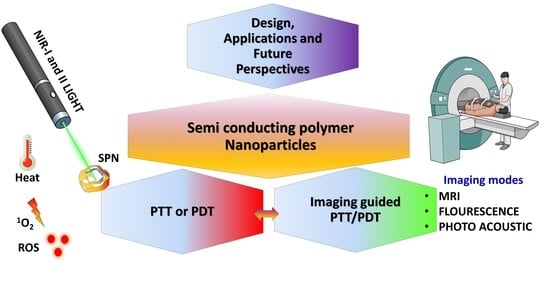
Recent Developments on Semiconducting Polymer Nanoparticles as Smart Photo-Therapeutic Agents for Cancer Treatments—A Review
Abstract
:1. Introduction
2. Semiconducting Polymer Nanoparticle (SPN)—How Do They Work?
3. Design and Formulation Strategies of SPN
4. SPN in Photo-Therapy
4.1. PTT
4.2. PDT
4.3. Combined PTT/PDT
4.4. Photo-Immuno Therapy
4.5. Photo-Radio Therapy
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Song, M. Cancer overtakes vascular disease as leading cause of excess death associated with diabetes. Lancet Diabetes Endocrinol. 2021, 9, 131–133. [Google Scholar] [CrossRef]
- Pinheiro, P.S.; Callahan, K.E.; Jones, P.D.; Morris, C.; Ransdell, J.M.; Kwon, D.; Brown, C.P.; Kobetz, E.N. Liver cancer: A leading cause of cancer death in the United States and the role of the 1945–1965 birth cohort by ethnicity. JHEP Rep. 2019, 1, 162–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demarinis, S. Cancer overtakes cardiovascular disease as leading cause of death in wealthy nations. Explore 2020, 16, 6–7. [Google Scholar] [CrossRef]
- Mahase, E. Cancer overtakes CVD to become leading cause of death in high income countries. BMJ 2019, 366, l5368. [Google Scholar] [CrossRef] [PubMed]
- Hastings, K.G.; Kapphahn, K.; Boothroyd, D.B.; Rehkopf, D.H.; Cullen, M.R.; Palaniappan, L. Transition From Heart Disease to Cancer as the Leading Cause of Death in the United States. Ann. Intern. Med. 2019, 171, 225. [Google Scholar] [CrossRef] [PubMed]
- Sidney, S.; Go, A.S.; Rana, J.S. Transition From Heart Disease to Cancer as the Leading Cause of Death in the United States. Ann. Intern. Med. 2019, 171, 225. [Google Scholar] [CrossRef] [PubMed]
- Hastings, K.G.; Boothroyd, D.B.; Kapphahn, K.; Hu, J.; Rehkopf, D.H.; Cullen, M.R.; Palaniappan, L. Socioeconomic Differences in the Epidemiologic Transition From Heart Disease to Cancer as the Leading Cause of Death in the United States, 2003 to 2015: An Observational Study. Ann. Intern. Med. 2019, 169, 836–844, Correction in 2019, 170, 220. [Google Scholar] [CrossRef]
- Harding, M.C.; Sloan, C.D.; Merrill, R.M.; Harding, T.M.; Thacker, B.J.; Thacker, E.L. Transitions From Heart Disease to Cancer as the Leading Cause of Death in US States, 1999–2016. Prev. Chronic Dis. 2018, 15, E158. [Google Scholar] [CrossRef] [Green Version]
- Pulvirenti, F.; Pecoraro, A.; Cinetto, F.; Milito, C.; Valente, M.; Santangeli, E.; Crescenzi, L.; Rizzo, F.; Tabolli, S.; Spadaro, G.; et al. Gastric Cancer Is the Leading Cause of Death in Italian Adult Patients With Common Variable Immunodeficiency. Front. Immunol. 2018, 9, 2546. [Google Scholar] [CrossRef]
- Stringhini, S.; Guessous, I. The Shift from Heart Disease to Cancer as the Leading Cause of Death in High-Income Countries: A Social Epidemiology Perspective. Ann. Intern. Med. 2018, 169, 877–878. [Google Scholar] [CrossRef]
- Pathak, E.B. Is Heart Disease or Cancer the Leading Cause of Death in United States Women? Womens Health Issues 2016, 26, 589–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, J.S. Lung cancer: Very high death rate with HIV, huge reduction possible with CT screening for early diagnosis. AIDS Treat. News 2006, 460, 5–6. [Google Scholar]
- Sofias, A.M.; Combes, F.; Koschmieder, S.; Storm, G.; Lammers, T. A paradigm shift in cancer nanomedicine: From traditional tumor targeting to leveraging the immune system. Drug Discov. Today 2021. [Google Scholar] [CrossRef] [PubMed]
- Vahedifard, F.; Chakravarthy, K. Nanomedicine for COVID-19: The role of nanotechnology in the treatment and diagnosis of COVID-19. Emergent Mater. 2021. [Google Scholar] [CrossRef]
- Cote, B.; Rao, D.; Alani, A.W.G. Nanomedicine for Drug Delivery throughout the Alimentary Canal. Mol. Pharm. 2021. [Google Scholar] [CrossRef]
- Faouzi, A.; Roullin, V.G. Think Big, Start Small: How Nanomedicine Could Alleviate the Burden of Rare CNS Diseases. Pharmaceuticals 2021, 14, 109. [Google Scholar] [CrossRef] [PubMed]
- Koo, K.M.; Wee, E.J.; Mainwaring, P.N.; Wang, Y.; Trau, M. Toward Precision Medicine: A Cancer Molecular Subtyping Nano-Strategy for RNA Biomarkers in Tumor and Urine. Small 2016, 12, 6233–6242. [Google Scholar] [CrossRef]
- Iqbal, S.; Fakhar, E.A.M.; Alimgeer, K.S.; Atif, M.; Hanif, A.; Yaqub, N.; Farooq, W.A.; Ahmad, S.; Chu, Y.M.; Suleman Rana, M.; et al. Mathematical modeling and experimental analysis of the efficacy of photodynamic therapy in conjunction with photo thermal therapy and PEG-coated Au-doped TiO2 nanostructures to target MCF-7 cancerous cells. Saudi J. Biol. Sci. 2021, 28, 1226–1232. [Google Scholar] [CrossRef]
- De Cordova, J.A. Role of Photo-Biomodulation Therapy in Facial Rejuvenation and Facial Plastic Surgery. Facial Plast. Surg. 2021. [Google Scholar] [CrossRef]
- He, Y.; Cong, C.; Zhao, S.; Li, Z.; Wang, D.; Gu, J.; Liu, L.; Gao, D. Gaseous microenvironmental remodeling of tumors for enhanced photo-gas therapy and real-time tracking. Biomater. Sci. 2021. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, H.; Zhao, J.; Yin, Y.; Zhang, Z.; Wang, S.; Lin, K. 2D LDH-MoS2 clay nanosheets: Synthesis, catalase-mimic capacity, and imaging-guided tumor photo-therapy. J. Nanobiotechnol. 2021, 19, 36. [Google Scholar] [CrossRef]
- Saatchian, E.; Ehsani, S.; Sarikhani, A.; Ghaznavi, H.; Montazerabadi, A.; Shakeri-Zadeh, A. Monitoring of the choline/lipid ratio by (1)H-MRS can be helpful for prediction and early detection of tumor response to nano-photo-thermal therapy. Lasers Med. Sci. 2021. [Google Scholar] [CrossRef]
- Sanoj Rejinold, N.; Choi, G.; Choy, J.-H. Recent trends in nano photo-chemo therapy approaches and future scopes. Coord. Chem. Rev. 2020, 411, 213252. [Google Scholar] [CrossRef]
- Yang, Y.; Tu, J.; Yang, D.; Raymond, J.L.; Roy, R.A.; Zhang, D. Photo- and Sono-Dynamic Therapy: A Review of Mechanisms and Considerations for Pharmacological Agents Used in Therapy Incorporating Light and Sound. Curr. Pharm. Des. 2019, 25, 401–412. [Google Scholar] [CrossRef]
- Liu, M.-H.; Zhang, Z.; Yang, Y.-C.; Chan, Y.-H. Polymethine-Based Semiconducting Polymer Dots with Narrow-Band Emission and Absorption/Emission Maxima at NIR-II for Bioimaging. Angew. Chem. Int. Ed. 2021, 60, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, Q.; Dai, X.; Ling, P.; Gao, F. Engineering fluorescent semiconducting polymer nanoparticles for biological applications and beyond. Chem. Commun. 2021, 57, 1989–2004. [Google Scholar] [CrossRef]
- Huang, J.; Yu, G. Recent progress in quinoidal semiconducting polymers: Structural evolution and insight. Mater. Chem. Front. 2021, 5, 76–96. [Google Scholar] [CrossRef]
- Lv, Q.; Zhu, Z.; Zhao, S.; Wang, L.; Zhao, Q.; Li, F.; Archer, L.A.; Chen, J. Semiconducting Metal-Organic Polymer Nanosheets for a Photoinvolved Li-O2 Battery under Visible Light. J. Am. Chem. Soc. 2021, 143, 1941–1947. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, D.; Zhong, J.; Wu, Y.; Shi, Y.; Yang, H.; Zhao, L.; Yang, K.; Lin, J. SPECT imaging and highly efficient therapy of rheumatoid arthritis based on hyperbranched semiconducting polymer nanoparticles. Biomater. Sci. 2021. [Google Scholar] [CrossRef]
- Phan, S.; Luscombe, C.K. Recent Advances in the Green, Sustainable Synthesis of Semiconducting Polymers. Trends Chem. 2020, 2, 86. [Google Scholar] [CrossRef] [Green Version]
- Geng, J.; Sun, C.; Liu, J.; Liao, L.-D.; Yuan, Y.; Thakor, N.; Wang, J.; Liu, B. Biocompatible Conjugated Polymer Nanoparticles for Efficient Photothermal Tumor Therapy. Small 2015, 11, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Liu, Z.; Wu, C.; Jiang, W.; Chen, L.; Shi, D.; Zhang, X.; Zhang, G.; Zhang, D. Simultaneous Incorporation of Two Types of Azo-Groups in the Side Chains of a Conjugated D-A Polymer for Logic Control of the Semiconducting Performance by Light Irradiation. Adv. Mater. 2021, 33, 2005613. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Chan, Y.H.; Saha, S.; Liu, M.H. Near-Infrared-II Semiconducting Polymer Dots for Deep-tissue Fluorescence Imaging. Chem. Asian J. 2021, 16, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Yang, Z.; Hu, X.; Wu, J.; Shou, K.; Ma, H.; Jian, C.; Zhao, Y.; Qi, B.; Hu, X.; et al. Organic Semiconducting Nanoparticles as Efficient Photoacoustic Agents for Lightening Early Thrombus and Monitoring Thrombolysis in Living Mice. ACS Nano 2017, 11, 3298–3310. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, F.; Wang, G.; Chen, H.; Zhang, X.; Qin, G.; Cheng, T. Passively Mode-Locked Operations Induced by Semiconducting Polymer Nanoparticles and a Side-Polished Fiber. ACS Appl. Mater. Interfaces 2020, 12, 57461–57467. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Tan, A.T.L.; Lee, J.; Park, J.E.; Won, S.; Kim, S.; Bedewy, M.; Go, J.; Kim, J.K.; Hart, A.J.; et al. High-Speed Production of Crystalline Semiconducting Polymer Line Arrays by Meniscus Oscillation Self-Assembly. ACS Nano 2020, 14, 17254–17261. [Google Scholar] [CrossRef]
- Pan, G.; Hu, L.; Su, S.; Yuan, J.; Li, T.; Xiao, X.; Chen, Q.; Zhang, F. Solvent Vapor-Assisted Magnetic Manipulation of Molecular Orientation and Carrier Transport of Semiconducting Polymers. ACS Appl. Mater. Interfaces 2020, 12, 29487–29496. [Google Scholar] [CrossRef]
- Galeotti, G.; De Marchi, F.; Hamzehpoor, E.; MacLean, O.; Rajeswara Rao, M.; Chen, Y.; Besteiro, L.V.; Dettmann, D.; Ferrari, L.; Frezza, F.; et al. Synthesis of mesoscale ordered two-dimensional pi-conjugated polymers with semiconducting properties. Nat. Mater. 2020, 19, 874–880. [Google Scholar] [CrossRef]
- Long, Z.; Dai, J.; Hu, Q.; Wang, Q.; Zhen, S.; Zhao, Z.; Liu, Z.; Hu, J.J.; Lou, X.; Xia, F. Nanococktail Based on AIEgens and Semiconducting Polymers: A Single Laser Excited Image-Guided Dual Photothermal Therapy. Theranostics 2020, 10, 2260–2272. [Google Scholar] [CrossRef]
- Mazzio, K.A.; Prasad, S.K.K.; Okamoto, K.; Hodgkiss, J.M.; Luscombe, C.K. End-Functionalized Semiconducting Polymers as Reagents in the Synthesis of Hybrid II-VI Nanoparticles. Langmuir 2018, 34, 9692–9700. [Google Scholar] [CrossRef]
- Li, W.-P.; Yen, C.-J.; Wu, B.-S.; Wong, T.-W. Recent Advances in Photodynamic Therapy for Deep-Seated Tumors with the Aid of Nanomedicine. Biomedicines 2021, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Zhen, X.; Miao, Q.; Lyu, Y.; Pu, K. Self-Assembled Semiconducting Polymer Nanoparticles for Ultrasensitive Near-Infrared Afterglow Imaging of Metastatic Tumors. Adv. Mater. 2018, 30, e1801331. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fang, X.; Jin, Y.; Hu, X.; Yin, M.; Men, X.; Chen, N.; Fan, C.; Chiu, D.T.; Wan, Y.; et al. Semiconducting Polymer Nanocavities: Porogenic Synthesis, Tunable Host-Guest Interactions, and Enhanced Drug/siRNA Delivery. Small 2018, 14, e1800239. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.; Pu, K.; Jiang, X. Photoacoustic Imaging and Photothermal Therapy of Semiconducting Polymer Nanoparticles: Signal Amplification and Second Near-Infrared Construction. Small 2021, 17, 2004723. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wu, M.; Lan, S.; Li, J.; Zhang, X.; Zhang, D.; Liu, X.; Liu, J. Semiconducting polymer-based nanoparticles for photothermal therapy at the second near-infrared window. Chem. Commun. 2018, 54, 13599–13602. [Google Scholar] [CrossRef]
- Wen, G.; Li, X.; Zhang, Y.; Han, X.; Xu, X.; Liu, C.; Chan, K.W.Y.; Lee, C.S.; Yin, C.; Bian, L.; et al. Effective Phototheranostics of Brain Tumor Assisted by Near-Infrared-II Light-Responsive Semiconducting Polymer Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 33492–33499. [Google Scholar] [CrossRef]
- Hu, L.; Chen, Z.; Liu, Y.; Tian, B.; Guo, T.; Liu, R.; Wang, C.; Ying, L. In Vivo Bioimaging and Photodynamic Therapy Based on Two-Photon Fluorescent Conjugated Polymers Containing Dibenzothiophene-S,S-dioxide Derivatives. ACS Appl. Mater. Interfaces 2020, 12, 57281–57289. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Hu, R.; Chen, H.; Li, M.; Wang, J.; Wang, Y.; Liu, L.; Lv, F.; Liang, X.-J.; et al. Near-Infrared (NIR)-Absorbing Conjugated Polymer Dots as Highly Effective Photothermal Materials for In Vivo Cancer Therapy. Chem. Mater. 2016, 28, 8669–8675. [Google Scholar] [CrossRef]
- Chang, K.; Liu, Y.; Hu, D.; Qi, Q.; Gao, D.; Wang, Y.; Li, D.; Zhang, X.; Zheng, H.; Sheng, Z.; et al. Highly Stable Conjugated Polymer Dots as Multifunctional Agents for Photoacoustic Imaging-Guided Photothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10, 7012–7021. [Google Scholar] [CrossRef]
- Hu, X.; Lu, F.; Chen, L.; Tang, Y.; Hu, W.; Lu, X.; Ji, Y.; Yang, Z.; Zhang, W.; Yin, C.; et al. Perylene Diimide-Grafted Polymeric Nanoparticles Chelated with Gd3+ for Photoacoustic/T1-Weighted Magnetic Resonance Imaging-Guided Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 30458–30469. [Google Scholar] [CrossRef]
- Zeng, W.; Wu, X.; Chen, T.; Sun, S.; Shi, Z.; Liu, J.; Ji, X.; Zeng, X.; Guan, J.; Mei, L.; et al. Renal-Clearable Ultrasmall Polypyrrole Nanoparticles with Size-Regulated Property for Second Near-Infrared Light-Mediated Photothermal Therapy. Adv. Funct. Mater. 2021, 2008362. [Google Scholar] [CrossRef]
- Li, J.; Zhen, X.; Lyu, Y.; Jiang, Y.; Huang, J.; Pu, K. Cell Membrane Coated Semiconducting Polymer Nanoparticles for Enhanced Multimodal Cancer Phototheranostics. ACS Nano 2018, 12, 8520–8530. [Google Scholar] [CrossRef]
- Lyu, Y.; Zeng, J.; Jiang, Y.; Zhen, X.; Wang, T.; Qiu, S.; Lou, X.; Gao, M.; Pu, K. Enhancing Both Biodegradability and Efficacy of Semiconducting Polymer Nanoparticles for Photoacoustic Imaging and Photothermal Therapy. ACS Nano 2018, 12, 1801–1810. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Yang, M.; Li, C.; Liu, G.; Sun, Q.; Luo, X.; Wu, F. Single molecular-based nanoparticles with aggregation-induced emission characteristics for fluorescence imaging and efficient cancer phototherapy. Dyes Pigment. 2021, 187, 109130. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, J.; Tong, L.; Su, P.; Liu, Y.; Gu, B.; Bao, B.; Wang, L. pH/NIR-responsive semiconducting polymer nanoparticles for highly effective photoacoustic image guided chemo-photothermal synergistic therapy. J. Control. Release 2019, 293, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Heeger, A.J. Semiconducting polymers: The Third Generation. Chem. Soc. Rev. 2010, 39, 2354–2371. [Google Scholar] [CrossRef]
- Huang, Y.C.; Chen, C.P.; Wu, P.J.; Kuo, S.Y.; Chan, Y.H. Coumarin dye-embedded semiconducting polymer dots for ratiometric sensing of fluoride ions in aqueous solution and bio-imaging in cells. J. Mater. Chem. B 2014, 2, 6188–6191. [Google Scholar] [CrossRef]
- Liu, S.; Ou, H.; Li, Y.; Zhang, H.; Liu, J.; Lu, X.; Kwok, R.T.K.; Lam, J.W.Y.; Ding, D.; Tang, B.Z. Planar and Twisted Molecular Structure Leads to the High Brightness of Semiconducting Polymer Nanoparticles for NIR-IIa Fluorescence Imaging. J. Am. Chem. Soc. 2020, 142, 15146–15156. [Google Scholar] [CrossRef]
- Wu, J.; Lee, H.J.; You, L.; Luo, X.; Hasegawa, T.; Huang, K.C.; Lin, P.; Ratliff, T.; Ashizawa, M.; Mei, J.; et al. Functionalized NIR-II Semiconducting Polymer Nanoparticles for Single-cell to Whole-Organ Imaging of PSMA-Positive Prostate Cancer. Small 2020, 16, e2001215. [Google Scholar] [CrossRef]
- Gong, X.T.; Xie, W.; Cao, J.J.; Zhang, S.; Pu, K.; Zhang, H.L. NIR-emitting semiconducting polymer nanoparticles for in vivo two-photon vascular imaging. Biomater. Sci. 2020, 8, 2666–2672. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, X.; Li, L.; Yang, Y.; Nuernisha, A.; Xue, D.; He, C.; Qian, J.; Hu, Q.; Chen, H.; et al. Semiconducting Polymer Nanoparticles as Theranostic System for Near-Infrared-II Fluorescence Imaging and Photothermal Therapy under Safe Laser Fluence. ACS Nano 2020, 14, 2509–2521. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, H.; Wu, C. Semiconducting polymer nanoparticles for amplified photoacoustic imaging. WIREs Nanomed. Nanobiotechnol. 2018, 10, e1510. [Google Scholar] [CrossRef]
- Chan, Y.-H.; Wu, P.-J. Semiconducting Polymer Nanoparticles as Fluorescent Probes for Biological Imaging and Sensing. Part. Part. Syst. Charact. 2015, 32, 11–28. [Google Scholar] [CrossRef]
- Pu, K.; Chattopadhyay, N.; Rao, J. Recent advances of semiconducting polymer nanoparticles in in vivo molecular imaging. J. Control. Release 2016, 240, 312–322. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Rao, J.; Pu, K. Recent progress on semiconducting polymer nanoparticles for molecular imaging and cancer phototherapy. Biomaterials 2018, 155, 217–235. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH). J. Chem. Soc. Chem. Commun. 1977, 578–580. [Google Scholar] [CrossRef]
- Gelmi, A.; Ljunggren, M.K.; Rafat, M.; Jager, E.W.H. Influence of conductive polymer doping on the viability of cardiac progenitor cells. J. Mater. Chem. B 2014, 2, 3860–3867. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Chiu, D.T. Highly fluorescent semiconducting polymer dots for biology and medicine. Angew. Chem. Int. Ed. Engl. 2013, 52, 3086–3109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pu, K.; Shuhendler, A.J.; Jokerst, J.V.; Mei, J.; Gambhir, S.S.; Bao, Z.; Rao, J. Semiconducting polymer nanoparticles as photoacoustic molecular imaging probes in living mice. Nat. Nanotechnol. 2014, 9, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Feng, L.; Zhang, G.; Wang, J.; Shen, S.; Li, D.; Yang, X. Semiconducting polymer-based nanoparticles with strong absorbance in NIR-II window for in vivo photothermal therapy and photoacoustic imaging. Biomaterials 2018, 155, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Wen, G.; Liu, C.; Yang, B.; Lin, S.; Huang, J.; Zhao, P.; Wong, S.H.D.; Zhang, K.; Chen, X.; et al. Organic Semiconducting Polymer Nanoparticles for Photoacoustic Labeling and Tracking of Stem Cells in the Second Near-Infrared Window. ACS Nano 2018, 12, 12201–12211. [Google Scholar] [CrossRef]
- Li, J.; Yu, X.; Jiang, Y.; He, S.; Zhang, Y.; Luo, Y.; Pu, K. Second Near-Infrared Photothermal Semiconducting Polymer Nanoadjuvant for Enhanced Cancer Immunotherapy. Adv. Mater. 2021, 33, 2003458. [Google Scholar] [CrossRef]
- Hornig, S.; Heinze, T.; Becer, C.R.; Schubert, U.S. Synthetic polymeric nanoparticles by nanoprecipitation. J. Mater. Chem. 2009, 19, 3838–3840. [Google Scholar] [CrossRef]
- Yao, D.; Wang, Y.; Zou, R.; Bian, K.; Liu, P.; Shen, S.; Yang, W.; Zhang, B.; Wang, D. Molecular Engineered Squaraine Nanoprobe for NIR-II/Photoacoustic Imaging and Photothermal Therapy of Metastatic Breast Cancer. ACS Appl. Mater. Interfaces 2020, 12, 4276–4284. [Google Scholar] [CrossRef]
- Bao, B.; Su, P.; Song, K.; Cui, Y.; Zhai, X.; Xu, Y.; Liu, J.; Wang, L. A Smart “Sense-and-Treat” Nanoplatform Based on Semiconducting Polymer Nanoparticles for Precise Photothermal-Photodynamic Combined Therapy. Biomacromolecules 2021, 22, 1137–1146. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, J.; Xu, C.; Pu, K. Activatable polymer nanoagonist for second near-infrared photothermal immunotherapy of cancer. Nat. Commun. 2021, 12, 742. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Duan, Y.K.; Liu, B. Recent advances of AIE dots in NIR imaging and phototherapy. Nanoscale 2019, 11, 19241–19250. [Google Scholar] [CrossRef]
- Lu, L.; Li, B.; Ding, S.; Fan, Y.; Wang, S.; Sun, C.; Zhao, M.; Zhao, C.-X.; Zhang, F. NIR-II bioluminescence for in vivo high contrast imaging and in situ ATP-mediated metastases tracing. Nat. Commun. 2020, 11, 4192. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, B.; Cudykier, A.; Singh, R.; Levi-Polyachenko, N.; Soker, S. Semiconducting polymer nanoparticles for photothermal ablation of colorectal cancer organoids. Sci. Rep. 2021, 11, 1532. [Google Scholar] [CrossRef] [PubMed]
- Ale, A.; Galdoporpora, J.M.; Mora, M.C.; de la Torre, F.R.; Desimone, M.F.; Cazenave, J. Mitigation of silver nanoparticle toxicity by humic acids in gills of Piaractus mesopotamicus fish. Environ. Sci. Pollut. Res. Int. 2021. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Patra, C.R. Nanoparticle-based angiogenesis for the recovery of heavy metal-induced vascular toxicity. Nanomedicine 2021, 16, 351–354. [Google Scholar] [CrossRef]
- Xiao, Z.; Yue, L.; Wang, C.; Chen, F.; Ding, Y.; Liu, Y.; Cao, X.; Chen, Z.; Rasmann, S.; Wang, Z. Downregulation of the photosynthetic machinery and carbon storage signaling pathways mediate La2O3 nanoparticle toxicity on radish taproot formation. J. Hazard. Mater. 2020, 411, 124971. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.T.A. Antagonistic effect of different selenium type on green synthesized silver nanoparticle toxicity on Oreochromis niloticus: Oxidative stress biomarkers. Environ. Sci. Pollut. Res. Int. 2021. [Google Scholar] [CrossRef] [PubMed]
- Mahana, A.; Guliy, O.I.; Mehta, S.K. Accumulation and cellular toxicity of engineered metallic nanoparticle in freshwater microalgae: Current status and future challenges. Ecotoxicol. Environ. Saf. 2021, 208, 111662. [Google Scholar] [CrossRef]
- Ahmed, T.; Noman, M.; Manzoor, N.; Shahid, M.; Abdullah, M.; Ali, L.; Wang, G.; Hashem, A.; Al-Arjani, A.F.; Alqarawi, A.A.; et al. Nanoparticle-based amelioration of drought stress and cadmium toxicity in rice via triggering the stress responsive genetic mechanisms and nutrient acquisition. Ecotoxicol. Environ. Saf. 2021, 209, 111829. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Dismuke, T.; Tikunov, A.; Rosen, E.P.; Kagel, J.R.; Ramsey, J.D.; Lim, C.; Zamboni, W.; Kabanov, A.V.; Gershon, T.R.; et al. Poly(2-oxazoline) nanoparticle delivery enhances the therapeutic potential of vismodegib for medulloblastoma by improving CNS pharmacokinetics and reducing systemic toxicity. Nanomedicine 2020, 32, 102345. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Gao, D.; Qi, Q.; Liu, Y.; Yuan, Z. Engineering biocompatible benzodithiophene-based polymer dots with tunable absorptions as high-efficiency theranostic agents for multiscale photoacoustic imaging-guided photothermal therapy. Biomater. Sci. 2019, 7, 1486–1492. [Google Scholar] [CrossRef]
- Men, X.; Wang, F.; Chen, H.; Liu, Y.; Men, X.; Yuan, Y.; Zhang, Z.; Gao, D.; Wu, C.; Yuan, Z. Ultrasmall Semiconducting Polymer Dots with Rapid Clearance for Second Near-Infrared Photoacoustic Imaging and Photothermal Cancer Therapy. Adv. Funct. Mater. 2020, 30, 1909673. [Google Scholar] [CrossRef]
- Larue, L.; Myrzakhmetov, B.; Ben-Mihoub, A.; Moussaron, A.; Thomas, N.; Arnoux, P.; Baros, F.; Vanderesse, R.; Acherar, S.; Frochot, C. Fighting Hypoxia to Improve PDT. Pharmaceuticals 2019, 12, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, D.; Huang, J.; Zhen, X.; Li, J.; Jiang, Y.; Pu, K. A Semiconducting Polymer Nano-prodrug for Hypoxia-Activated Photodynamic Cancer Therapy. Angew. Chem. Int. Ed. 2019, 58, 5920–5924. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chen, H.; Chang, K.; Liu, Z.; Wang, Y.; Qu, S.; Xu, H.; Wu, C. Photo-Cross-Linkable Polymer Dots with Stable Sensitizer Loading and Amplified Singlet Oxygen Generation for Photodynamic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 3419–3431. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pu, K. Semiconducting Polymer Nanomaterials as Near-Infrared Photoactivatable Protherapeutics for Cancer. Acc. Chem. Res. 2020, 53, 752–762. [Google Scholar] [CrossRef]
- Hu, W.; Ma, H.; Hou, B.; Zhao, H.; Ji, Y.; Jiang, R.; Hu, X.; Lu, X.; Zhang, L.; Tang, Y.; et al. Engineering Lysosome-Targeting BODIPY Nanoparticles for Photoacoustic Imaging and Photodynamic Therapy under Near-Infrared Light. ACS Appl. Mater. Interfaces 2016, 8, 12039–12047. [Google Scholar] [CrossRef]
- Feng, Z.; Tao, P.; Zou, L.; Gao, P.; Liu, Y.; Liu, X.; Wang, H.; Liu, S.; Dong, Q.; Li, J.; et al. Hyperbranched Phosphorescent Conjugated Polymer Dots with Iridium(III) Complex as the Core for Hypoxia Imaging and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 28319–28330. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Meng, Z.-H.; Xu, H.; Wu, C.-F. Semiconducting polymer dots with photosensitizer loading and peptide modification for enhanced cell penetration and photodynamic effect. Chin. Chem. Lett. 2017, 28, 2164–2168. [Google Scholar] [CrossRef]
- Thambi, T.; Deepagan, V.G.; Yoon, H.Y.; Han, H.S.; Kim, S.H.; Son, S.; Jo, D.G.; Ahn, C.H.; Suh, Y.D.; Kim, K.; et al. Hypoxia-responsive polymeric nanoparticles for tumor-targeted drug delivery. Biomaterials 2014, 35, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.G.; Surendran, S.P.; Jeong, Y.Y. Tumor Microenvironment-Stimuli Responsive Nanoparticles for Anticancer Therapy. Front. Mol. Biosci. 2020, 7, 610533. [Google Scholar] [CrossRef]
- Fu, W.; Zhang, X.; Mei, L.; Zhou, R.; Yin, W.; Wang, Q.; Gu, Z.; Zhao, Y. Stimuli-Responsive Small-on-Large Nanoradiosensitizer for Enhanced Tumor Penetration and Radiotherapy Sensitization. ACS Nano 2020, 14, 10001–10017. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, Y.; Wu, Y.; Yin, L. Hypoxia-Induced Pro-Protein Therapy Assisted by a Self-Catalyzed Nanozymogen. Angew. Chem. Int. Ed. Engl. 2020, 59, 22544–22553. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, J.; Xu, X.; Shi, R.; Saw, P.E.; Wang, J.; Chung, S.; Li, W.; Aljaeid, B.M.; Lee, R.J.; et al. Dual Hypoxia-Targeting RNAi Nanomedicine for Precision Cancer Therapy. Nano Lett. 2020, 20, 4857–4863. [Google Scholar] [CrossRef]
- Yang, G.; Phua, S.Z.F.; Lim, W.Q.; Zhang, R.; Feng, L.; Liu, G.; Wu, H.; Bindra, A.K.; Jana, D.; Liu, Z.; et al. A Hypoxia-Responsive Albumin-Based Nanosystem for Deep Tumor Penetration and Excellent Therapeutic Efficacy. Adv. Mater. 2019, 31, e1901513. [Google Scholar] [CrossRef] [PubMed]
- Bennie, L.A.; McCarthy, H.O.; Coulter, J.A. Enhanced nanoparticle delivery exploiting tumour-responsive formulations. Cancer Nanotechnol. 2018, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liang, H.; Jiang, P.; Zhang, K.Y.; Liu, S.; Yang, T.; Zhao, Q.; Yang, L.; Lv, W.; Yu, Q.; et al. Multifunctional Phosphorescent Conjugated Polymer Dots for Hypoxia Imaging and Photodynamic Therapy of Cancer Cells. Adv. Sci. 2016, 3, 1500155. [Google Scholar] [CrossRef] [Green Version]
- Wen, K.; Xu, X.; Chen, J.; Lv, L.; Wu, L.; Hu, Y.; Wu, X.; Liu, G.; Peng, A.; Huang, H. Triplet Tellurophene-Based Semiconducting Polymer Nanoparticles for Near-Infrared-Mediated Cancer Theranostics. ACS Appl. Mater. Interfaces 2019, 11, 17884–17893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, A.; Feng, J.; Yi, J.; Peng, L.; Chen, J.; Ke, Z.; Yang, J.; Dai, Y.; Zou, D. A heavy atom free semiconducting polymer with high singlet oxygen quantum yield for photodynamic and photothermal synergistic therapy. Mater. Des. 2021, 197, 109263. [Google Scholar] [CrossRef]
- Yang, M.; Deng, J.; Su, H.; Gu, S.; Zhang, J.; Zhong, A.; Wu, F. Small organic molecule-based nanoparticles with red/near-infrared aggregation-induced emission for bioimaging and PDT/PTT synergistic therapy. Mater. Chem. Front. 2021, 5, 406–417. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, D.-W.; Yang, G.-L.; Zhu, Y.-C.; Tian, J.; Cao, H.-L.; Gao, Y.; Zhang, W.-A. A Single-wavelength NIR-triggered Polymer for in Situ Generation of Peroxynitrite (ONOO−) to Enhance Phototherapeutic Efficacy. Chin. J. Polym. Sci. 2021. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, M.; Zeng, Y.; Liao, N.; Cai, Z.; Liu, G.; Liu, X.; Liu, J. Lipid micelles packaged with semiconducting polymer dots as simultaneous MRI/photoacoustic imaging and photodynamic/photothermal dual-modal therapeutic agents for liver cancer. J. Mater. Chem. B 2016, 4, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Zhou, F.; Doughty, A.; Hoover, A.R.; Nordquist, R.E.; Chen, W.R. Nanotechnology-based photoimmunological therapies for cancer. Cancer Lett. 2019, 442, 429–438. [Google Scholar] [CrossRef]
- Xu, X.; Lu, H.; Lee, R. Near Infrared Light Triggered Photo/Immuno-Therapy Toward Cancers. Front. Bioeng. Biotechnol. 2020, 8, 488. [Google Scholar] [CrossRef]
- Choi, G.; Rejinold, N.S.; Piao, H.; Choy, J.-H. Inorganic–inorganic nanohybrids for drug delivery, imaging and photo-therapy: Recent developments and future scope. Chem. Sci. 2021. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, C.; Li, J.; Cui, D.; Jiang, Y.; Pu, K. Activatable Polymer Nanoenzymes for Photodynamic Immunometabolic Cancer Therapy. Adv. Mater. 2021, 33, 2007247. [Google Scholar] [CrossRef]
- Tekin, V.; Aweda, T.; Kozgus Guldu, O.; Biber Muftuler, F.Z.; Bartels, J.; Lapi, S.E.; Unak, P. A novel anti-angiogenic radio/photo sensitizer for prostate cancer imaging and therapy: (89)Zr-Pt@TiO2-SPHINX, synthesis and in vitro evaluation. Nucl. Med. Biol. 2021, 94–95, 20–31. [Google Scholar] [CrossRef]
- Bakhshizadeh, M.; Mohajeri, S.A.; Esmaily, H.; Aledavood, S.A.; Varshoei Tabrizi, F.; Seifi, M.; Hadizadeh, F.; Sazgarnia, A. Utilizing photosensitizing and radiosensitizing properties of TiO2-based mitoxantrone imprinted nanopolymer in fibrosarcoma and melanoma cells. Photodiagnosis Photodyn. Ther. 2019, 25, 472–479. [Google Scholar] [CrossRef]
- Tang, W.; Dong, Z.; Zhang, R.; Yi, X.; Yang, K.; Jin, M.; Yuan, C.; Xiao, Z.; Liu, Z.; Cheng, L. Multifunctional Two-Dimensional Core-Shell MXene@Gold Nanocomposites for Enhanced Photo-Radio Combined Therapy in the Second Biological Window. ACS Nano 2019, 13, 284–294. [Google Scholar] [CrossRef]
- Sandwall, P.A.; Bastow, B.P.; Spitz, H.B.; Elson, H.R.; Lamba, M.; Connick, W.B.; Fenichel, H. Radio-Fluorogenic Gel Dosimetry with Coumarin. Bioengineering 2018, 5, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, X.; Yong, Y.; Dai, Y.; Song, X.; Yang, G.; Pan, Y.; Ge, C. Enhanced Radiotherapy using Bismuth Sulfide Nanoagents Combined with Photo-thermal Treatment. Theranostics 2017, 7, 4087–4098. [Google Scholar] [CrossRef]
- Singh, B.N.; Gupta, V.K.; Chen, J.; Atanasov, A.G. Organic Nanoparticle-Based Combinatory Approaches for Gene Therapy. Trends Biotechnol. 2017, 35, 1121–1124. [Google Scholar] [CrossRef]
- Beik, J.; Abed, Z.; Ghoreishi, F.S.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S.K. Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. J. Control. Release 2016, 235, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Alikaniotis, K.; Borla, O.; Monti, V.; Vivaldo, G.; Zanini, A.; Giannini, G. Radiotherapy dose enhancement using BNCT in conventional LINACs high-energy treatment: Simulation and experiment. Rep. Pract. Oncol. Radiother. 2016, 21, 117–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sørensen, B.S.; Horsman, M.R. Tumor Hypoxia: Impact on Radiation Therapy and Molecular Pathways. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhong, D.; Hua, S.; Du, Z.; Zhou, M. Biomineralized Biohybrid Algae for Tumor Hypoxia Modulation and Cascade Radio-Photodynamic Therapy. ACS Appl. Mater. Interfaces 2020, 12, 44541–44553. [Google Scholar] [CrossRef]
- Song, C.; Xu, W.; Wei, Z.; Ou, C.; Wu, J.; Tong, J.; Cai, Y.; Dong, X.; Han, W. Anti-LDLR modified TPZ@Ce6-PEG complexes for tumor hypoxia-targeting chemo-/radio-/photodynamic/photothermal therapy. J. Mater. Chem. B 2020, 8, 648–654. [Google Scholar] [CrossRef]
- Wang, Z.; Chang, Z.M.; Shao, D.; Zhang, F.; Chen, F.; Li, L.; Ge, M.F.; Hu, R.; Zheng, X.; Wang, Y.; et al. Janus Gold Triangle-Mesoporous Silica Nanoplatforms for Hypoxia-Activated Radio-Chemo-Photothermal Therapy of Liver Cancer. ACS Appl. Mater. Interfaces 2019, 11, 34755–34765. [Google Scholar] [CrossRef] [PubMed]
- Al Tameemi, W.; Dale, T.P.; Al-Jumaily, R.M.K.; Forsyth, N.R. Hypoxia-Modified Cancer Cell Metabolism. Front. Cell Dev. Biol. 2019, 7, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.; Yang, Z.; He, L.; Deng, L.; Fathi, P.; Zhu, S.; Li, L.; Shen, B.; Wang, Z.; Jacobson, O.; et al. A hybrid semiconducting organosilica-based O2 nanoeconomizer for on-demand synergistic photothermally boosted radiotherapy. Nat. Commun. 2021, 12, 523. [Google Scholar] [CrossRef] [PubMed]









| Materials | Specification | (PTCE) * | SPN Formulation Method | Applications | Ref(s) |
|---|---|---|---|---|---|
| Diketopyrrolopyrrole polymer P(AcIIDDPP) | 200 nm sized SPN with very good stability | 49.5% | Stille cross-coupling reaction followed an emulsification method | Improved PTT on human epithelial cervix adenocarcinoma (HeLa) bearing mouse model | [45] |
| Thiadiazoloquinoxaline-based semiconducting polymer | Hydrodynamic size of SPNs is 58.9 ± 1.4 nm and the PDI is 0.35. | 21.2% | Nanoprecipitation method | PTT on human brain glioblastoma cell line (U87) xenograft model | [46] |
| Dibenzothiophene-S,S-dioxide derivatives | High photostability, improved tissue penetration | 66% ** | Nano-precipitation method | Improved PDT on human epithelial cervix adenocarcinoma (HeLa) bearing mouse model | [47] |
| Thiophene based conjugate polymers | 30 nm sized conjugated polymer | 65% | Nano-precipitation method | Improved PTT effects on mouse epithelial mammary gland metastatic cancer cells (4T1) bearing tumor model | [48] |
| 4,8-bis[5-(2-ethylhexyl)thiophen-2-yl]-2,6-bis(trimethylstannyl)benzo[1,2-b:4,5-b′]dithiophene-6,6′-dibromo-N,N′-(2-ethylhexyl)isoindigo (BDT-IID) Pdots | 20 nm sized Pdots | 45% | Nanoprecipitation method | PTT on Human epithelial mammary gland adenocarcinoma cell line (MCF7) bearing tumor model | [49] |
| Gd3+-PMA–PDI–PEG NPs (Gd3+-chelated poly(isobutylene-alt-maleic anhydride) (PMA) framework pendent with perylene-3,4,9,10-tetracarboxylic diimide (PDI) derivatives and poly(ethylene glycol) (PEG)) | 101.9 ± 2.8 nm (PMA–PDI–PEG NPs)72.6 ± 2.4 nm (Gd3+-PMA–PDI–PEG NPs) | 40% | Nanoprecipitation method | PTT on human epithelial cervix adenocarcinoma (HeLa) tumor model | [50] |
| PolyPyrrole-PEG NPss | 7 nm | 33.35% (808 nm), 41.97% (1064 nm) | Self assembling method | Multimodal imaging and PTT on human brain glioblastoma cell line bearing mouse tumor model | [51] |
| poly(cyclopentadithiophene-alt-benzothiadiazole) (PCPDTBT) | 47 nm with −20 mV | Not given | Nanoprecipitation method | Combined PTT/PDT on mouse epithelial mammary gland metastatic cancer cells (4T1) tumor bearing mouse | [52] |
| Poly vinylene based SPN | 36 nm | Not given | Nanoprecipitation method | Improved PAI/PTT effects on mouse epithelial mammary gland metastatic cancer cells (4T1) tumor bearing model | [53] |
| BODIPY-TPA (Triphenylamine) | 80 nm in size with −35.5 mV surface charge | 20.7% | Nano-precipitation method | Improved PTT/PDT in-vitro on human epithelial lung carcinoma cell line (A549) | [54] |
| diketopyrrolopyrrole-based semiconducting polymer and polystyrene-b-poly(N-isopropyl acrylamide-co-acrylic acid) (PDPP3T@PSNiAA NPs) | 70 nm sized NPs showed photo chemo effects in-vitro and in-vivo | 34.1% | Co-precipitation method | High photo chemo effects on human epithelial cervix adenocarcinoma (HeLa) bearing tumor model | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rejinold, N.S.; Choi, G.; Choy, J.-H. Recent Developments on Semiconducting Polymer Nanoparticles as Smart Photo-Therapeutic Agents for Cancer Treatments—A Review. Polymers 2021, 13, 981. https://doi.org/10.3390/polym13060981
Rejinold NS, Choi G, Choy J-H. Recent Developments on Semiconducting Polymer Nanoparticles as Smart Photo-Therapeutic Agents for Cancer Treatments—A Review. Polymers. 2021; 13(6):981. https://doi.org/10.3390/polym13060981
Chicago/Turabian StyleRejinold, N. Sanoj, Goeun Choi, and Jin-Ho Choy. 2021. "Recent Developments on Semiconducting Polymer Nanoparticles as Smart Photo-Therapeutic Agents for Cancer Treatments—A Review" Polymers 13, no. 6: 981. https://doi.org/10.3390/polym13060981
APA StyleRejinold, N. S., Choi, G., & Choy, J. -H. (2021). Recent Developments on Semiconducting Polymer Nanoparticles as Smart Photo-Therapeutic Agents for Cancer Treatments—A Review. Polymers, 13(6), 981. https://doi.org/10.3390/polym13060981