Effect of Deterpenated Origanum majorana L. Essential Oil on the Physicochemical and Biological Properties of Chitosan/β-Chitin Nanofibers Nanocomposite Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
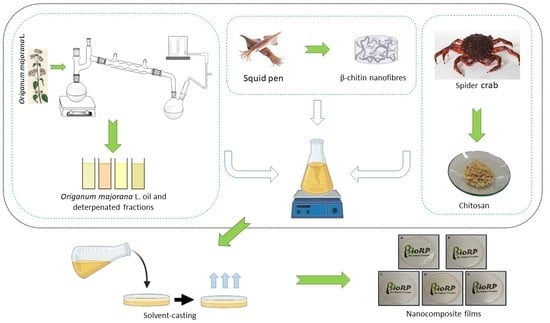
2.2. Preparation of the Nanocomposite Films
2.3. Characterization of the Nanocomposite Films
2.3.1. Physicochemical Characterization
Thickness
Moisture Content
Water Solubility
Color of the Samples
Transmittance and Opacity
Water Contact Angle
Attenuated Total Reflection-Fourier Transform Infrared Radiation (ATR-FTIR)
2.3.2. Morphology
2.3.3. Thermogravimetric Analysis (TGA) and Mechanical Properties
2.3.4. Antifungal Properties
2.3.5. Cytotoxicity Assay
2.3.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization
3.1.1. Moisture Content and Water Solubility
3.1.2. Color Properties
3.1.3. Optical Properties
3.1.4. ATR-FTIR
3.1.5. Water Contact Angle
3.2. Morphology
3.3. Thermogravimetric Analysis and Mechanical Properties
3.4. Antifungal Properties
3.5. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Santos, R.; Andrade, M.; De Melo, N.R.; Sanches-Silva, A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Fernández-Marín, R.; Labidi, J.; Andrés, M.Á.; Fernandes, S.C.M. Using α-chitin nanocrystals to improve the final properties of poly (vinyl alcohol) films with Origanum vulgare essential oil. Polym. Degrad. Stab. 2020, 179. [Google Scholar] [CrossRef]
- Lago, S.; Rodríguez, H.; Soto, A.; Arce, A. Deterpenation of citrus essential oil by liquid-liquid extraction with 1-alkyl-3-methylimidazolium bis(trifluoromethylsulfonyl)amide ionic liquids. J. Chem. Eng. Data 2011, 56, 1273–1281. [Google Scholar] [CrossRef]
- Silvestre, W.P.; Agostini, F.; Muniz, L.A.R.; Pauletti, G.F. Fractionating of green Mandarin (Citrus deliciosa Tenore) essential oil by vacuum fractional distillation. J. Food Eng. 2016, 178, 90–94. [Google Scholar] [CrossRef]
- Francisco, M.; Lago, S.; Soto, A.; Arce, A. Essential oil deterpenation by solvent extraction using 1-ethyl-3-methylimidazolium 2-(2-methoxyethoxy) ethylsulfate ionic liquid. Fluid Phase Equilibria 2010, 296, 149–153. [Google Scholar] [CrossRef]
- Perini, J.F.; Silvestre, W.P.; Agostini, F.; Toss, D.; Pauletti, G.F. Fractioning of orange (Citrus sinensis L.) essential oil using vacuum fractional distillation. Sep. Sci. Technol. 2017, 52, 1397–1403. [Google Scholar] [CrossRef]
- Gonçalves, D.; Costa, P.; Rodrigues, C.E.C.; Rodrigues, A.E. Effect of citrus sinensis essential oil deterpenation on the aroma profile of the phases obtained by solvent extraction. J. Chem. Thermodyn. 2018, 116, 166–175. [Google Scholar] [CrossRef]
- Sakamoto, K.; Fujii, K.; Inoue, A.; Kozuka, H.; Ohta, H. Differential recovery of terpene hydrocarbons and oxygenated compounds from condensates containing essential oil discharged during concentration of citrus juices using a ceramic membrane. Food Sci. Technol. Res. 2003, 9, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Ben Salha, G.; Herrera Díaz, R.; Labidi, J.; Abderrabba, M. Deterpenation of Origanum majorana L. essential oil by reduced pressure steam distillation. Ind. Crops Prod. 2017, 109, 116–122. [Google Scholar] [CrossRef]
- Bouyahya, A.; Chamkhi, I.; Benali, T.; Guaouguaou, F.E.; Balahbib, A.; El Omari, N.; Taha, D.; Belmehdi, O.; Ghokhan, Z.; El Menyiy, N. Traditional use, phytochemistry, toxicology, and pharmacology of Origanum majorana L. J. Ethnopharmacol. 2021, 265, 113318. [Google Scholar] [CrossRef]
- Fernández-Marín, R.; Fernandes, S.C.M.; McReynolds, C.; Labidi, J.; Sánchez Andrés, M.Á. Chapter 22-Chitosan-Based Materials as Templates for Essential Oils. In Handbook of Chitin and Chitosan Volume 3: Chitina-and Chitosan-Based Polymer Materials for Varius Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 689–720. [Google Scholar]
- Jahed, E.; Khaledabad, M.A.; Almasi, H.; Hasanzadeh, R. Physicochemical properties of Carum copticum essential oil loaded chitosan films containing organic nanoreinforcements. Carbohydr. Polym. 2017, 164, 325–338. [Google Scholar] [CrossRef]
- Perdones, Á.; Vargas, M.; Atarés, L.; Chiralt, A. Physical, antioxidant and antimicrobial properties of chitosan e cinnamon leaf oil fi lms as affected by oleic acid. Food Hydrocoll. 2014, 36, 256–264. [Google Scholar] [CrossRef]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. In vitro antibacterial and antioxidant properties of chitosan edible fi lms incorporated with Thymus moroderi or Thymus piperella essential oils. Food Control 2013, 30, 386–392. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Labidi, J.; Fernandes, S.C.M. Different routes to turn chitin into stunning nano-objects. Eur. Polym. J. 2015, 68, 503–515. [Google Scholar] [CrossRef]
- Kaya, M.; Ravikumar, P.; Ilk, S.; Mujtaba, M.; Akyuz, L.; Labidi, J.; Salaberria, A.M.; Cakmak, Y.S.; Erkul, S.K. Production and characterization of chitosan based edible films from Berberis crataegina’s fruit extract and seed oil. Innov. Food Sci. Emerg. Technol. 2018, 45, 287–297. [Google Scholar] [CrossRef]
- Salari, M.; Sowti Khiabani, M.; Rezaei Mokarram, R.; Ghanbarzadeh, B.; Samadi Kafil, H. Development and evaluation of chitosan based active nanocomposite films containing bacterial cellulose nanocrystals and silver nanoparticles. Food Hydrocoll. 2018, 84, 414–423. [Google Scholar] [CrossRef]
- Zubillaga, V.; Alonso-Varona, A.; Fernandes, S.C.M.; Salaberria, A.M.; Palomares, T. Adipose-derived mesenchymal stem cell chondrospheroids cultured in hypoxia and a 3D porous chitosan/chitin nanocrystal scaffold as a platform for cartilage tissue engineering. Int. J. Mol. Sci. 2020, 21, 1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zubillaga, V.; Salaberria, A.M.; Palomares, T.; Alonso-Varona, A.; Kootala, S.; Labidi, J.; Fernandes, S.C.M. Chitin Nanoforms Provide Mechanical and Topological Cues to Support Growth of Human Adipose Stem Cells in Chitosan Matrices. Biomacromolecules 2018, 19, 3000–3012. [Google Scholar] [CrossRef] [PubMed]
- Salaberría, A.M.; Teruel-Juanes, R.; Badia, J.D.; Fernandes, S.C.M.; Sáenz de Juano-Arbona, V.; Labidi, J.; Ribes-Greus, A. Influence of chitin nanocrystals on the dielectric behaviour and conductivity of chitosan-based bionanocomposites. Compos. Sci. Technol. 2018, 167, 323–330. [Google Scholar] [CrossRef]
- Robles, E.; Salaberria, A.M.; Herrera, R.; Fernandes, S.C.M.; Labidi, J. Self-bonded composite films based on cellulose nanofibers and chitin nanocrystals as antifungal materials. Carbohydr. Polym. 2016, 144, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Salaberria, A.M.; Labidi, J.; Fernandes, S.C.M. Chitin nanocrystals and nanofibers as nano-sized fillers into thermoplastic starch-based biocomposites processed by melt-mixing. Chem. Eng. J. 2014, 256, 356–364. [Google Scholar] [CrossRef]
- Saralegi, A.; Fernandes, S.C.M.; Alonso-Varona, A.; Palomares, T.; Foster, E.J.; Weder, C.; Eceiza, A.; Corcuera, M.A. Shape-memory bionanocomposites based on chitin nanocrystals and thermoplastic polyurethane with a highly crystalline soft segment. Biomacromolecules 2013, 14, 4475–4482. [Google Scholar] [CrossRef] [PubMed]
- Kasaai, M.R. Determination of the degree of N -acetylation for chitin and chitosan by various NMR spectroscopy techniques: A review. Carbohydr. Polym. 2010, 79, 801–810. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Diaz, R.H.; Labidi, J.; Fernandes, S.C.M. Preparing valuable renewable nanocomposite films based exclusively on oceanic biomass—Chitin nanofillers and chitosan. React. Funct. Polym. 2015, 89, 31–39. [Google Scholar] [CrossRef]
- Sahraee, S.; Milani, J.M.; Ghanbarzadeh, B.; Hamishehkar, H. Effect of corn oil on physical, thermal, and antifungal properties of gelatin-based nanocomposite films containing nano chitin. LWT Food Sci. Technol. 2017, 76, 33–39. [Google Scholar] [CrossRef]
- Sani, I.K.; Pirsa, S.; Tağı, Ş. Preparation of chitosan/zinc oxide/Melissa officinalis essential oil nano-composite film and evaluation of physical, mechanical and antimicrobial properties by response surface method. Polym. Test. 2019, 79. [Google Scholar] [CrossRef]
- Pereda, M.; Dufresne, A.; Aranguren, M.I.; Marcovich, N.E. Polyelectrolyte films based on chitosan/olive oil and reinforced with cellulose nanocrystals. Carbohydr. Polym. 2014, 101, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Poloni, T.; Lourenço, R.V.; Sobral, P.J.A. Antioxidant potential of eugenol and ginger essential oils with gelatin / chitosan films. Food Biosci. J. 2018, 23, 107–114. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Y. Combined effects of two kinds of essential oils on physical, mechanical and structural properties of chitosan films. Food Hydrocoll. 2014, 36, 287–293. [Google Scholar] [CrossRef]
- Hafsa, J.; Ali Smach, M.; Ben Khedher, M.R.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Physical, antioxidant and antimicrobial properties of chitosan films containing Eucalyptus globulus essential oil. LWT Food Sci. Technol. 2016, 68, 356–364. [Google Scholar] [CrossRef]
- Riaz, A.; Lei, S.; Akhtar, S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Muhammad, M.; Zeng, X. Preparation and characterization of chitosan-based antimicrobial active food packaging fi lm incorporated with apple peel polyphenols. Int. J. Biol. Macromol. 2018, 114, 547–555. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Harte, B.R. Physical properties and antioxidant activity of an active fi lm from chitosan incorporated with green tea extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Wu, J.; Ge, S.; Liu, H.; Wang, S.; Chen, S.; Wang, J.; Li, J.; Zhang, Q. Properties and antimicrobial activity of silver carp (Hypophthalmichthys molitrix) skin gelatin-chitosan films incorporated with oregano essential oil for fish preservation. Food Packag. Shelf Life 2014, 2, 7–16. [Google Scholar] [CrossRef]
- Cuong, H.N.; Minh, N.C.; Van Hoa, N.; Trung, T.S. Preparation and characterization of high purity β-chitin from squid pens (Loligo chenisis). Int. J. Biol. Macromol. 2016, 93, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Reddy, J.P.; Rhim, J.W.; Kim, H.Y. Preparation, characterization, and antimicrobial activity of chitin nanofibrils reinforced carrageenan nanocomposite films. Carbohydr. Polym. 2015, 117, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Oberemko, A.; Salaberria, A.M.; Saule, R.; Saulis, G.; Kaya, M. Physicochemical and in vitro cytotoxic properties of chitosan from mushroom species (Boletus bovinus and Laccaria laccata). Carbohydr. Polym. 2019, 221, 1–9. [Google Scholar] [CrossRef]
- Shin, C.; Kim, D.; Shin, W. Characterization of chitosan extracted from Mealworm Beetle (Tenebrio molitor, Zophobas morio) and Rhinoceros Beetle (Allomyrina dichotoma) and their antibacterial activities. Int. J. Biol. Macromol. 2019, 125, 72–77. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar Goel, N.; Gautam, S.S. Volatile Constituents of Curcuma caesia Roxb. Rhizome from North India. Natl. Acad. Sci. Lett. 2020, 43, 607–610. [Google Scholar] [CrossRef]
- Munhuweyi, K.; Caleb, O.J.; Lennox, C.L.; Van Reenen, A.J.; Opara, U.L. In vitro and in vivo antifungal activity of chitosan-essential oils against pomegranate fruit pathogens. Postharvest Biol. and Technol. 2017, 129, 9–22. [Google Scholar] [CrossRef]
- Morales, A.; Andrés, M.Á.; Labidi, J.; Gullón, P. UV–vis protective poly(vinyl alcohol)/bio-oil innovative films. Ind. Crops Prod. 2019, 131, 281–292. [Google Scholar] [CrossRef]
- Hajji, S.; Kchaou, H.; Bkhairia, I.; Ben Slama-Ben Salem, R.; Boufi, S.; Debeaufort, F.; Moncef, N. Conception of active food packaging films based on 1 crab chitosan and 2 gelatin enriched with crustacean protein hydrolysates with improved 3 functional and biological properties. Food Hydrocoll. 2021, 116, 106639. [Google Scholar] [CrossRef]
- Cunha, A.G.; Fernandes, S.C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A. What is the real value of chitosan’s surface energy? Biomacromolecules 2008, 9, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.G.L.; Pires, J.R.A.; Freitas Rodrigues, P.; Lopes, A.A.S.; Fernandes, F.M.B.; Paula, M.; Coelhoso, I.M.; Luisa, A. Bionanocomposites of chitosan/montmorillonite incorporated with Rosmarinus o ffi cinalis essential oil: Development and physical characterization. Food Packag. Shelf Life 2018, 16, 148–156. [Google Scholar] [CrossRef]
- Valizadeh, S.; Naseri, M.; Babaei, S.; Hosseini, S.M.H.; Imani, A. Development of bioactive composite films from chitosan and carboxymethyl cellulose using glutaraldehyde, cinnamon essential oil and oleic acid. Int. J. Biol. Macromol. 2019, 134, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, R.A.; Salmieri, S.; Le Tien, C.; Riedl, B.; Bouchard, J.; Chauve, G.; Tan, V.; Kamal, M.R.; Lacroix, M. Mechanical and barrier properties of nanocrystalline cellulose reinforced chitosan based nanocomposite films. Carbohydr. Polym. 2012, 90, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Noshirvani, N.; Ghanbarzadeh, B.; Gardrat, C. Cinnamon and ginger essential oils to improve antifungal, physical and mechanical properties of chitosan-carboxymethyl cellulose fi lms. Food Hydrocoll. 2017, 70, 36–45. [Google Scholar] [CrossRef]
- Silva Damasceno, E.T.; Almeida, R.R.; De Carvalho, S.Y.B.; De Carvalho, G.S.G.; Mano, V.; Pereira, A.C.; De Lima Guimarães, L.G. Lippia origanoides Kunth. essential oil loaded in nanogel based on the chitosan and ρ-coumaric acid: Encapsulation efficiency and antioxidant activity. Ind. Crops Prod. 2018, 125, 85–94. [Google Scholar] [CrossRef]
- Shen, Z.; Kamdem, D.P. Development and characterization of biodegradable chitosan films containing two essential oils. Int. J. Biol. Macromol. 2015, 74, 289–296. [Google Scholar] [CrossRef]
- Ardekani Torabi, N.; Khorram, M.; Zomorodian, K.; Yazdanpanah, S.; Veisi, H.; Hojat, V. Evaluation of electrospun poly (vinyl alcohol)-based nano fi ber mats incorporated with Zataria multi fl ora essential oil as potential wound dressing. Int. J. Biol. Macromol. 2019, 125, 743–750. [Google Scholar] [CrossRef]
- Mohammadi, M.; Mirabzadeh, S.; Shahvalizadeh, R.; Hamishehkar, H. Development of novel active packaging films based on whey protein isolate incorporated with chitosan nanofiber and nano-formulated cinnamon oil. Int. J. Biol. Macromol. 2020, 149, 11–20. [Google Scholar] [CrossRef]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of essential oils and homogenization conditions on properties of chitosan-based films. Food Hydrocoll. 2012, 26. [Google Scholar] [CrossRef]
- Kulisic, T.; Radonic, A.; Katalinic, V.; Milos, M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004, 85, 633–640. [Google Scholar] [CrossRef]
- Inouye, S.; Uchida, K.; Yamaguchi, H.; Miyara, T.; Gomi, S.; Amano, M. Volatile aroma constituents of three labiatae herbs growing wild in the Karakoram-Himalaya district and their antifungal activity by vapor contact. J. Essent. Oil Res. 2001, 13, 68–72. [Google Scholar] [CrossRef]
- Patil Shriniwas, P.; Kumbar Subhash, T. Antioxidant, antibacterial and cytotoxic potential of silver nanoparticles synthesized using terpenes rich extract of Lantana camara L. leaves. Biochem. Biophys. Rep. 2017, 10, 76–81. [Google Scholar] [CrossRef]








| Samples | Samples Identification | β-CHNF (% w/v) * | Fractions and Essential Oil (% v/v) * |
|---|---|---|---|
| Chitosan + β-CHNF | CSNF | 0.5 | - |
| Chitosan + β-CHNF + F1 | CSNF-F1 | 0.5 | 0.25 |
| Chitosan + β-CHNF + F2 | CSNF-F2 | 0.5 | 0.25 |
| Chitosan + β-CHNF + F3 | CSNF-F3 | 0.5 | 0.25 |
| Chitosan + β-CHNF +OM | CSNF-OM | 0.5 | 0.25 |
| Samples | Thickness (μm) | Moisture Content % | Water Solubility % |
|---|---|---|---|
| CSNF | 41.33 ± 1.97 a | 54.98 ± 2.48 a | 57.87 ± 4.78 a |
| CSNF-F1 | 45.00 ± 3.03 b | 40.41 ± 7.96 b | 32.53 ± 1.30 b |
| CSNF-F2 | 41.83 ± 5.34 a | 45.20 ± 9.03 b | 34.56 ± 3.07 b |
| CSNF-F3 | 44.33 ± 4.41 b,c | 53.79 ± 1.48 b | 49.27 ± 3.60 b |
| CSNF-OM | 42.83 ± 5.19 c | 42.72 ± 7.45 b | 32.37 ± 4.87 b |
| Samples | L* | a* | b* | ΔE | Opacity |
|---|---|---|---|---|---|
| CSNF | 91.83 ± 0.69 a | 1.23 ± 0.11 a | 6.02 ± 0.69 a | 1.73 ± 0.56 a | 4.76 ± 0.42 a |
| CSNF-F1 | 90.20 ± 0.23 a | 1.35 ± 0.12 a | 11.02 ± 0.51 b | 6.68 ± 1.05 b | 7.08 ± 0.78 b |
| CSNF-F2 | 91.95 ± 0.47 b | 0.91 ± 0.06 b | 6.84 ± 1.07 a | 2.21 ± 1.05 a | 7.47 ± 0.77 b |
| CSNF-F3 | 91.42 ± 1.12 a | 1.23 ± 0.36 a | 7.13 ± 2.09 a | 2.85 ± 2.21 a | 7.90 ± 0.35 b |
| CSNF-OM | 91.97 ± 0.33 b | 0.94 ± 0.05 b | 6.56 ± 0.83 a | 1.94 ± 0.83 a | 6.61 ± 0.53 b |
| Samples | TS (MPa) | YM (MPa) | E % |
|---|---|---|---|
| CSNF | 15.25 ± 1.86 a | 328.25 ± 18.67 a | 44.87 ± 8.12 a |
| CSNF-F1 | 17.88 ± 5.32 b | 310.98 ± 61.05 b | 64.40 ± 14.64 b |
| CSNF-F2 | 11.52 ± 3.97 b,c | 77.09 ± 11.77 c | 67.92 ± 33.13 b |
| CSNF-F3 | 12.20 ± 3.56 c | 153.39 ± 48.92 d | 46.90 ± 15.01 a,c |
| CSNF-OM | 11.49 ± 3.27 b,c | 133.89 ± 33.38 d | 50.66 ± 12.62 b,c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Marín, R.; Mujtaba, M.; Cansaran-Duman, D.; Ben Salha, G.; Andrés Sánchez, M.Á.; Labidi, J.; Fernandes, S.C.M. Effect of Deterpenated Origanum majorana L. Essential Oil on the Physicochemical and Biological Properties of Chitosan/β-Chitin Nanofibers Nanocomposite Films. Polymers 2021, 13, 1507. https://doi.org/10.3390/polym13091507
Fernández-Marín R, Mujtaba M, Cansaran-Duman D, Ben Salha G, Andrés Sánchez MÁ, Labidi J, Fernandes SCM. Effect of Deterpenated Origanum majorana L. Essential Oil on the Physicochemical and Biological Properties of Chitosan/β-Chitin Nanofibers Nanocomposite Films. Polymers. 2021; 13(9):1507. https://doi.org/10.3390/polym13091507
Chicago/Turabian StyleFernández-Marín, Rut, Muhammad Mujtaba, Demet Cansaran-Duman, Ghada Ben Salha, Mª Ángeles Andrés Sánchez, Jalel Labidi, and Susana C. M. Fernandes. 2021. "Effect of Deterpenated Origanum majorana L. Essential Oil on the Physicochemical and Biological Properties of Chitosan/β-Chitin Nanofibers Nanocomposite Films" Polymers 13, no. 9: 1507. https://doi.org/10.3390/polym13091507
APA StyleFernández-Marín, R., Mujtaba, M., Cansaran-Duman, D., Ben Salha, G., Andrés Sánchez, M. Á., Labidi, J., & Fernandes, S. C. M. (2021). Effect of Deterpenated Origanum majorana L. Essential Oil on the Physicochemical and Biological Properties of Chitosan/β-Chitin Nanofibers Nanocomposite Films. Polymers, 13(9), 1507. https://doi.org/10.3390/polym13091507