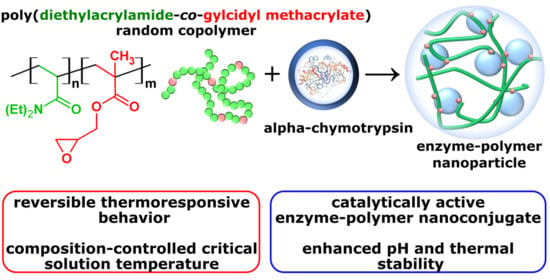
Thermoresponsive Poly(N,N-diethylacrylamide-co-glycidyl methacrylate) Copolymers and Its Catalytically Active α-Chymotrypsin Bioconjugate with Enhanced Enzyme Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis Methods
2.2.1. Synthesis of PDEAAm Homopolymer and P(DEAAm-co-GMA) Copolymers by Free Radical Polymerization
2.2.2. Synthesis of Enzyme–Copolymer Nanoparticle (EPNP)
2.3. Characterization
2.3.1. Gel Permeation Chromatography (GPC)
2.3.2. 1H NMR Spectroscopy
2.3.3. Thermoresponsive Behavior
2.3.4. Dynamic Light Scattering (DLS)
2.3.5. Quantification of the Enzyme Content in the Nanoparticles
2.3.6. Catalytic Activity Assay
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tasdelen, M.A.; Kahveci, M.U.; Yagci, Y. Telechelic polymers by living and controlled/living polymerization methods. Prog. Polym. Sci. 2011, 36, 455–567. [Google Scholar] [CrossRef]
- Bokern, S.; Gries, K.; Görtz, H.H.; Warzelhan, V.; Agarwal, S.; Greiner, A. Precisely designed gold nanoparticles by surface polymerization-artificial molecules as building blocks for novel materials. Adv. Funct. Mater. 2011, 21, 3753–3759. [Google Scholar] [CrossRef]
- Gao, H.; Matyjaszewski, K. Synthesis of functional polymers with controlled architecture by CRP of monomers in the presence of cross-linker: From stars to gels. Prog. Polym. Sci. 2009, 34, 317–350. [Google Scholar] [CrossRef]
- Kennedy, J.P.; Iván, B. Designed Polymers by Carbocationic Macromolecular Engineering: Theory and Practice; Hanser Publisher: New York, NY, USA, 1992. [Google Scholar]
- He, X.-Y.; Liu, B.-Y.; Ai, S.-L.; Xu, L.; Zhuo, R.-X.; Cheng, S.-X. Functional polymer/inorganic hybrid nanoparticles for macrophage targeting delivery of oligodeoxynucleotides in cancer immuniotherapy. Mater. Today Chem. 2017, 4, 106–116. [Google Scholar] [CrossRef]
- Fu, H.-L.; Cheng, S.-X.; Zhang, X.-Z.; Zhuo, R.-X. Dendrimer/DNA complexes encapsulated functional biodegradable polymer for substrate-mediated gene delivery. J. Gene Med. 2008, 10, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-Q.; Wang, C.; Kim, N.-Y. High-sensitivity and low-hysteresis porous MIM-type capacitive humidity sensor using functional polymer mixed with TiO2 microparticles. Sensors 2017, 17, 284. [Google Scholar] [CrossRef]
- Takeuchi, T.; Hayashi, T.; Ichikawa, S.; Kaji, A.; Masui, M.; Matsumoto, H.; Sasao, R. Molecularly imprinted tailor-made functional polymer receptors for highly sensitive and selective separation and detection of target molecules. Chromatography 2016, 37, 43–64. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Yan, C.; Zheng, Z. Functional polymer surfaces for controlling cell behaviors. Mater. Today 2018, 21, 38–59. [Google Scholar] [CrossRef]
- Zhao, Y.; Weix, D.J. Nickel-catalyzed regiodivergent opening of epoxides with aryl halides: Co-catalysis controls regioselectivity. J. Am. Chem. Soc. 2014, 136, 48–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, S.; Jang, J. Protein immobilization on aminated poly(glycidyl methacrylate) nanofibers as polymeric carriers. Biomacromolecules 2007, 8, 1400–1403. [Google Scholar] [CrossRef]
- Gadwal, I.I.; Stuparu, M.C.; Khan, A. Homopolymer biofunctionalization through sequential thiol-epoxy and esterification reactions: An optimization, quantification, and structural elucidation study. Polym. Chem. 2015, 6, 1393–1404. [Google Scholar] [CrossRef]
- Undin, J.; Finne-Wistrand, A.; Albertsson, A.C. Copolymerization of 2-methylene-1,3-dioxepane and glycidyl methacrylate, a well-defined and efficient process for achieving functionalized polyesters for covalent binding of bioactive molecules. Biomacromolecules 2013, 14, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, S.K.; Murphy, E.L.; Gopalan, P. Crosslinked PEG mats for peptide immobilization and stem cell adhesion. J. Mater. Chem. B 2013, 1, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Kasza, G.Y.; Szarka, G.Y.; Bodor, A.; Kali, G.; Iván, B. In situ terminal functionalization of polystyrene obtained by quasiliving ATRP and subsequent derivatization. ACS Symp. Ser. 2018, 1285, 281–295. [Google Scholar]
- Iván, B.; Fónagy, T. Quantitative Derivatizations of 1-Chloro-1-phenylethyl Chain End of Polystyrene Obtained by Quasiliving Atom Transfer Radical Polymerization. ACS Symp. Ser. 2000, 768, 372–383. [Google Scholar]
- Sane, P.S.; Palaskar, D.V.; Wadgaonkar, P.P. Synthesis of bis-allyloxy functionalized polystyrene and poly(methyl methacrylate) macromonomers using a new ATRP initiator. Eur. Polym. J. 2011, 47, 1621–1629. [Google Scholar] [CrossRef]
- Hirao, A.; Shimohara, N.; Ryu, S.W.; Sugiyama, K. Synthesis of highly branched comblike polymers having one branch in each repeating unit by linking reaction of polystyryllithium with well-defined new epoxy-functionalized polystyrene. Macromol. Smyp. 2004, 214, 17–28. [Google Scholar] [CrossRef]
- Muzammil, E.M.; Khan, A.; Stuparu, M.C. Post-polymerization modification reactions of poly(glycidyl methacrylate)s. RSC Adv. 2017, 7, 55874–55884. [Google Scholar] [CrossRef] [Green Version]
- Stuparu, M.C.; Khan, A. Thiol-epoxy “click” chemistry: Application in preparation and postpolymerization modification of polymers. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3057–3070. [Google Scholar] [CrossRef]
- Kuroishi, P.K.; Bennison, M.J.; Dove, A.P. Synthesis and post-polymerisation modification of an epoxy-functional polycarbonate. Polym. Chem. 2016, 46, 7108–7115. [Google Scholar] [CrossRef]
- McLeod, D.C.; Tsarevsky, N.V. 4-Vinylphenyl glycidyl ether: Synthesis, RAFT polymerization and postpolymerization modifications with alcohols. Macromolecules 2016, 49, 1135–1142. [Google Scholar] [CrossRef]
- Gunaydin, O.; Yilmaz, F. Copolymers of glycidyl methacrylate with 3-methylthienyl methacrylate: Synthesis, characterization and reactivity ratios. Polym. J. 2007, 39, 579–588. [Google Scholar] [CrossRef] [Green Version]
- Maciejewska, M. Thermal properties of TRIM-GMA copolymers with pendant amine groups. J. Therm. Anal. Calorim. 2016, 126, 1777–1785. [Google Scholar] [CrossRef] [Green Version]
- Chou, Y.-N.; Wen, T.-C.; Chang, Y. Zwitterionic surface grafting of epoxylated sulfobetaine copolymers for the development of stealth biomaterial interfaces. Acta Biomater. 2016, 40, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Brito, G.F.; Agrawal, P.; Mélo, T.J.A. Mechanical and morphological properties of PLA/BioPE blen compatibilized with E-GMA and EMA-GMA copolymers. Macromol. Symp. 2016, 367, 176–182. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H.T.; Chen, J.L.; Fu, Z.A.; Zhao, X.W.; Li, Y.J. Copolymers containing two types of reactive groups: New compatibilizer for immiscible PLLA/PA11 polymer blends. Polymer 2019, 177, 139–148. [Google Scholar] [CrossRef]
- Acikbas, Y.; Capan, R.; Erdogan, M.; Bulut, L.; Soykan, C. Optical characterization and swelling behavior of Langmuir-Blodgett thin films of a novel poly[(styrene (ST)-co-glycidyl methacrylate (GMA)]. Sens. Actuators B 2017, 241, 1111–1120. [Google Scholar] [CrossRef]
- Lei, Z.; Gao, J.; Liu, X.; Liu, D.; Wang, Z. Poly(glycidyl methacrylate-co-2-hydroxyethyl methacrylate) brushes as peptide/protein microarray substrate for improving protein binding and functionality. ACS Appl. Mater. Interfaces 2016, 8, 10174–10182. [Google Scholar] [CrossRef]
- Nastasovic, A.B.; Ekmescic, B.M.; Sandic, Z.P.; Randelovic, D.V.; Mozetic, M.; Vesel, A.; Onjia, A.E. Mechanism of Cu(II), Cd(II) and Pb(II) ions sorption from aqueous solution by macroporous poly(glycidyl methacrylate-co-ethylene glycol dimethacrylate). Appl. Surf. Sci. 2016, 385, 605–615. [Google Scholar] [CrossRef]
- Chauhan, G.S.; Guleria, L.; Sharma, R. Synthesis, characterization and metal ion sorption studies of graft copolymers of cellulose with glycidyl methacrylate and some comonomers. Cellulose 2005, 12, 97–110. [Google Scholar] [CrossRef]
- Hus, S.; Kolar, M.; Krajnc, P. Separation of heavy metals from water by functionalized glycidyl methacrylate poly(high internal phase emulsions). J. Chromatogr. A 2016, 1437, 168–175. [Google Scholar] [CrossRef]
- Li, X.; Xu, Z.; Yin, H.; Feng, Y.; Quan, H. Comparative Studies on Enhanced Oil Recovery: Thermoviscosifying Polymer Versus Polyacrylamide. Energy Fuels 2017, 31, 2479–2487. [Google Scholar] [CrossRef]
- Li, X.; Yin, H.-Y.; Zhang, R.-S.; Cui, J.; Wu, J.-W.; Feng, Y.-J. A salt-induced viscosifying smart polymer for fracturing inter-salt shale oil reservoirs. Petroleum Sci. 2019, 16, 816–829. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Feng, Y. Thermoviscosifying Smart Polymers for Oil and Gas Production: State of the Art. Chem. Phys. Chem. 2018, 19, 1941–1955. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Park, H.-K.; Park, H.; Lee, S.-H. Snapshot of phase transition in thermoresponsive hydrogel PNIPAM: Role in drug delivery and tissue engineering. Macromol. Res. 2016, 24, 297–304. [Google Scholar] [CrossRef]
- Utrata-Wesolek, A.; Oleszko-Torbus, N.; Bochenek, M.; Kosowski, D.; Kowalczuk, A.; Trzebicka, B.; Dworak, A. Thermoresponsive polymer surfaces and their application in tissue engineering. Polimery 2018, 5, 325–406. [Google Scholar] [CrossRef]
- Song, X.; Zhu, J.-L.; Wen, Y.; Zhao, F.; Zhang, Z.-X.; Li, J. Thermoresponsive supramolecular micellar drug delivery system based on star-linear pseudo-block polymer consisting of β-cyclodextrin-poly(N-isopropylacrylamide) and adamantyl-poly(ethylene glycol). J. Colloid Interface Sci. 2017, 490, 372–379. [Google Scholar] [CrossRef]
- Gandhi, A.; Paul, A.; Sen, S.O.; Sen, K.K. Studies on thermoresponsive polymers: Phase behavior, drug delivery and biomedical applications. Asian J. Pharm. Sci. 2015, 10, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Gong, D.; Cao, T.; Han, S.-C.; Zhu, X.; Iqbal, A.; Liu, W.; Qin, W.; Guo, H. Fluorescence enhancement thermoresponsive polymer luminescent sensors based on BODIPY for intracellular temperature. Sens. Actuators B 2017, 252, 577–583. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Baek, P.; De la Rosa, V.R.; Barker, D.; Hoogenboom, R.; Travas-Sejdic, J. Thermoresponsive laterally-branched polythiophene phenylene derivative as water-soluble temperature sensor. Polym. Chem. 2017, 8, 4352–4359. [Google Scholar] [CrossRef] [Green Version]
- Owusu-Nkwantabisah, S.; Gillmor, J.; Switalski, S.; Mis, M.R.; Bennett, G.; Moody, R.; Antalek, B.; Gutierrez, R.; Slater, G. Synergistic thermoresponsive optical properties of a composite self-healing hydrogel. Macromolecules 2017, 50, 3671–3679. [Google Scholar] [CrossRef]
- Vidal, F.; Lin, H.; Morales, C.; Jakle, F. Polysiloxane/polystyrene thermos-responsive and self-healing polymer network via Lewis asic-Lewis base pair formation. Molecules 2018, 23, 405. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-H.; Jung, Y.; Lee, D.; Jang, W.-D. Thermoresponsive polymer and fluorescent dye hybrids for tunable multicolour emission. Adv. Mater. 2016, 28, 3499–3503. [Google Scholar] [CrossRef]
- Chin, S.M.; Synatschke, C.V.; Liu, S.; Nap, R.J.; Sather, N.A.; Wang, Q.; Álvarez, Z.; Edelbrock, A.N.; Fyrner, T.; Palmer, L.C.; et al. Covalent-supramolecular hybrid polymers as muscle-inspired anisotropic actuators. Nat. Commun. 2018, 9, 2395. [Google Scholar] [CrossRef] [Green Version]
- Osváth, Z.; Iván, B. The Dependence of the Cloud Point, Clearing Point, and Hysteresis of Poly(N-isopropylacrylamide) on Experimental Conditions: The Need for Standardization of Thermoresponsive Transition Determinations. Macromol. Chem. Phys. 2017, 218, 1600470. [Google Scholar] [CrossRef] [Green Version]
- Osváth, Z.; Tóth, T.; Iván, B. Synthesis, characterization, LCST-type behavior and unprecedented heating-cooling hysteresis of poly(N-isopropylacrylamide-co-3-(trimethoxysilyl) propyl methacrylate) copolymers. Polymer 2017, 108, 395–399. [Google Scholar] [CrossRef] [Green Version]
- Osváth, Z.; Tóth, T.; Iván, B. Sustained Drug Release by Thermoresponsive Sol-Gel Hybrid Hydrogels of Poly(N-Isopropylacrylamide-co-3-(Trimethoxysilyl) Propyl Methacrylate) Copolymers. Macromol. Rapid Commun. 2017, 38, 1600724. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, Y.; Fu, W.; Yao, M.; Ding, Z.; Xuan, J.; Li, D.; Wang, S.; Xia, Y.; Cao, M. Poly(N-isopropylacrylamide)-based thermoresponsive composite hydrogels for biomedical applications. Polymers 2020, 12, 580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barsbay, M.; Güven, O. Modification of Polystyrene Cell-Culture-Dish Surfaces by Consecutive Grafting of Poly(acrylamide)/Poly(N-isopropylacrylamide) via Reversible Addition-Fragmentation Chain Transfer-Mediated Polymerization. Eur. Polym. J. 2021, 147, 110330. [Google Scholar] [CrossRef]
- Luo, G.F.; Chen, W.H.; Zhang, X.Z. 100th Anniversary of Macromolecular Science Viewpoint: Poly(N-isopropylacrylamide)-Based Thermally Responsive Micelles. ACS Macro Lett. 2020, 9, 872–881. [Google Scholar] [CrossRef]
- Hirayama, S.; Oohora, K.; Uchihashi, T.; Hayashi, T. Thermoresponsive micellar assembly constructed from a hexameric hemoprotein modified with poly(N-isopropylacrylamide) toward an artificial light-harvesting system. J. Am. Chem. Soc. 2020, 142, 1822–1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, T.; Demco, D.E.; Fechete, R.; Möller, M.; Singh, S. Poly(vinylamine-co-N-isopropylacrylamide) linear polymer and hydrogels with tuned thermoresponsivity. Soft Matter 2020, 16, 6549–6562. [Google Scholar] [CrossRef]
- Tang, L.; Wang, L.; Yang, X.; Feng, Y.; Li, Y.; Feng, W. Poly(N-isopropylacrylamide)-based smart hydrogels: Design, properties and applications. Prog. Mater. Sci. 2020, 100702. [Google Scholar] [CrossRef]
- Halperin, A.; Kröger, M.; Winnik, F.M. Poly(N-isopropylacrylamide) phase diagrams: Fifty years of research. Angew. Chem. Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef]
- Virtanen, J.; Tenhu, H. Studies on copolymerization of N-isopropylacrylamide and glycidyl methacrylate. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 3716–3725. [Google Scholar] [CrossRef]
- Venugopal, B.; Shenoy, S.J.; Mohan, S.; Anil Kumar, P.R.; Kumary, T.V. Bioengineered corneal epithelial cell sheet from mesenchymal stem cells—A functional alternative to limbal stem cells for ocular surface reconstruction. J. Biomed. Mater. Res. Part B 2020, 108, 1033–1045. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Kohestanian, M.; Streb, C. pH and thermal dual-responsive poly(NIPAM-co-GMA)-coated magnetic nanoparticles via surface-initiated RAFT polymerization for controlled drug delivery. Mater. Sci. Eng. C 2020, 108, 110418. [Google Scholar] [CrossRef]
- Li, P.; Xu, R.; Wang, W.; Li, X.; Xu, Z.; Yeung, K.W.; Chu, P.K. Thermosensitive poly(N-isopropylacrylamide-co-glycidyl methacrylate) microgels for controlled drug release. Colloids Surf. B 2013, 101, 251–255. [Google Scholar] [CrossRef]
- Jiang, X.; Xiong, D.A.; An, Y.; Zheng, P.; Zhang, W.; Shi, L. Thermoresponsive hydrogel of poly(glycidyl methacrylate-co-N-isopropylacrylamide) as a nanoreactor of gold nanoparticles. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 2812–2819. [Google Scholar] [CrossRef]
- Chen, L.; Dong, J.; Ding, Y.; Han, W. Environmental responses of poly(N-isopropylacrylamide-co-glycidyl methacrylate) derivatized dextran hydrogels. J. Appl. Polym. Sci. 2005, 96, 2435–2439. [Google Scholar] [CrossRef]
- Nguyen, A.L.; Luong, J.H.T. Syntheses and applications of water-soluble reactive polymers for purification and immobilization of biomolecules. Biotechnol. Bioeng. 1989, 34, 1186–1190. [Google Scholar] [CrossRef]
- Fang, S.-J.; Kawaguchi, H. A thermoresponsive amphoteric microsphere and its potential application as a biological carrier. Colloid Polym. Sci. 2002, 280, 984–989. [Google Scholar] [CrossRef]
- Peng, H.; Rübsam, K.; Hu, C.; Jakob, F.; Schwaneberg, U.; Pich, A. Stimuli-Responsive Poly(N-Vinyllactams) with Glycidyl Side Groups: Synthesis, Characterization, and Conjugation with Enzymes. Biomacromolecules 2019, 20, 992–1006. [Google Scholar] [CrossRef]
- Panayiotou, M.; Pöhner, C.; Vandevyver, C.; Wandrey, C.; Hilbrig, F.; Freitag, R. Synthesis and characterisation of thermo-responsive poly(N,N′-diethylacrylamide) microgels. React. Funct. Polym. 2007, 67, 807–819. [Google Scholar] [CrossRef]
- Ida, S.; Harada, H.; Sakai, K.; Atsumi, K.; Tani, Y.; Tanimoto, S.; Hirokawa, Y. Shape and size regulation of gold nanoparticles by poly(N,N-diethylacrylamide) microgels. Chem. Lett. 2017, 46, 760–763. [Google Scholar] [CrossRef] [Green Version]
- Isiklan, N.; Kazan, H. Thermoresponsive and biocompatible poly(vinyl alcohol)-graft-poly(N,N-diethylacrylamide) copolymer: Microwave-assisted synthesis, characterization, and swelling behavior. J. Appl. Polym. Sci. 2018, 135, 45969. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Z.; Xie, D.; Liu, D.; Chen, Z.; Li, K.; Li, Z.; Tichnell, B.; Liu, Z. Design and synthesis study of the thermo-sensitive poly(N-vinylpyrrolidone-b-N,N-diethylacrylamide). Des. Monomers Polym. 2018, 21, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Tada, T.; Asoh, T.A.; Shoji, T.; Nishiyama, T.; Horibe, H.; Katsumoto, Y.; Tsuboi, Y. Dynamics of the phase separation in a thermoresponsive polymer: Accelerated phase separation of stereocontrolled poly(N,N-diethylacrylamide) in water. Langmuir 2018, 34, 13690–13696. [Google Scholar] [CrossRef] [Green Version]
- Havanur, S.; Farheenand, V.; JagadeeshBabu, P.E. Synthesis and optimization of poly(N,N-diethylacrylamide) hydrogel and evaluation of its anticancer drug doxorubicin’s release behavior. Iran. Polym. J. 2019, 28, 99–112. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, H.; Liu, H. Study of phase separation behavior of poly(N,N-diethylacrylamide) in aqueous solution prepared by RAFT polymerization. Polym. Bull. 2019, 76, 825–848. [Google Scholar] [CrossRef]
- Li, J.; Kikuchi, S.; Sato, S.I.; Chen, Y.; Xu, L.; Song, B.; Duan, Q.; Wang, Y.; Kakuchi, T.; Shen, X. Core-First Synthesis and Thermoresponsive Property of Three-, Four-, and Six-Arm Star-Shaped Poly(N,N-diethylacrylamide)s and Their Block Copolymers with Poly(N,N-dimethylacrylamide). Macromolecules 2019, 52, 7207–7217. [Google Scholar] [CrossRef]
- Luo, G.; Lu, Y.; Wu, S.; Shen, X.; Zhu, M.; Li, S. Hierarchical polymer composites as smart reactor for formulating simple/tandem-commutative catalytic ability. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4394–4407. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, L.; Xu, S.; Kuchel, R.P.; Dai, Y.; Jung, K.; Boyer, C. Dual Role of Doxorubicin for Photopolymerization and Therapy. Biomacromolecules 2020, 21, 3887–3897. [Google Scholar] [CrossRef] [PubMed]
- Paneysar, J.S.; Jain, S.; Ahmed, N.; Barton, S.; Ambre, P.; Coutinho, E. Novel smart composite materials for industrial wastewater treatment and reuse. SN Appl. Sci. 2020, 2, 1–12. [Google Scholar] [CrossRef]
- Li, S.; Wang, F.; Yang, Z.; Xu, J.; Liu, H.; Zhang, L.; Xu, W. Emulsifying performance of near-infrared light responsive polydopamine-based silica particles to control drug release. Powder Technol. 2020, 359, 17–26. [Google Scholar] [CrossRef]
- Baert, M.; Wicht, K.; Hou, Z.; Szucs, R.; Prez, F.D.; Lynen, F. Exploration of the selectivity and retention behavior of alternative polyacrylamides in temperature responsive liquid chromatography. Anal. Chem. 2020, 92, 9815–9822. [Google Scholar] [CrossRef]
- Lee, C.H.; Bae, Y.C. Thermodynamic framework for switching the lower critical solution temperature of thermo-sensitive particle gels in aqueous solvent. Polymer 2020, 195, 122428. [Google Scholar] [CrossRef]
- Ma, Y.; Li, M.; Shi, K.; Chen, Z.; Yang, B.; Rao, D.; Li, X.; Ma, W.; Hou, S.; Gou, G.; et al. Multiple stimuli-switchable electrocatalysis and logic gates of rutin based on semi-interpenetrating polymer network hydrogel films. New J. Chem. 2020, 44, 16045–16053. [Google Scholar] [CrossRef]
- Ma, C.; Tchameni, A.P.; Pan, L.; Su, C.; Zhou, C. A thermothickening polymer as a novel flocculant for oily wastewater treatment. Sep. Sci. Technol. 2020, 55, 123–134. [Google Scholar] [CrossRef]
- Ni, M.; Xu, Y.; Wang, C.; Zhao, P.; Yang, P.; Chen, C.; Zheng, K.; Wang, H.; Sun, X.; Lia, C.; et al. A novel thermo-controlled acetaminophen electrochemical sensor based on carboxylated multi-walled carbon nanotubes and thermosensitive polymer. Diam. Relat. Mater. 2020, 107, 107877. [Google Scholar] [CrossRef]
- Zhang, X.; Burton, T.F.; In, M.; Begu, S.; Aubert-Pouëssel, A.; Robin, J.J.; Mongea, S.; Giani, O. Synthesis and behaviour of PEG-b-PDEAm block copolymers in aqueous solution. Mater. Today Commun. 2020, 24, 100987. [Google Scholar] [CrossRef]
- Chen, L.; Chen, X.; Gong, Y.; Shao, T.; Zhang, X.; Chen, D.; Cai, Z. Study on Influence of Monobutyl Itaconate and N,N-diethylacrylamide on Acrylic Latex. Prot. Met. Phys. Chem. Surf. 2020, 56, 740–745. [Google Scholar] [CrossRef]
- Hanyková, L.; Krakovský, I.; Šestáková, E.; Šťastná, J.; Labuta, J. Poly(N,N′-Diethylacrylamide)-Based Thermoresponsive Hydrogels with Double Network Structure. Polymers 2020, 12, 2502. [Google Scholar] [CrossRef]
- Tuan, H.N.A.; Nhu, V.T.T. Synthesis and properties of pH-thermo dual responsive semi-IPN hydrogels based on N,N′-diethylacrylamide and itaconamic acid. Polymers 2020, 12, 1139. [Google Scholar] [CrossRef]
- Yan, X.; Chu, Y.; Liu, B.; Ru, G.; Di, Y.; Feng, J. Dynamic mechanism of halide salts on the phase transition of protein models, poly(N-isopropylacrylamide) and poly(N,N-diethylacrylamide). Phys. Chem. Chem. Phys. 2020, 22, 12644–12650. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, R.; Ou, X.; Zhang, X.; Liu, P.; Chen, Z.; Zhang, B.; Liu, C.; Zhao, S.; Chen, Z.; et al. Bio-inspired synthesis of thermo-responsive imprinted composite membranes for selective recognition and separation of ReO4−. Sep. Purif. Technol. 2021, 259, 118165. [Google Scholar] [CrossRef]
- Drozdov, A.D.; Christiansen, J.D. Modulation of the volume phase transition temperature of thermo-responsive gels. J. Mech. Behav. Biomed. 2021, 114, 104215. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Mao, J.; Cai, Y.; Li, S.; Fu, Y.; Liu, X.; Liang, H.; Zhao, T.; Liu, M.; Jiang, L. Euryhaline Hydrogel with Constant Swelling and Salinity-Enhanced Mechanical Strength in a Wide Salinity Range. Adv. Funct. Mater. 2021, 31, 2007664. [Google Scholar] [CrossRef]
- Xie, B.; Tchameni, A.P.; Luo, M.; Wen, J. A novel thermo-associating polymer as rheological control additive for bentonite drilling fluid in deep offshore drilling. Mater. Lett. 2021, 284, 128914. [Google Scholar] [CrossRef]
- Li, K.; Liu, X.T.; Zhang, Y.F.; Liu, D.; Zhang, X.Y.; Ma, S.M.; Ruso, J.M.; Tang, Z.H.; Chen, Z.B.; Liu, Z. The engineering and immobilization of penicillin G acylase onto thermo-sensitive tri-block copolymer system. Polym. Adv. Technol. 2019, 30, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Shan, G.; Ma, X.; Zhang, X.; Chen, Z.; Tang, Z.; Liu, Z. Study of target spacing of thermo-sensitive carrier on the activity recovery of immobilized penicillin G acylase. Colloids Surf. B. 2019, 179, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Idziak, I.; Avoce, D.; Lessard, D.; Gravel, D.; Zhu, X.X. Thermosensitivity of Aqueous Solutions of Poly(N,N-diethylacrylamide). Macromolecules 1999, 32, 1260–1263. [Google Scholar] [CrossRef]
- Lessard, D.G.; Ousalem, M.; Zhu, X.X. Effect of the molecular weight on the lower critical solution temperature of poly(N,N-diethylacrylamide) in aqueous solutions. Can. J. Chem. 2001, 79, 1870–1874. [Google Scholar] [CrossRef]
- Lessard, D.G.; Ousalem, M.; Zhu, X.X.; Eisenberg, A.; Carreau, P.J. Study of the phase transition of poly(N,N-diethylacrylamide) in water by rheology and dynamic light scattering. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 1627–1637. [Google Scholar] [CrossRef]
- Zhou, K.; Lu, Y.; Li, J.; Shen, L.; Zhang, G.; Xie, Z.; Wu, C. The coil-to-globule-to-coil transition of linear polymer chains in dilute aqueous solutions: Effect of intrachain hydrogen bonding. Macromolecules 2008, 41, 8927–8931. [Google Scholar] [CrossRef]
- Trzebicka, B.; Szweda, R.; Kosowski, D.; Szweda, D.; Otulakowski, Ł.; Haladjova, E.; Dworak, A. Thermoresponsive polymer-peptide/protein conjugates. Prog. Polym. Sci. 2017, 68, 35–76. [Google Scholar] [CrossRef]
- Burridge, K.M.; Page, R.C.; Konkolewicz, D. Bioconjugates—From a specialized past to a diverse future. Polymer 2020, 211, 123062. [Google Scholar] [CrossRef]
- Wright, T.A.; Page, R.C.; Konkolewicz, D. Polymer conjugation of proteins as a synthetic post-translational modification to impact their stability and activity. Polym. Chem. 2019, 10, 434–454. [Google Scholar] [CrossRef]
- Chen, C.; Ng, D.Y.W.; Weil, T. Polymer bioconjugates: Modern design concepts toward precision hybrid materials. Prog. Polym. Sci. 2020, 105, 101241. [Google Scholar] [CrossRef]
- Rodriguez-Abetxuko, A.; Sánchez-de Alcázar, D.; Muñumer, P.; Beloqui, A. Tunable polymeric scaffolds for enzyme immobilization. Front. Bioeng. Biotechnol. 2020, 8, 830. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, C. Site-specific conjugation of polymers to proteins. Biomacromolecules 2018, 19, 1804–1825. [Google Scholar] [CrossRef]
- Messina, M.S.; Messina, K.M.; Bhattacharya, A.; Montgomery, H.R.; Maynard, H.D. Preparation of biomolecule-polymer conjugates by grafting-from using ATRP, RAFT, or ROMP. Prog. Polym. Sci. 2020, 100, 101186. [Google Scholar] [CrossRef] [PubMed]
- Carmali, S.; Murata, H.; Cummings, C.; Matyjaszewski, K.; Russell, A.J. Polymer-Based Protein Engineering: Synthesis and Characterization of Armored, High Graft Density Polymer–Protein Conjugates. Methods Enzymol. 2017, 590, 347–380. [Google Scholar] [PubMed]
- Baker, S.L.; Kaupbayeva, B.; Lathwal, S.; Das, S.R.; Russell, A.J.; Matyjaszewski, K. Atom transfer radical polymerization for biorelated hybrid materials. Biomacromolecules 2019, 20, 4272–4298. [Google Scholar] [CrossRef] [PubMed]
- Cummings, C.; Murata, H.; Koepsel, R.; Russell, A.J. Dramatically increased pH and temperature stability of chymotrypsin using dual block polymer-based protein engineering. Biomacromolecules 2014, 15, 763–771. [Google Scholar] [CrossRef]
- Kaupbayeva, B.; Russell, A.J. Polymer-enhanced biomacromolecules. Prog. Polym. Sci. 2020, 101, 101194. [Google Scholar] [CrossRef]
- Shakya, A.K.; Nandakumar, K.S. An update on smart biocatalysts for industrial and biomedical applications. J. R. Soc. Interface 2018, 15, 20180062. [Google Scholar] [CrossRef]
- Rottke, F.O.; Heyne, M.V.; Reinicke, S. Switching enzyme activity by a temperature responsive inhibitor modified polymer. Chem. Commun. 2020, 56, 2459–2462. [Google Scholar] [CrossRef]
- Chapman, R.; Stenzel, M.H. All wrapped up: Stabilization of enzymes within single enzyme nanoparticles. J. Am. Chem. Soc. 2019, 141, 2754–2769. [Google Scholar] [CrossRef]
- Yadavalli, N.S.; Borodinov, N.; Choudhury, C.K.; Quiñones-Ruiz, T.; Laradji, A.M.; Tu, S.; Lednev, I.K.; Kuksenok, O.; Luzinov, I.; Minko, S. Thermal stabilization of enzymes with molecular brushes. ACS Catal. 2017, 7, 8675–8684. [Google Scholar] [CrossRef]
- Pasparakis, G.; Tsitsilianis, C. LCST polymers: Thermoresponsive nanostructured assemblies towards bioapplications. Polymer 2020, 11, 123146. [Google Scholar] [CrossRef]
- Chado, G.R.; Holland, E.N.; Tice, A.K.; Stoykovich, M.P.; Kaar, J.L. Exploiting the benefits of homogeneous and heterogeneous biocatalysis: Tuning the molecular interaction of enzymes with solvents via polymer modification. ACS Catal. 2018, 8, 11579–11588. [Google Scholar] [CrossRef]
- Bilici, Z.; Camli, S.T.; Unsal, E.; Tuncel, A. Activity behacior of a HPLC column including α-chymotrypsin immobilized monosized-porous particles. Anal. Chim. Acta 2004, 516, 125–133. [Google Scholar] [CrossRef]
- Meller, K.; Pomastowski, P.; Grzywinski, D.; Szumski, M.; Buszewski, B. Preparation and evaluation of dual-enzyme microreactor with co-immobilized trypsin and chymotrypsin. J. Chromatogr. A 2016, 1440, 45–54. [Google Scholar] [CrossRef]
- Munasinghe, A.; Baker, S.L.; Lin, P.; Russell, A.J.; Colina, C.M. Structure–function–dynamics of α-chymotrypsin based conjugates as a function of polymer charge. Soft Matter 2020, 16, 456–465. [Google Scholar] [CrossRef]
- Mukhopadhayay, A.; Singh, D.; Sharma, K.P. Neat Ionic liquid and α-Chymotrypsin-Polymer Surfactant Conjugate-Based Biocatalytic Solvent. Biomacromolecules 2020, 21, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Cummings, C.S.; Koepsel, R.R.; Russell, A.J. Rational tailoring of substrate and inhibitor affinity via ATRP polymer-based protein engineering. Biomacromolecules 2014, 15, 2817–2823. [Google Scholar] [CrossRef]
- Hegedüs, I.; Nagy, E. Improvement of chymotrypsin enzyme stability as single enzyme nanoparticles. Chem. Eng. Sci. 2009, 64, 1053–1060. [Google Scholar] [CrossRef]
- Hegedüs, I.; Vitai, M.; Jakab, M.; Nagy, E. Study of Prepared α-Chymotrypsin as Enzyme Nanoparticles and of Biocatalytic Membrane Reactor. Catalysts 2020, 10, 1454. [Google Scholar] [CrossRef]
- Wang, X.; Yadavalli, N.S.; Laradji, A.M.; Minko, S. Grafting through method for implanting of lysozyme enzyme in molecular brush for improved biocatalytic activity and thermal stability. Macromolecules 2018, 51, 5039–5047. [Google Scholar] [CrossRef]
- Rahman, M.S.; Brown, J.; Murphy, R.; Carnes, S.; Carey, B.; Averick, S.; Konkolewicz, D.; Page, R.C. Polymer Modification of Lipases, Substrate Interactions, and Potential Inhibition. Biomacromolecules 2021, 22, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Wirnt, R.; Bergmeyer, H.U. Chymotrypsin, in Methods of Enzymatic Analysis; Academic Press: New York, NY, USA, 1974; pp. 1009–1012. [Google Scholar]
- Yin, X.; Stöver, H.D. Hydrogel microspheres by thermally induced coacervation of poly(N,N-dimethylacrylamide-co-glycidyl methacrylate) aqueous solutions. Macromolecules 2003, 36, 9817–9822. [Google Scholar] [CrossRef]
- Ghaouar, N.; Elmissaoui, S.; Aschi, A.; Gharbi, A. Concentration regimes and denaturation effects on the conformational changes of α-chymotrypsin by viscosity and dynamic light scattering measurements. Int. J. Biol. Macromol. 2010, 47, 425–430. [Google Scholar] [CrossRef] [PubMed]








| Sample | Molar Feed Ratio AIBN:DEAAm:GMA | Yield % | Mn (g/mol) | Mp (g/mol) | Đ |
|---|---|---|---|---|---|
| A | 1:95:5 | 62.7 | 6650 | 7530 | 1.75 |
| B | 1:90:10 | 69.6 | 6525 | 7620 | 1.88 |
| C | 1:190:10 | 75.6 | 8620 | 20,170 | 2.31 |
| D | 1:180:20 | 86.8 | 7520 | 21,470 | 2.80 |
| PDEAAm | 1:100:0 | 81.5 | 9820 | 18,840 | 1.90 |
| Sample | DEAAm/GMA Comonomerfeed Ratio | DEAAm/GMA Ratio in the Copolymers a | XGMA (%) | TCP (°C) | TCL (°C) |
|---|---|---|---|---|---|
| A | 19:1 | 17.06:1 | 5.5 | 31.2 | 30.8 |
| B | 9:1 | 8.91:1 | 10.1 | 27.2 | 26.2 |
| C | 19:1 | 15.25:1 | 6.2 | 30.6 | 30.1 |
| D | 9:1 | 7.80:1 | 11.4 | 24.8 | 24.6 |
| PDEAAm | - | - | 0 | 37.4 | 36.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasza, G.; Stumphauser, T.; Bisztrán, M.; Szarka, G.; Hegedüs, I.; Nagy, E.; Iván, B. Thermoresponsive Poly(N,N-diethylacrylamide-co-glycidyl methacrylate) Copolymers and Its Catalytically Active α-Chymotrypsin Bioconjugate with Enhanced Enzyme Stability. Polymers 2021, 13, 987. https://doi.org/10.3390/polym13060987
Kasza G, Stumphauser T, Bisztrán M, Szarka G, Hegedüs I, Nagy E, Iván B. Thermoresponsive Poly(N,N-diethylacrylamide-co-glycidyl methacrylate) Copolymers and Its Catalytically Active α-Chymotrypsin Bioconjugate with Enhanced Enzyme Stability. Polymers. 2021; 13(6):987. https://doi.org/10.3390/polym13060987
Chicago/Turabian StyleKasza, György, Tímea Stumphauser, Márk Bisztrán, Györgyi Szarka, Imre Hegedüs, Endre Nagy, and Béla Iván. 2021. "Thermoresponsive Poly(N,N-diethylacrylamide-co-glycidyl methacrylate) Copolymers and Its Catalytically Active α-Chymotrypsin Bioconjugate with Enhanced Enzyme Stability" Polymers 13, no. 6: 987. https://doi.org/10.3390/polym13060987
APA StyleKasza, G., Stumphauser, T., Bisztrán, M., Szarka, G., Hegedüs, I., Nagy, E., & Iván, B. (2021). Thermoresponsive Poly(N,N-diethylacrylamide-co-glycidyl methacrylate) Copolymers and Its Catalytically Active α-Chymotrypsin Bioconjugate with Enhanced Enzyme Stability. Polymers, 13(6), 987. https://doi.org/10.3390/polym13060987