Preparation and Gas Sensing Properties of PANI/SnO2 Hybrid Material
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
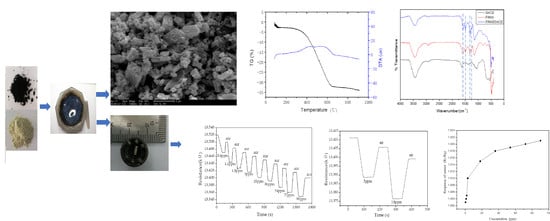
3.1. Characterization of Materials
3.2. Test of Gas Sensing Properties
3.2.1. Ammonia Gas Sensing Properties
3.2.2. Ammonia Sensing Mechanism
3.2.3. Benzene Vapor Gas Sensing Properties
3.2.4. Benzene Vapor Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korotcenkov, G.; Han, S.-D.; Cho, B.K.; Brinzari, V. Grain size effects in sensor response of nanostructured SnO2 and In2O3-based conductometric thin film gas sensor. Crit. Rev. Solid State Mater. Sci. 2009, 34, 1–17. [Google Scholar] [CrossRef]
- Yang, W.; Shen, H.; Ge, J.; Xu, B. Improving TiO2 gas sensing selectivity to acetone and other gases via a molecular imprinting method. Nanotechnology 2021, 32, 155503. [Google Scholar] [CrossRef] [PubMed]
- Atique, M.; Mehmood, M.; Shabbir, G. Fabrication and characterization of organic semiconductor based gas sensor. Key Eng. Mater. 2010, 421–422, 344–347. [Google Scholar] [CrossRef]
- Li, Z.; Wu, X.-H.; Jiang, W.-R.; Shi, Y.-B. Preparation and gas sensitivity study of new phthalocyanine organic semiconductor materials. Mater. Sci. Technol. 2011, 19, 108–112. [Google Scholar]
- Lin, J.; Moller, S.; Obermeier, E. Thin-film gas sensors with organically modified silicates for the measurement of SO2. Sens. Actuators B Chem. 1991, B5, 219–221. [Google Scholar] [CrossRef]
- Xu, H.; Chen, X.; Zhang, J. NO2 gas sensing with SnO2-ZnO/PANI composite thick film fabricated from porous nanosolid. Sens. Actuators B Chem. 2013, 176, 166–173. [Google Scholar] [CrossRef]
- Quan, L.; Sun, J.; Bai, S.; Luo, R.; Li, D.; Chen, A.; Liu, C.C. A flexible sensor based on polyaniline hybrid using ZnO as template and sensing properties to triethylamine at room temperature. Appl. Surf. Sci. 2017, 399, 583–591. [Google Scholar] [CrossRef]
- Saravanan, K.K.; Karthik, P.S.; Mirtha, P.R.; Balaji, J.; Rajeshkanna, B. A one-pot hydrothermal-induced PANI/SnO2 and PANI/SnO2/rGO ternary composites for high-performance chemiresistive-based H2S and NH3 gas sensors. J. Mater. Sci. Mater. Electron. 2020, 31, 8825–8836. [Google Scholar] [CrossRef]
- Shrivastav, A.M.; Sharma, G.; Rathore, A.S. Hypersensitive and Selective Interferometric Nose for Ul-tratrace Ammonia Detection with Fast Response Utilizing PANI@SnO2 Nanocomposite. ACS Photonics 2018, 5, 4402–4412. [Google Scholar] [CrossRef]
- Kulkarni, S.; Patil, P.; Mujumdar, A.; Naik, J. Synthesis and evaluation of gas sensing properties of PANI, PANI/SnO2 and PANI/SnO2/rGO nanocomposites at room temperature. Inorg. Chem. Commun. 2018, 96, 90–96. [Google Scholar] [CrossRef]
- Khuspe, G.D.; Navale, S.T.; Bandgar, D.K.; Sakhare, R.D.; Chougule, M.A.; Patil, V.B. SnO2 nanoparticles-modified polyaniline films as highly selective, sensitive, reproducible and stable ammonia sensors. Electron. Mater. Lett. 2014, 10, 191–197. [Google Scholar] [CrossRef]
- Khuspe, G.D.; Navale, S.T.; Chougule, M.A. Ammonia gas sensing properties of CSA doped PANi-SnO2 nanohybrid thin films. Synth. Met. 2013, 185, 1–8. [Google Scholar] [CrossRef]
- Singh, B.; Das, B. The synthesis and study of structural, optical and electrical behaviors of tin oxide/polyaniline nanocompo-sites. Pramana—J. Phys. 2019, 93, 32. [Google Scholar] [CrossRef]
- Geng, L.-N. Gas sensitivity of polyaniline/SnO2 hybrids to volatile organic compounds. Trans. Nonferrous Met. Soc. China 2009, 19 (Suppl. 3), 678–683. [Google Scholar] [CrossRef]
- Pattanauwat, P.; Aht-Ong, D. In-Situ Electrochemical Synthesis of Novel Sensitive Layer of Polyaniline/Multiwall Carbon Nanotube/Tin Oxide Hybrid Materials for Ethylene Gas Detection. Polym.-Plast. Technol. Eng. 2013, 52, 189–194. [Google Scholar] [CrossRef]
- Venkatachalaiah, C.; Venkataraman, U.; Sellappan, R. PANI/TiO2 nanocomposite-based chemiresistive gas sensor for the detection of E. coli bacteria. IET Nanobiotechnol. 2020, 14, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Bahru, R.; Zamri, M.F.M.A.; Shamsuddin, A.H.; Mohamed, M.A. Structural analyses of polyaniline–titanium oxide composite for acetone detection. J. Mater. Sci. Mater. Electron. 2020, 31, 1574–1584. [Google Scholar] [CrossRef]
- Seif, A.M.; Nikfarjam, A.; Hajghassem, H. UV enhanced ammonia gas sensing properties of PANI/TiO2 core-shell nanofibers. Sens. Actuators B Chem. 2019, 298, 126906. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, H.; Li, X.; Guo, T.; Zhu, L.; Xue, Q. Layer-by-layer self-assembly of polyaniline nanofibers/TiO2 nanotubes heterojunction thin film for ammonia detection at room temperature. Nanotechnology 2019, 30, 135501. [Google Scholar] [CrossRef]
- Zhu, C.; Cheng, X.; Dong, X.; Xu, Y.M. Enhanced Sub-ppm NH3 Gas Sensing Performance of PANI/TiO2 Nanocomposites at Room Temperature. Front. Chem. 2018, 6, 493. [Google Scholar] [CrossRef]
- Gawri, I.; Ridhi, R.; Singh, K.P.; Tripathi, S.K. Chemically synthesized TiO2 and PANI/TiO2 thin films for ethanol sensing applications. Mater. Res. Express 2018, 5, 025303. [Google Scholar] [CrossRef]
- Harb, M.E.; Ebrahim, S.; Soliman, M.; Shabana, M. Fabrication of Organic Transistors Using Nanomaterials for Sensing Applications. J. Electron. Mater. 2018, 47, 353–358. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, X.; Huang, C.; Chen, X.; Dai, W.; Fu, X. CO gas sensitivity and its oxidation over TiO2 modified by PANI under UV irradiation at room temperature. Appl. Catal. B Environ. 2017, 219, 379–390. [Google Scholar] [CrossRef]
- Ma, X.; Wang, M.; Li, G.; Chen, H.; Bai, R. Preparation of polyaniline-TiO2 composite film with in situ polymerization approach and its gas-sensitivity at room temperature. Mater. Chem. Phys. 2006, 98, 241–247. [Google Scholar] [CrossRef]
- Wei, X.; Jiao, L.; Sun, J.; Liu, S.; Yuan, H. Synthesis, electrochemical, and gas sensitivity performance of polyaniline/MoO3 hybrid materials. J. Solid State Electrochem. 2010, 14, 197–202. [Google Scholar] [CrossRef]
- Xie, Y.; Shi, Z.; Liu, J. Polyaniline/nano-In2O3 composites used as a sensitive material to NH3. Adv. Mater. Res. 2011, 239–242, 322–327. [Google Scholar] [CrossRef]
- Xu, L.-H.; Wu, T.-M. Synthesis of highly sensitive ammonia gas sensor of polyaniline/graphene nanoribbon/indium oxide composite at room temperature. J. Mater. Sci. Mater. Electron. 2020, 31, 7276–7283. [Google Scholar] [CrossRef]
- Sridhar, C.B.; Sasikala, M.; Husain, J. Synthesis, characterization, electrical and sensor behavior of PANI/In2O3 nanocomposites. Ferroelectrics 2016, 502, 159–169. [Google Scholar] [CrossRef]
- Al-Hartomy, O.A.; Khasim, S.; Roy, A.; Pasha, A. Highly conductive polyaniline/graphene nano-platelet composite sensor towards detection of toluene and benzene gases. Appl. Phys. A Mater. Sci. Process. 2019, 125, 12. [Google Scholar] [CrossRef]
- Ihdene, Z.; Mekki, A.; Mettai, B.; Mahmoud, R.; Hamada, B.; Chehimi, M.M. Quartz crystal microbalance VOCs sensor based on dip coated polyaniline emeraldine salt thin films. Sens. Actuators B Chem. 2014, 203, 647–654. [Google Scholar] [CrossRef]













| Test Gas | PANI | PANI/SnO2 | [29] |
|---|---|---|---|
| Benzene gas concentration | 0–36 ppm | 0–90 ppm | 0–22,000 ppm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Q.; Zhang, H.; Shi, Y.; Yu, X.; Lan, G. Preparation and Gas Sensing Properties of PANI/SnO2 Hybrid Material. Polymers 2021, 13, 1360. https://doi.org/10.3390/polym13091360
Feng Q, Zhang H, Shi Y, Yu X, Lan G. Preparation and Gas Sensing Properties of PANI/SnO2 Hybrid Material. Polymers. 2021; 13(9):1360. https://doi.org/10.3390/polym13091360
Chicago/Turabian StyleFeng, Qiaohua, Huanhuan Zhang, Yunbo Shi, Xiaoyu Yu, and Guangdong Lan. 2021. "Preparation and Gas Sensing Properties of PANI/SnO2 Hybrid Material" Polymers 13, no. 9: 1360. https://doi.org/10.3390/polym13091360
APA StyleFeng, Q., Zhang, H., Shi, Y., Yu, X., & Lan, G. (2021). Preparation and Gas Sensing Properties of PANI/SnO2 Hybrid Material. Polymers, 13(9), 1360. https://doi.org/10.3390/polym13091360