derived epoxy resin and renewable phenolic hardeners. Eur. Polym. J. 2017, 92, 165–173. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, L.; Zhang, Y.; Liu, Q.; Ru, C.; Zhang, W.; Zhao, C. Novel biobased epoxy resin thermosets derived from eugenol and vanillin. Polym. Degrad. Stab. 2019, 160, 45–52. [Google Scholar] [CrossRef]
- Guzmán, D.; Ramis, X.; Fernández-Francos, X.; de La Flor, S.; Serra, A. New bio-based materials obtained by thiol-ene/thiol-epoxy dual curing click procedures from eugenol derivates. Eur. Polym. J. 2017, 93, 530–544. [Google Scholar] [CrossRef]
- Guzmán, D.; Ramis, X.; Fernández-Francos, X.; de La Flor, S.; Serra, A. Preparation of new biobased coatings from a triglycidyl eugenol derivative through thiol-epoxy click reaction. Prog. Org. Coat. 2018, 114, 259–267. [Google Scholar] [CrossRef]
- Guzmán, D.; Serra, A.; Ramis, X.; Fernández-Francos, X.; de La Flor, S. Fully renewable thermosets based on bis-eugenol prepared by thiol-click chemistry. React. Funct. Polym. 2019, 136, 153–166. [Google Scholar] [CrossRef]
- Guzmán, D.; Santiago, D.; Serra, À.; Ferrando, F. Novel Bio-Based Epoxy Thermosets Based on Triglycidyl Phloroglucinol Prepared by Thiol-Epoxy Reaction. Polymers 2020, 12, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fourcade, D.; Ritter, B.S.; Walter, P.; Schönfeld, R.; Mülhaupt, R. Renewable resource-based epoxy resins derived from multifunctional poly(4-hydroxybenzoates). Green Chem. 2013, 15, 910–918. [Google Scholar] [CrossRef]
- Güner, F.S.; Yağcı, Y.; Erciyes, A.T. Polymers from triglyceride oils. Prog. Polym. Sci. 2006, 31, 633–670. [Google Scholar] [CrossRef]
- Xia, Y.; LaRock, R.C. Vegetable oil-based polymeric materials: Synthesis, properties, and applications. Green Chem. 2010, 12, 1893–1909. [Google Scholar] [CrossRef]
- Zhang, C.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R. Recent advances in vegetable oil-based polymers and their composites. Prog. Polym. Sci. 2017, 71, 91–143. [Google Scholar] [CrossRef]
- Llevot, A. Sustainable Synthetic Approaches for the Preparation of Plant Oil-Based Thermosets. J. Am. Oil Chem. Soc. 2016, 94, 169–186. [Google Scholar] [CrossRef]
- Ng, F.; Couture, G.; Philippe, C.; Boutevin, B.; Caillol, S. Bio-Based Aromatic Epoxy Monomers for Thermoset Materials. Molecules 2017, 22, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, S.K.; Mohanty, S.; Nayak, S.K. Synthesis and characterization of bio-based epoxy blends from renewable resource based epoxidized soybean oil as reactive diluent. Chin. J. Polym. Sci. 2014, 33, 137–152. [Google Scholar] [CrossRef]
- Kumar, S.; Krishnan, S.; Mohanty, S.; Nayak, S.K. Synthesis and characterization of petroleum and biobased epoxy resins: A review. Polym. Int. 2018, 67, 815–839. [Google Scholar] [CrossRef]
- John, G.; Nagarajan, S.; Vemula, P.K.; Silverman, J.R.; Pillai, C. Natural monomers: A mine for functional and sustainable materials—Occurrence, chemical modification and polymerization. Prog. Polym. Sci. 2019, 92, 158–209. [Google Scholar] [CrossRef]
- Yim, Y.-J.; Rhee, K.Y.; Park, S.-J. Fracture toughness and ductile characteristics of diglycidyl ether of bisphenol-A resins modified with biodegradable epoxidized linseed oil. Compos. Part B Eng. 2017, 131, 144–152. [Google Scholar] [CrossRef]
- Feng, Y.; Hu, Y.; Man, L.; Yuan, T.; Zhang, C.; Yang, Z. Biobased thiol-epoxy shape memory networks from gallic acid and vegetable oils. Eur. Polym. J. 2019, 112, 619–628. [Google Scholar] [CrossRef]
- Lebedevaite, M.; Ostrauskaite, J. Influence of photoinitiator and temperature on photocross-linking kinetics of acrylated epoxidized soybean oil and properties of the resulting polymers. Ind. Crop. Prod. 2021, 161, 113210. [Google Scholar] [CrossRef]
- Krongauz, V.V. Diffusion in polymers dependence on crosslink density. J. Therm. Anal. Calorim. 2010, 102, 435–445. [Google Scholar] [CrossRef]
- DeVoe, R.J.; Kalweit, H.W.; Leatherdale, C.A.; Williams, T.R. Voxel Shapes in Two-Photon Microfabrication. In Multiphoton Absorption and Nonlinear Transmission Processes: Materials, Theory, and Applications, Proceedings of the International Symposium on Optical Science and Technology, Seattle, WA, USA, 14 February 2003; SPIE: Bellingham, WA, USA, 2003. [Google Scholar] [CrossRef]
- Lebedevaite, M.; Ostrauskaite, J.; Skliutas, E.; Malinauskas, M. Photoinitiator Free Resins Composed of Plant-Derived Monomers for the Optical µ-3D Printing of Thermosets. Polymers 2019, 11, 116. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, D.; Ramis, X.; Fernández-Francos, X.; Serra, A. Enhancement in the Glass Transition Temperature in Latent Thiol-Epoxy Click Cured Thermosets. Polymers 2015, 7, 680–694. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, D.; Ramis, X.; Fernández-Francos, X.; Serra, A. Preparation of click thiol-ene/thiol-epoxy thermosets by controlled photo/thermal dual curing sequence. RSC Adv. 2015, 5, 101623–101633. [Google Scholar] [CrossRef] [Green Version]
- Rothammer, M.; Heep, M.-C.; Von Freymann, G.; Zollfrank, C. Enabling direct laser writing of cellulose-based submicron architectures. Cellulose 2018, 25, 6031–6039. [Google Scholar] [CrossRef]
- Skliutas, E.; Lebedevaite, M.; Kasetaite, S.; Rekštytė, S.; Lileikis, S.; Ostrauskaite, J.; Malinauskas, M. A Bio-Based Resin for a Multi-Scale Optical 3D Printing. Sci. Rep. 2020, 10, 9758. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, B.; Gu, M. Engineering stop gaps of inorganic-organic polymeric 3D woodpile photonic crystals with post-thermal treatment. Opt. Express 2008, 16, 20073–20080. [Google Scholar] [CrossRef]
- Seniutinas, G.; Weber, A.; Padeste, C.; Sakellari, I.; Farsari, M.; David, C. Beyond 100 nm resolution in 3D laser lithography—Post processing solutions. Microelectron. Eng. 2018, 191, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Gailevičius, D.; Padolskytė, V.; Mikoliūnaitė, L.; Šakirzanovas, S.; Juodkazis, S.; Malinauskas, M. Additive-manufacturing of 3D glass-ceramics down to nanoscale resolution. Nanoscale Horiz. 2018, 4, 647–651. [Google Scholar] [CrossRef]
- Sharipova, M.I.; Baluyan, T.G.; Abrashitova, K.A.; Kulagin, G.E.; Petrov, A.K.; Chizhov, A.S.; Shatalova, T.B.; Chubich, D.; Kolymagin, D.A.; Vitukhnovsky, A.G.; et al. Effect of pyrolysis on microstructures made of various photoresists by two-photon polymerization: Comparative study. Opt. Mater. Express 2021, 11, 371–384. [Google Scholar] [CrossRef]







| Resin * | Thiol | Amount of AESO, (wt.%) | Amount of ELO, (wt.%) | Amount of thiol, (wt.%) | Amount of TPOL, (wt.%) | Amount of 1MI, (wt.%) | Viscosity η, Pa·s |
|---|---|---|---|---|---|---|---|
| 100A | 1,3BDT | 88.16 | 0 | 10.53 | 1.31 | 0 | 17.26 ± 0.40 |
| 75A/25B | 65.56 | 17.03 | 15.26 | 0.98 | 1.17 | 13.32 ± 0.23 | |
| 50A/50B | 43.26 | 33.75 | 19.98 | 0.58 | 2.43 | 3.71 ± 0.69 | |
| 25A/75B | 21.43 | 50.16 | 24.51 | 0.29 | 3.61 | 0.54 ± 0.01 | |
| 100B | 0 | 66.41 | 29.01 | 0 | 4.58 | 0.12 ± 0.01 | |
| 100C | PETMP | 81.57 | 0 | 17.43 | 1.00 | 0 | 10.06 ± 0.02 |
| 75C/25D | 60.61 | 13.93 | 23.54 | 0.75 | 1.17 | 4.03 ± 0.02 | |
| 50C/50D | 40.00 | 27.67 | 29.42 | 0.49 | 2.42 | 2.10 ± 0.09 | |
| 25C/75D | 19.80 | 41.09 | 35.24 | 0.25 | 3.62 | 1.90 ± 0.10 | |
| 100D | 0 | 54.20 | 41.22 | 0 | 4.58 | 0.55 ± 0.02 |
| Resin | Proportion of 1MI (phr 1) | ∆h 2 (J/g) | ∆h 3 (kJ/eq) |
|---|---|---|---|
| 100B | 1 | 400.1 | 93.5 |
| 2 | 405.6 | 95.8 | |
| 3 | 409.3 | 97.5 | |
| 4 | 427.4 | 102.9 | |
| 5 | 443.8 | 107.8 | |
| 100D | 1 | 139.4 | 39.7 |
| 2 | 190.0 | 57.1 | |
| 3 | 207.0 | 60.6 | |
| 4 | 225.6 | 66.7 | |
| 5 | 256.3 | 76.5 |
| Resin | Storage Modulus G′ (MPa) | Loss Modulus G″ (kPa) | Complex Viscosity η* (MPa·s) | Gel Point tgel (s) |
|---|---|---|---|---|
| 100A | 1.56 ± 0.05 | 257.72 ± 27.04 | 25.15 ± 0.85 | 3.5 ± 0.5 |
| 75A/25B | 2.44 ± 0.00 | 10.25 ± 1.38 | 38.85 ± 0.01 | 7.0 ± 0.0 |
| 50A/50B | 1.30 ± 0.10 | 2.98 ± 0.09 | 20.55 ± 1.45 | 1340.1 ± 35.7 |
| 25A/75B | 3.15 ± 0.00 | 60.58 ± 0.00 | 50.10 ± 0.00 | 1390.2 ± 0.0 |
| 100B | 5.19 ± 0.60 | 106.77 ± 0.61 | 82.55 ± 9.55 | 786.6 ± 15.6 |
| 100C | 3.79 ± 0.30 | 1415 ± 385 | 64.50 ± 6.60 | 2.0 ± 0.0 |
| 75C/25D | 3.96 ± 0.00 | 12.56 ± 0.00 | 63.03 ± 0.00 | 2.0 ± 0.0 |
| 50C/50D | 3.87 ± 0.00 | 11.37 ± 0.00 | 61.62 ± 0.00 | 2.0 ± 0.0 |
| 25C/75D | 2.70 ± 0.00 | 9.94 ± 0.00 | 42.90 ± 0.00 | 1692.6 ± 0.0 |
| 100D | 3.36 ± 0.29 | 25.80 ± 0.00 | 53.35 ± 4.55 | 1170.0 ± 0.0 |
| Polymer | DMTA | TGA | |||
|---|---|---|---|---|---|
| Tg1 (°C) | Er2 (MPa) | νe3 (mol/m3) | Tdec.−5%4 (°C) | Char yield 5 (%) | |
| 100A | −4 | 3.98 | 479 | 343 | 0.7 |
| 75A/25B | 4 | 5.10 | 614 | 344 | 1.3 |
| 100B | 60 | 6.16 | 742 | 308 | 2.2 |
| 100C | 1 | 5.64 | 679 | 354 | 0.9 |
| 75C/25D | −4 | 6.38 | 768 | 358 | 1.1 |
| 100D | 29 | 3.92 | 472 | 317 | 2.4 |
| Polymer | Elongation at Break (%) | Tensile Strength (MPa) | Young’s Modulus (MPa) |
|---|---|---|---|
| 100A | 5.75 ± 0.23 | 0.65 ± 0.03 | 9.53 ± 4.42 |
| 75A/25B | 3.26 ± 0.03 | 0.91 ± 0.09 | 13.46 ± 1.81 |
| 100B | 1.92 ± 0.06 | 31.54 ± 2.72 | 2458 ± 43.90 |
| 100C | 1.95 ± 0.82 | 0.82 ± 0.50 | 4.00 ± 2.09 |
| 75C/25D | 4.97 ± 1.11 | 0.87 ± 0.01 | 8.76 ± 2.22 |
| 100D | 7.03 ± 1.05 | 1.44 ± 0.43 | 23.3 ± 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grauzeliene, S.; Navaruckiene, A.; Skliutas, E.; Malinauskas, M.; Serra, A.; Ostrauskaite, J. Vegetable Oil-Based Thiol-Ene/Thiol-Epoxy Resins for Laser Direct Writing 3D Micro-/Nano-Lithography. Polymers 2021, 13, 872. https://doi.org/10.3390/polym13060872
Grauzeliene S, Navaruckiene A, Skliutas E, Malinauskas M, Serra A, Ostrauskaite J. Vegetable Oil-Based Thiol-Ene/Thiol-Epoxy Resins for Laser Direct Writing 3D Micro-/Nano-Lithography. Polymers. 2021; 13(6):872. https://doi.org/10.3390/polym13060872
Chicago/Turabian StyleGrauzeliene, Sigita, Aukse Navaruckiene, Edvinas Skliutas, Mangirdas Malinauskas, Angels Serra, and Jolita Ostrauskaite. 2021. "Vegetable Oil-Based Thiol-Ene/Thiol-Epoxy Resins for Laser Direct Writing 3D Micro-/Nano-Lithography" Polymers 13, no. 6: 872. https://doi.org/10.3390/polym13060872
APA StyleGrauzeliene, S., Navaruckiene, A., Skliutas, E., Malinauskas, M., Serra, A., & Ostrauskaite, J. (2021). Vegetable Oil-Based Thiol-Ene/Thiol-Epoxy Resins for Laser Direct Writing 3D Micro-/Nano-Lithography. Polymers, 13(6), 872. https://doi.org/10.3390/polym13060872