Cyclodextrins in Polymer-Based Active Food Packaging: A Fresh Look at Nontoxic, Biodegradable, and Sustainable Technology Trends
Abstract
:1. Introduction
2. Cyclodextrins (CDs) and Their Derivatives
3. Polymers Used in Active Packaging
3.1. Nonbiodegradable Polymers
3.2. Biodegradable Polymers
- Those obtained directly from biomass (for example, starch, protein, and cellulose).
- Those produced through chemical synthesis from bioderived monomers (for example, polylactic acid (PLA) and biobased polyethylene (PE)).
- Those produced through microbial fermentation (for example, polyhydroxy-alkanoates).
- Those produced through chemical synthesis from bioderived monomers and monomers based on petroleum, butylene polysuccinate (PBS), or polytrimethylene terephthalate (PTT) [51].
4. Incorporation of Active Substances in Polymeric Matrix Composites for Active Packaging Applications
4.1. Cyclodextrins in Food Packaging Technologies
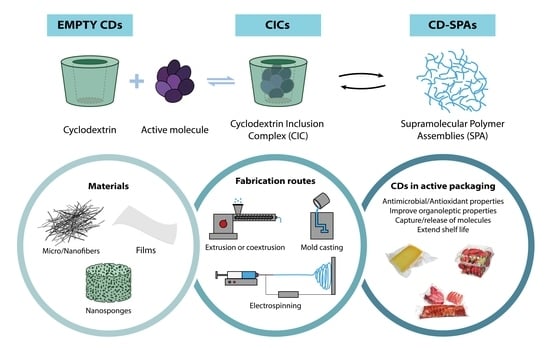
4.1.1. Empty Cyclodextrins
4.1.2. Active Molecule Encapsulation and Cyclodextrin Inclusion Complex for Active Food Packaging Applications
4.1.3. Cyclodextrin and Their Derivatives for the Formation of Supramolecular Polymer Assemblies
5. Methods of Incorporating CDs in Polymer-Based Active Food Packaging
5.1. Electrospun Micro-/Nanofibers and Mats
5.2. Nanosponges
5.3. Nanotechnology and Cyclodextrins in Active Food Packaging Applications
6. Toxicity and Legislation of Cyclodextrins in Polymer-Based Active Food Packaging
7. Commercial Products and Patents
8. Conclusions and Future Trends
Funding
Conflicts of Interest
Abbreviations
| ADI | Acceptable daily intake |
| AFP | Active food packaging |
| ARP | Active-releasing packaging |
| ASP | Active-scavenging packaging |
| CDs | Cyclodextrins |
| CD-NSs | Cyclodextrin-based nanosponges |
| CI | Citral |
| CICs | Cyclodextrin inclusion complexes |
| DMDS | Dimethyl disulfide |
| EOs | Essential oils |
| EVA | Polyethylene-co-vinyl acetate |
| FDA | Food and Drug Administration |
| GRAS | Generally Recognized as Safe |
| HDPE | High-density polyethylene |
| JECFA | Joint Expert Committee on Food Additives |
| LDPE | Low-density polyethylene |
| MAP | Modified atmospheric storage |
| MB | Masterbatch |
| PBS | Butylene polysuccinate |
| PE | Polyethylene |
| PET | Polyethylene terephthalate |
| PLA | Polylactic acid |
| PP | Polypropylene |
| PTT | Polytrimethylene terephthalate |
| PVA | Poly(vinyl acetate) |
| SCF | Scientific Committee on Food |
| TA-β-CD | Triacetyl-β-cyclodextrin |
| TC | Trans-cinnamaldehyde |
References
- Han, J.W.; Ruiz-Garcia, L.; Qian, J.P.; Yang, X.T. Food Packaging: A Comprehensive Review and Future Trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moeini, A.; Germann, N.; Malinconico, M.; Santagata, G. Formulation of secondary compounds as additives of biopolymer-based food packaging: A review. Trends Food Sci. Technol. 2021, 114, 342–354. [Google Scholar] [CrossRef]
- Beltran Sanahuja, A.; Valdes Garcia, A. New Trends in the Use of Volatile Compounds in Food Packaging. Polymers 2021, 13, 1053. [Google Scholar] [CrossRef] [PubMed]
- Soltani Firouz, M.; Mohi-Alden, K.; Omid, M. A critical review on intelligent and active packaging in the food industry: Research and development. Food Res. Int. 2021, 141, 110113. [Google Scholar] [CrossRef] [PubMed]
- Hosseinnejad, M. Active packaging for food applications—A review. Int. J. Adv. Biol. Biomed. Res. 2014, 2, 1174–1180. [Google Scholar]
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Applications of cyclodextrins in food science. A review. Trends Food Sci. Technol. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Hashemi, S.M.B.; Limbo, S. Antimicrobial agents and packaging systems in antimicrobial active food packaging: An overview of approaches and interactions. Food Bioprod. Process. 2018, 111, 1–19. [Google Scholar] [CrossRef]
- Limbo, S.; Khaneghah, A.M. Active packaging of foods and its combination with electron beam processing. In Electron Beam Pasteurization and Complementary Food Processing Technologies; Woodhead Publishing: Cambridge, UK, 2015; pp. 195–217. [Google Scholar]
- Mallardo, S.; De Vito, V.; Malinconico, M.; Volpe, M.G.; Santagata, G.; Di Lorenzo, M.L. Poly(butylene succinate)-based composites containing β-cyclodextrin/d-limonene inclusion complex. Eur. Polym. J. 2016, 79, 82–96. [Google Scholar] [CrossRef]
- Troise, A.D.; Fogliano, V. Reactants encapsulation and Maillard Reaction. Trends Food Sci. Technol. 2013, 33, 63–74. [Google Scholar] [CrossRef]
- Szente, L.; Fenyvesi, E. Cyclodextrin-Enabled Polymer Composites for Packaging (dagger). Molecules 2018, 23, 1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aytac, Z.; Ipek, S.; Durgun, E.; Tekinay, T.; Uyar, T. Antibacterial electrospun zein nanofibrous web encapsulating thymol/cyclodextrin-inclusion complex for food packaging. Food Chem. 2017, 233, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, B. Cellulose sulfate based film with slow-release antimicrobial properties prepared by incorporation of mustard essential oil and β-cyclodextrin. Food Hydrocoll. 2016, 55, 100–107. [Google Scholar] [CrossRef]
- Marques, H.M.C. A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Fragr. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gándara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- Harada, A.; Takashima, Y.; Yamaguchi, H. Cyclodextrin-based supramolecular polymers. Chem. Soc. Rev. 2009, 38, 875–882. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Schardinger, F. Über thermophile Bakterien aus verschiedenen Speisen und Milch. Zeitschrift für Untersuchung der Nahrungs- und Genußmittel 1903, 6, 865–880. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K.A. Phase Solubility Techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Becerril, R.; Nerin, C.; Silva, F. Encapsulation Systems for Antimicrobial Food Packaging Components: An Update. Molecules 2020, 25, 1134. [Google Scholar] [CrossRef] [Green Version]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Kfoury, M.; Geagea, C.; Ruellan, S.; Greige-Gerges, H.; Fourmentin, S. Effect of cyclodextrin and cosolvent on the solubility and antioxidant activity of caffeic acid. Food Chem. 2019, 278, 163–169. [Google Scholar] [CrossRef]
- Hedges, A. Cyclodextrins: Properties and Applications. In Starch: Chemistry and Technology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 833–851. [Google Scholar]
- Cravotto, G.; Binello, A.; Baranelli, E.; Carraro, P.; Trotta, F. Cyclodextrins as Food Additives and in Food Processing. Curr. Nutr. Food Sci. 2006, 2, 343–350. [Google Scholar] [CrossRef]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Leblanc, J.C.; et al. Re-evaluation of β-cyclodextrin (E 459) as a food additive. EFSA J. 2016, 14, e04628. [Google Scholar]
- Martina, K.; Binello, A.; Lawson, D.; Jicsinszky, L.; Cravotto, G. Recent Applications of Cyclodextrins as Food Additives and in Food Processing. Curr. Nutr. Food Sci. 2013, 9, 167–179. [Google Scholar] [CrossRef]
- Matencio, A.; Garcia-Carmona, F.; Lopez-Nicolas, J.M. The inclusion complex of oxyresveratrol in modified cyclodextrins: A thermodynamic, structural, physicochemical, fluorescent and computational study. Food Chem. 2017, 232, 177–184. [Google Scholar] [CrossRef]
- Poverenov, E.; Granit, R.; Gabai, S. Encapsulation and controlled release of antifungal propionic acid utilizing biodegradable active films based on natural polymers. Eur. Food Res. Technol. 2013, 237, 19–26. [Google Scholar] [CrossRef]
- Shin, J.; Lee, E.J.; Ahn, D.U. Electrospinning of tri-acetyl-β-cyclodextrin (TA-β-CD) functionalized low-density polyethylene to minimize sulfur odor volatile compounds. Food Packag. Shelf Life 2018, 18, 107–114. [Google Scholar] [CrossRef]
- Gonzalez Pereira, A.; Carpena, M.; Garcia Oliveira, P.; Mejuto, J.C.; Prieto, M.A.; Simal Gandara, J. Main Applications of Cyclodextrins in the Food Industry as the Compounds of Choice to Form Host-Guest Complexes. Int. J. Mol. Sci. 2021, 22, 1339. [Google Scholar] [CrossRef]
- Tian, B.; Xiao, D.; Hei, T.; Ping, R.; Hua, S.; Liu, J. The application and prospects of cyclodextrin inclusion complexes and polymers in the food industry: A review. Polym. Int. 2020, 69, 597–603. [Google Scholar] [CrossRef]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in aqueous solution: A review. J. Pharm. Biomed. Anal. 2014, 101, 238–250. [Google Scholar] [CrossRef]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in the solid state: A review. J. Pharm. Biomed. Anal. 2015, 113, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sameen, D.E.; Ahmed, S.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. Recent advances in cyclodextrin-based films for food packaging. Food Chem. 2021, 370, 131026. [Google Scholar] [CrossRef]
- Yildirim, S.; Rocker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [Green Version]
- Rooney, M.L. Active Food Packaging; Springer: Boston, MA, USA, 1995; p. 260. [Google Scholar]
- Da Costa, J.P.; Rocha-Santos, T.; Duarte, A.C. The Environmental Impacts of Plastics and Micro-Plastics Use, Waste and Pollution: EU and National Measures; European Parliament: Strasbourg, France, 2020. [Google Scholar]
- Silvestre, C.; Duraccio, D.; Cimmino, S. Food packaging based on polymer nanomaterials. Prog. Polym. Sci. 2011, 36, 1766–1782. [Google Scholar] [CrossRef]
- Tajeddin, B.; Arabkhedri, M. Polymers and food packaging. In Polymer Science and Innovative Applications; Elsevier: London, UK, 2020; pp. 525–543. [Google Scholar]
- Wang, K.; Deng, Q. The Thermal and Mechanical Properties of Poly(ethylene-co-vinyl acetate) Random Copolymers (PEVA) and its Covalently Crosslinked Analogues (cPEVA). Polymers 2019, 11, 1055. [Google Scholar] [CrossRef] [Green Version]
- Frine, V.C.; Hector, A.P.; Manuel, N.S.; Estrella, N.D.; Antonio, G.J. Development and Characterization of a Biodegradable PLA Food Packaging Hold Monoterpene-Cyclodextrin Complexes against Alternaria alternata. Polymers 2019, 11, 1720. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Huan, C.; Liang, X.; Fang, S.; Wang, J.; Chen, J. Development of Starch-Based Antifungal Coatings by Incorporation of Natamycin/Methyl-beta-Cyclodextrin Inclusion Complex for Postharvest Treatments on Cherry Tomato against Botrytis cinerea. Molecules 2019, 24, 3962. [Google Scholar] [CrossRef] [Green Version]
- Boonnattakorn, R.; Chonhenchob, V.; Siddiq, M.; Singh, S.P. Controlled Release of Mangiferin Using Ethylene Vinyl Acetate Matrix for Antioxidant Packaging. Packag. Technol. Sci. 2015, 28, 241–252. [Google Scholar] [CrossRef]
- Sonia, A.K.; Dasan, K.P. Feasibility studies of cellulose microfiber (CMF) reinforced poly(ethylene-co-vinyl acetate) (EVA) composites for food packaging applications. Sci. Eng. Compos. Mater. 2016, 23, 489–494. [Google Scholar] [CrossRef]
- Reesha, K.V.; Panda, S.K.; Bindu, J.; Varghese, T.O. Development and characterization of an LDPE/chitosan composite antimicrobial film for chilled fish storage. Int. J. Biol. Macromol. 2015, 79, 934–942. [Google Scholar] [CrossRef]
- Suppakul, P.; Sonneveld, K.; Bigger, S.W.; Miltz, J. Efficacy of polyethylene-based antimicrobial films containing principal constituents of basil. LWT-Food Sci. Technol. 2008, 41, 779–788. [Google Scholar] [CrossRef]
- Wang, L.; Mu, R.-J.; Li, Y.; Lin, L.; Lin, Z.; Pang, J. Characterization and antibacterial activity evaluation of curcumin loaded konjac glucomannan and zein nanofibril films. Lwt 2019, 113, 108293. [Google Scholar] [CrossRef]
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.K.; Mahanti, N.K. Application of Biodegradable Polymers in Food Packaging Industry: A Comprehensive Review. J. Packag. Technol. Res. 2018, 3, 77–96. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Byun, Y.; Kim, Y.T. Bioplastics for Food Packaging. In Innovations in Food Packaging; Elsevier: Tokyo, Japan, 2014; pp. 353–368. [Google Scholar]
- Mittal, V. Characterization Techniques for Polymer Nanocomposites; Wiley VCH: Singapore, 2012. [Google Scholar]
- Mano, J.F.; Gómez Ribelles, J.L.; Alves, N.M.; Salmerón Sanchez, M. Glass transition dynamics and structural relaxation of PLLA studied by DSC: Influence of crystallinity. Polymers 2005, 46, 8258–8265. [Google Scholar] [CrossRef] [Green Version]
- Velazquez-Contreras, F.; Garcia-Caldera, N.; Padilla de la Rosa, J.D.; Martinez-Romero, D.; Nunez-Delicado, E.; Gabaldon, J.A. Effect of PLA Active Packaging Containing Monoterpene-Cyclodextrin Complexes on Berries Preservation. Polymers 2021, 13, 1399. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Barnett, I. The Global Outlook for Biodegradable Packaging; Business Insights Ltd.: Burnaby, BC, Canada, 2011. [Google Scholar]
- Gotro, J. Thermoplastic Starch: A Renewable, Biodegradable Bioplastic. In Polymer Innovation Blog; InnoCentrix, LLC: Rancho Santa Margarita, CA, USA, 2013. [Google Scholar]
- Restrepo-Osorio, A.; Cruz Riaño, L.J.; Alvarez-Lopéz, C.; Rios Osorio, A.D. Revisión: Fibroína de seda y sus potenciales aplicaciones en empaques biodegradables para alimentos/Review: Silk fibroin and their potential applications on biodegradable food packaging. Prospectiva 2017, 15, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and new opportunities on barrier performance of biodegradable polymers for sustainable packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Wei, X.W.; Guo, G.; Gong, C.Y.; Gou, M.L.; Yong Qian, Z. Biodegradable Polymers: Research and Applications. In A Handbook of Applied Biopolymer Technology; Springer: Berlin/Heidelberg, Germany, 2011; Chapter 12; pp. 365–387. [Google Scholar]
- Tang, X.Z.; Kumar, P.; Alavi, S.; Sandeep, K.P. Recent advances in biopolymers and biopolymer-based nanocomposites for food packaging materials. Crit. Rev. Food Sci. Nutr. 2012, 52, 426–442. [Google Scholar] [CrossRef]
- Södergård, A.; Stolt, M. Poly(lactic acid): Synthesis, Structures, Properties, Processing, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Goni-Ciaurriz, L.; Senosiain-Nicolay, M.; Velaz, I. Aging Studies on Food Packaging Films Containing beta-Cyclodextrin-Grafted TiO2 Nanoparticles. Int. J. Mol. Sci. 2021, 22, 2257. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.; Thomas, R.; Byun, Y.; Whiteside, S. Improved flexibility of thermally stable poly-lactic acid (PLA). Carbohydr. Polym. 2012, 88, 165–172. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Almasi, H.; Jahanbakhsh Oskouie, M.; Saleh, A. A review on techniques utilized for design of controlled release food active packaging. Crit. Rev. Food Sci. Nutr. 2021, 61, 2601–2621. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M.C.d. Nanocomposites for food packaging applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef] [Green Version]
- Echegoyen, Y.; Fabra, M.J.; Castro-Mayorga, J.L.; Cherpinski, A.; Lagaron, J.M. High throughput electro-hydrodynamic processing in food encapsulation and food packaging applications: Viewpoint. Trends Food Sci. Technol. 2017, 60, 71–79. [Google Scholar] [CrossRef]
- Patino Vidal, C.; Lopez de Dicastillo, C.; Rodriguez-Mercado, F.; Guarda, A.; Galotto, M.J.; Munoz-Shuguli, C. Electrospinning and cyclodextrin inclusion complexes: An emerging technological combination for developing novel active food packaging materials. Crit. Rev. Food Sci. Nutr. 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, M.; Boonruang, K.; Chinsirikul, W.; Hararak, B.; Kerddonfag, N.; Chonhenchob, V.; Kenawy, E.-R. Antifungal Poly(lactic acid) Films Containing Thymol and Carvone. MATEC Web Conf. 2016, 67, 6107. [Google Scholar]
- Chen, H.; Li, L.; Ma, Y.; McDonald, T.P.; Wang, Y. Development of active packaging film containing bioactive components encapsulated in β-cyclodextrin and its application. Food Hydrocoll. 2019, 90, 360–366. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Hashemi, S.M.B.; Es, I.; Fracassetti, D.; Limbo, S. Efficacy of Antimicrobial Agents for Food Contact Applications: Biological Activity, Incorporation into Packaging, and Assessment Methods: A Review. J. Food Prot. 2018, 81, 1142–1156. [Google Scholar] [CrossRef]
- Sanches-Silva, A.; Costa, D.; Albuquerque, T.G.; Buonocore, G.G.; Ramos, F.; Castilho, M.C.; Machado, A.V.; Costa, H.S. Trends in the use of natural antioxidants in active food packaging: A review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 2014, 31, 374–395. [Google Scholar] [CrossRef] [PubMed]
- Joo, M.; Auras, R.; Almenar, E. Preparation and characterization of blends made of poly(l-lactic acid) and β-cyclodextrin: Improvement of the blend properties by using a masterbatch. Carbohydr. Polym. 2011, 86, 1022–1030. [Google Scholar] [CrossRef]
- Wen, P.; Wen, Y.; Zong, M.H.; Linhardt, R.J.; Wu, H. Encapsulation of Bioactive Compound in Electrospun Fibers and Its Potential Application. J. Agric. Food Chem. 2017, 65, 9161–9179. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Guijarro, M.; García-Gómez, P.; Almela, L.; Nuñez-Delicado, E.; Gabaldón, J.A. Improvement of the shelf life of minimally processed artichoke through antimicrobial and antioxidants agents. Acta Hortic. 2020, 1284, 205–220. [Google Scholar] [CrossRef]
- Petitjean, M.; Garcia-Zubiri, I.X.; Isasi, J.R. History of cyclodextrin-based polymers in food and pharmacy: A review. Environ. Chem. Lett. 2021, 19, 3465–3476. [Google Scholar] [CrossRef] [PubMed]
- Arruda, T.R.; Marques, C.S.; Soares, N.F.F. Native Cyclodextrins and Their Derivatives as Potential Additives for Food Packaging: A Review. Polysaccharides 2021, 2, 825–842. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Winterton, P.; Fourmentin, S.; Wilson, L.D.; Fenyvesi, É.; Crini, G. Water-insoluble β-cyclodextrin–epichlorohydrin polymers for removal of pollutants from aqueous solutions by sorption processes using batch studies: A review of inclusion mechanisms. Prog. Polym. Sci. 2018, 78, 1–23. [Google Scholar] [CrossRef]
- Matencio, A.; Hoti, G.; Monfared, Y.K.; Rezayat, A.; Pedrazzo, A.R.; Caldera, F.; Trotta, F. Cyclodextrin Monomers and Polymers for Drug Activity Enhancement. Polymers 2021, 13, 1684. [Google Scholar] [CrossRef]
- Hu, Y.; Qiu, C.; Qin, Y.; Xu, X.; Fan, L.; Wang, J.; Jin, Z. Cyclodextrin–phytochemical inclusion complexes: Promising food materials with targeted nutrition and functionality. Trends Food Sci. Technol. 2021, 109, 398–412. [Google Scholar] [CrossRef]
- Lopez-de-Dicastillo, C.; Jorda, M.; Catala, R.; Gavara, R.; Hernandez-Munoz, P. Development of active polyvinyl alcohol/beta-cyclodextrin composites to scavenge undesirable food components. J. Agric. Food Chem. 2011, 59, 11026–11033. [Google Scholar] [CrossRef]
- López-de-Dicastillo, C.; Gallur, M.; Catalá, R.; Gavara, R.; Hernandez-Muñoz, P. Immobilization of β-cyclodextrin in ethylene-vinyl alcohol copolymer for active food packaging applications. J. Membr. Sci. 2010, 353, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef] [Green Version]
- González-Louzao, R.; Lucas-Abellán, C.; Pérez-Sánchez, H.; Pedro Cerón-Carrasco, J.; Antonio Gabaldón, J.; López-Miranda, S.; Josefa Yáñez-Gascón, M.; Asín-Llorca, M.; Núñez-Delicado, E. Encapsulation of finasteride with native and modified γ-cyclodextrins. Extensive characterization of the complexes. Int. J. Pharm. 2020, 587, 119619. [Google Scholar] [CrossRef] [PubMed]
- Folch-Cano, C.; Yazdani-Pedram, M.; Olea-Azar, C. Inclusion and functionalization of polymers with cyclodextrins: Current applications and future prospects. Molecules 2014, 19, 14066–14079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charasphat, P.; Warinyupa, M.; Manchumas, P.; Ekasit, N.; Krisana, S. Enhancing stability and antioxidant efficacy of fisetin by encapsulating as β-cyclodextrin inclusion complex with porous polylactic acid film from breath figure. J. Met. Mater. Miner. 2021, 31, 81–87. [Google Scholar]
- de Castro, D.O.; Tabary, N.; Martel, B.; Gandini, A.; Belgacem, N.; Bras, J. Controlled release of carvacrol and curcumin: Bio-based food packaging by synergism action of TEMPO-oxidized cellulose nanocrystals and cyclodextrin. Cellulose 2018, 25, 1249–1263. [Google Scholar] [CrossRef]
- Lavoine, N.; Givord, C.; Tabary, N.; Desloges, I.; Martel, B.; Bras, J. Elaboration of a new antibacterial bio-nano-material for food-packaging by synergistic action of cyclodextrin and microfibrillated cellulose. Innov. Food Sci. Emerg. Technol. 2014, 26, 330–340. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, D.H.; Feng, K.; Liu, F.J.; Lou, W.Y.; Li, N.; Zong, M.H.; Wu, H. Fabrication of electrospun polylactic acid nanofilm incorporating cinnamon essential oil/beta-cyclodextrin inclusion complex for antimicrobial packaging. Food Chem. 2016, 196, 996–1004. [Google Scholar] [CrossRef]
- Prakash, B.; Kujur, A.; Yadav, A.; Kumar, A.; Singh, P.P.; Dubey, N.K. Nanoencapsulation: An efficient technology to boost the antimicrobial potential of plant essential oils in food system. Food Control 2018, 89, 1–11. [Google Scholar] [CrossRef]
- Zanetti, M.; Carniel, T.K.; Dalcanton, F.; dos Anjos, R.S.; Gracher Riella, H.; de Araújo, P.H.H.; de Oliveira, D.; Antônio Fiori, M. Use of encapsulated natural compounds as antimicrobial additives in food packaging: A brief review. Trends Food Sci. Technol. 2018, 81, 51–60. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Y.; Cui, H. Electrospun thyme essential oil/gelatin nanofibers for active packaging against Campylobacter jejuni in chicken. LWT 2018, 97, 711–718. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Carvalho-Costa, D.; Cavaleiro, C.; Costa, H.S.; Albuquerque, T.G.; Castilho, M.C.; Ramos, F.; Melo, N.R.; Sanches-Silva, A. A novel insight on an ancient aromatic plant: The rosemary (Rosmarinus officinalis L.). Trends Food Sci. Technol. 2015, 45, 355–368. [Google Scholar] [CrossRef]
- Dias Antunes, M.; da Silva Dannenberg, G.; Fiorentini, A.M.; Pinto, V.Z.; Lim, L.T.; da Rosa Zavareze, E.; Dias, A.R.G. Antimicrobial electrospun ultrafine fibers from zein containing eucalyptus essential oil/cyclodextrin inclusion complex. Int. J. Biol. Macromol. 2017, 104 Pt A, 874–882. [Google Scholar] [CrossRef]
- Higueras, L.; Lopez-Carballo, G.; Hernandez-Munoz, P.; Catala, R.; Gavara, R. Antimicrobial packaging of chicken fillets based on the release of carvacrol from chitosan/cyclodextrin films. Int. J. Food Microbiol. 2014, 188, 53–59. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Lin, L. Plasma-treated poly(ethylene oxide) nanofibers containing tea tree oil/beta-cyclodextrin inclusion complex for antibacterial packaging. Carbohydr. Polym. 2018, 179, 360–369. [Google Scholar] [CrossRef]
- Munhuweyi, K.; Caleb, O.J.; van Reenen, A.J.; Opara, U.L. Physical and antifungal properties of β-cyclodextrin microcapsules and nanofibre films containing cinnamon and oregano essential oils. LWT 2018, 87, 413–422. [Google Scholar] [CrossRef]
- Pan, J.; Ai, F.; Shao, P.; Chen, H.; Gao, H. Development of polyvinyl alcohol/beta-cyclodextrin antimicrobial nanofibers for fresh mushroom packaging. Food Chem. 2019, 300, 125249. [Google Scholar] [CrossRef]
- Favre, L.C.; Dos Santos, C.; Lopez-Fernandez, M.P.; Mazzobre, M.F.; Buera, M.D.P. Optimization of beta-cyclodextrin-based extraction of antioxidant and anti-browning activities from thyme leaves by response surface methodology. Food Chem. 2018, 265, 86–95. [Google Scholar] [CrossRef]
- da Rocha Neto, A.C.; Beaudry, R.; Maraschin, M.; Di Piero, R.M.; Almenar, E. Double-bottom antimicrobial packaging for apple shelf-life extension. Food Chem. 2019, 279, 379–388. [Google Scholar] [CrossRef]
- Zhou, C.; Abdel-Samie, M.A.; Li, C.; Cui, H.; Lin, L. Active packaging based on swim bladder gelatin/galangal root oil nanofibers: Preparation, properties and antibacterial application. Food Packag. Shelf Life 2020, 26, 100586. [Google Scholar] [CrossRef]
- Marques, C.S.; Carvalho, S.G.; Bertoli, L.D.; Villanova, J.C.O.; Pinheiro, P.F.; Dos Santos, D.C.M.; Yoshida, M.I.; de Freitas, J.C.C.; Cipriano, D.F.; Bernardes, P.C. beta-Cyclodextrin inclusion complexes with essential oils: Obtention, characterization, antimicrobial activity and potential application for food preservative sachets. Food Res. Int. 2019, 119, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Simionato, I.; Domingues, F.C.; Nerin, C.; Silva, F. Encapsulation of cinnamon oil in cyclodextrin nanosponges and their potential use for antimicrobial food packaging. Food Chem. Toxicol. 2019, 132, 110647. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Enescu, D.; Torres-Giner, S.; Cabedo, L.; Cerqueira, M.A.; Pastrana, L.; Fuciños, P.; Lagaron, J.M. Development of electrospun active films of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by the incorporation of cyclodextrin inclusion complexes containing oregano essential oil. Food Hydrocoll. 2020, 108, 106013. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Preparation and antibacterial activity of Litsea cubeba essential oil/dandelion polysaccharide nanofiber. Ind. Crops Prod. 2019, 140, 111739. [Google Scholar] [CrossRef]
- Adel, A.M.; Ibrahim, A.A.; El-Shafei, A.M.; Al-Shemy, M.T. Inclusion complex of clove oil with chitosan/β-cyclodextrin citrate/oxidized nanocellulose biocomposite for active food packaging. Food Packag. Shelf Life 2019, 20, 100307. [Google Scholar] [CrossRef]
- Lopez-Gomez, A.; Ros-Chumillas, M.; Buendia-Moreno, L.; Navarro-Segura, L.; Martinez-Hernandez, G.B. Active Cardboard Box with Smart Internal Lining Based on Encapsulated Essential Oils for Enhancing the Shelf Life of Fresh Mandarins. Foods 2020, 9, 590. [Google Scholar] [CrossRef]
- Muñoz-Shugulí, C.; Vidal, C.P.; Cantero-López, P.; Lopez-Polo, J. Encapsulation of plant extract compounds using cyclodextrin inclusion complexes, liposomes, electrospinning and their combinations for food purposes. Trends Food Sci. Technol. 2021, 108, 177–186. [Google Scholar] [CrossRef]
- Reque, P.M.; Brandelli, A. Encapsulation of probiotics and nutraceuticals: Applications in functional food industry. Trends Food Sci. Technol. 2021, 114, 1–10. [Google Scholar] [CrossRef]
- Rajkumar, T.; Kukkar, D.; Kim, K.-H.; Sohn, J.R.; Deep, A. Cyclodextrin-metal–organic framework (CD-MOF): From synthesis to applications. J. Ind. Eng. Chem. 2019, 72, 50–66. [Google Scholar] [CrossRef]
- Yao, X.; Huang, P.; Nie, Z. Cyclodextrin-based polymer materials: From controlled synthesis to applications. Prog. Polym. Sci. 2019, 93, 1–35. [Google Scholar] [CrossRef]
- Zou, Y.; Yuan, C.; Cui, B.; Sha, H.; Liu, P.; Lu, L.; Wu, Z. High-Amylose Corn Starch/Konjac Glucomannan Composite Film: Reinforced by Incorporating beta-Cyclodextrin. J. Agric. Food Chem. 2021, 69, 2493–2500. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.A.; Zeng, X.; Zhu, Q.; Lu, K.; Xu, Q.; Ye, C. Development of an active packaging with molecularly imprinted polymers for beef preservation. Packag. Technol. Sci. 2018, 31, 213–220. [Google Scholar] [CrossRef]
- Del Nobile, M.A.; Conte, A.; Buonocore, G.G.; Incoronato, A.L.; Massaro, A.; Panza, O. Active packaging by extrusion processing of recyclable and biodegradable polymers. J. Food Eng. 2009, 93, 1–6. [Google Scholar] [CrossRef]
- El Fawal, G. Polymer nanofibers electrospinning: A review. Egypt. J. Chem. 2019, 63, 1279–1303. [Google Scholar] [CrossRef]
- Narvaez-Muñoz, C.P.; Carrion-Matamoros, L.M.; Vizuete, K.; Debut, A.; Arroyo, C.R.; Guerrero, V.; Almeida-Naranjo, C.E.; Morales-Flórez, V.; Mowbray, D.J.; Zamora-Ledezma, C. Tailoring Organic–Organic Poly(vinylpyrrolidone) Microparticles and Fibers with Multiwalled Carbon Nanotubes for Reinforced Composites. ACS Appl. Nano Mater. 2019, 2, 4302–4312. [Google Scholar] [CrossRef]
- Peng, H.; Liu, Y.; Ramakrishna, S. Recent development of centrifugal electrospinning. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Lin, Y.; Yao, Y.; Yang, X.; Wei, N.; Li, X.; Gong, P.; Li, R.; Wu, D. Preparation of poly(ether sulfone) nanofibers by gas-jet/electrospinning. J. Appl. Polym. Sci. 2008, 107, 909–917. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Zhang, Z.; Wang, L.; Tan, R.; Zhang, D. Controllable generation of nanofibers through a magnetic-field-assisted electrospinning design. Mater. Lett. 2019, 247, 19–24. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, F.; Zhang, H. Emulsion electrospinning: Fundamentals, food applications and prospects. Trends Food Sci. Technol. 2018, 80, 175–186. [Google Scholar] [CrossRef]
- Dalton, P.D.; Grafahrend, D.; Klinkhammer, K.; Klee, D.; Möller, M. Electrospinning of polymer melts: Phenomenological observations. Polymer 2007, 48, 6823–6833. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Cyclodextrin-functionalized mesostructured silica nanoparticles for removal of polycyclic aromatic hydrocarbons. J. Colloid Interface Sci. 2017, 497, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Topuz, F.; Uyar, T. Antioxidant, antibacterial and antifungal electrospun nanofibers for food packaging applications. Food Res. Int. 2020, 130, 108927. [Google Scholar] [CrossRef]
- Tian, J.; Deng, H.; Huang, M.; Liu, R.; Yi, Y.; Dong, X. Electrospun Nanofibers for Food and Food Packaging Technology. In Electrospinning: Nanofabrication and Application; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 455–516. [Google Scholar]
- Aytac, Z.; Xu, J.; Raman Pillai, S.K.; Eitzer, B.D.; Xu, T.; Vaze, N.; Ng, K.W.; White, J.C.; Chan-Park, M.B.; Luo, Y.; et al. Enzyme- and Relative Humidity-Responsive Antimicrobial Fibers for Active Food Packaging. ACS Appl. Mater. Interfaces 2021, 13, 50298–50308. [Google Scholar] [CrossRef]
- Sharif, N.; Golmakani, M.-T.; Hajjari, M.M.; Aghaee, E.; Ghasemi, J.B. Antibacterial cuminaldehyde/hydroxypropyl-β-cyclodextrin inclusion complex electrospun fibers mat: Fabrication and characterization. Food Packag. Shelf Life 2021, 29, 100738. [Google Scholar] [CrossRef]
- Li, C.; Chen, W.; Siva, S.; Cui, H.; Lin, L. Electrospun phospholipid nanofibers encapsulated with cinnamaldehyde/HP-β-CD inclusion complex as a novel food packaging material. Food Packag. Shelf Life 2021, 28, 100647. [Google Scholar] [CrossRef]
- Narayanan, V.; Alam, M.; Ahmad, N.; Balakrishnan, S.B.; Ganesan, V.; Shanmugasundaram, E.; Rajagopal, B.; Thambusamy, S. Electrospun poly (vinyl alcohol) nanofibers incorporating caffeic acid/cyclodextrins through the supramolecular assembly for antibacterial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 249, 119308. [Google Scholar] [CrossRef]
- Silva, F.; Caldera, F.; Trotta, F.; Nerín, C.; Domingues, F.C. Encapsulation of coriander essential oil in cyclodextrin nanosponges: A new strategy to promote its use in controlled-release active packaging. Innov. Food Sci. Emerg. Technol. 2019, 56, 102177. [Google Scholar] [CrossRef]
- Trotta, F.; Zanetti, M.; Cavalli, R. Cyclodextrin-based nanosponges as drug carriers. Beilstein J. Org. Chem. 2012, 8, 2091–2099. [Google Scholar] [CrossRef]
- Sherje, A.P.; Dravyakar, B.R.; Kadam, D.; Jadhav, M. Cyclodextrin-based nanosponges: A critical review. Carbohydr. Polym. 2017, 173, 37–49. [Google Scholar] [CrossRef]
- Gokularaman, S.; Stalin Cruz, A.; Pragalyaashree, M.; Nishadh, A. Nanotechnology Approach in Food Packaging Review. J. Pharm. Sci. Res. 2017, 9, 1743–1749. [Google Scholar]
- Kolahalam, L.A.; Kasi Viswanath, I.V.; Diwakar, B.S.; Govindh, B.; Reddy, V.; Murthy, Y.L.N. Review on nanomaterials: Synthesis and applications. Mater. Today Proc. 2019, 18, 2182–2190. [Google Scholar] [CrossRef]
- Laux, P.; Tentschert, J.; Riebeling, C.; Braeuning, A.; Creutzenberg, O.; Epp, A.; Fessard, V.; Haas, K.H.; Haase, A.; Hund-Rinke, K.; et al. Nanomaterials: Certain aspects of application, risk assessment and risk communication. Arch. Toxicol. 2018, 92, 121–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.; Mahajan, P.; Kaur, R.; Gautam, S. Nanotechnology and its challenges in the food sector: A review. Mater. Today Chem. 2020, 17, 100332. [Google Scholar] [CrossRef]
- Mlalila, N.; Kadam, D.M.; Swai, H.; Hilonga, A. Transformation of food packaging from passive to innovative via nanotechnology: Concepts and critiques. J. Food Sci. Technol. 2016, 53, 3395–3407. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, G.; Shen, J.; Matai, I.; Sachdev, A.; Boy, R.; Tonelli, A.E. Cyclodextrin-based nanostructures. Prog. Mater. Sci. 2021, 100869. [Google Scholar] [CrossRef]
- Lin, L.; Xue, L.; Duraiarasan, S.; Haiying, C. Preparation of ε-polylysine/chitosan nanofibers for food packaging against Salmonella on chicken. Food Packag. Shelf Life 2018, 17, 134–141. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver nanoparticles as potential antiviral agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [Green Version]
- Hegab, H.M.; ElMekawy, A.; Zou, L.; Mulcahy, D.; Saint, C.P.; Ginic-Markovic, M. The controversial antibacterial activity of graphene-based materials. Carbon 2016, 105, 362–376. [Google Scholar] [CrossRef]
- Magne, T.M.; de Oliveira Vieira, T.; Costa, B.; Alencar, L.M.R.; Ricci-Junior, E.; Hu, R.; Qu, J.; Zamora-Ledezma, C.; Alexis, F.; Santos-Oliveira, R. Factors affecting the biological response of Graphene. Colloids Surf. B Biointerfaces 2021, 203, 111767. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Chicaiza-Zambrano, A.; Santiago Vispo, N.; Debut, A.; Vizuete, K.; Guerrero, V.H.; Almeida, C.E.; Alexis, F. Frequency Based Control of Antifouling Properties Using Graphene Nanoplatelet/Poly(Lactic-co-Glycolic Acid) Composite Films. Compos. Interfaces 2021, 28, 1137–1153. [Google Scholar] [CrossRef]
- Boutillier, S.; Fourmentin, S.; Laperche, B. Food additives and the future of health: An analysis of the ongoing controversy on titanium dioxide. Futures 2020, 122, 102598. [Google Scholar] [CrossRef]
- Ju, J.; Chen, X.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of essential oil as a sustained release preparation in food packaging. Trends Food Sci. Technol. 2019, 92, 22–32. [Google Scholar] [CrossRef]
- Kathuria, A.; Pauwels, A.-K.; Buntinx, M.; Shin, J.; Harding, T. Inclusion of ethanol in a nano-porous, bio-based metal organic framework. J. Incl. Phenom. Macrocycl. Chem. 2019, 95, 91–98. [Google Scholar] [CrossRef]
- Adeli, M.; Ghobadi, M.; Ghanbarzadeh, B.; Alizadeh, A.; Ghasemi, P. Effect of novel bioactive coating enriched with nanoemulsion of mustard essential oil on the quality of turkey meat. J. Food Nutr. Res. 2020, 59, 71–80. [Google Scholar]
- Aliabbasi, N.; Emam-Djomeh, Z.; Amighi, F. Active food packaging with nano/microencapsulated ingredients. In Application of Nano/Microencapsulated Ingredients in Food Products; Academic Press: Cambridge, MA, USA, 2021; pp. 171–210. [Google Scholar]
- Wu, M.-H.; Zhu, L.; Zhou, Z.-Z.; Zhang, Y.-Q. Coimmobilization of Naringinases on Silk Fibroin Nanoparticles and Its Application in Food Packaging. J. Nanopart. 2013, 2013, 901401. [Google Scholar] [CrossRef]
- Lozano-Pérez, A.A.; Montalbán, M.G.; Aznar-Cervantes, S.D.; Cragnolini, F.; Cenis, J.L.; Víllora, G. Production of silk fibroin nanoparticles using ionic liquids and high-power ultrasounds. J. Appl. Polym. Sci. 2014, 132. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Fourmentin, S.; Fenyvesi, É.; Lichtfouse, E.; Torri, G.; Fourmentin, M.; Crini, G. 130 years of cyclodextrin discovery for health, food, agriculture, and the industry: A review. Environ. Chem. Lett. 2021, 19, 2581–2617. [Google Scholar] [CrossRef]
- Jing, D.; Wen, L. Active Packaging Film Based On Essential Oil/Beta-cyclodextrin Inclusion Compound and Preparation Method for Active Packaging Film. Patent No. CN102585412A, 18 July 2012. [Google Scholar]
- Ge, L.; Li, D.; Mu, C.; Ye, Y. Functional Gelatin Food Packaging Film and Preparation Method. Patent No. CN104693811A, 10 June 2015. [Google Scholar]
- Keute, J.; Kuduk, W.; Wood, W. Cyclodextrin Compositions, Articles, and Methods. Patent No. AU2016206334A1, 4 August 2016. [Google Scholar]
- Xu, J. Antibacterial Quality-Guarantee Food Packaging Bag and Preparation Method Thereof. Patent No. CN105623067A, 1 June 2016. [Google Scholar]
- Zapata Ramirez, P.A.; Yañez Sanchez, M.E. Degradable Packaging Film for Fruit and Vegetables. U.S. Patent Application No WO 2017/106984 Al, 22 December 2017. [Google Scholar]
- Li, J. Clove Essential Oil Contained Sterilization Plastic Wrap and Preparation Method Thereof. Patent No. CN106967226A, 21 July 2017. [Google Scholar]
- Li, W.; Yang, Y. Method for Preparing Antibacterial Food Packaging Preservation Film by Doping Garlic Oil-Beta Cyclodextrin Inclusion Compound-Clove Oil. Patent No. CN109233161A, 18 January 2019. [Google Scholar]
- Xie, Q. Environment-Friendly Food Packaging Plastic and Preparation Method Thereof. Patent No. CN112048102A, 8 December 2020. [Google Scholar]
- Bian, X.; Liang, N.; Shen, J.; Sun, Y.; Wang, X. Application of Hydroxypropyl-beta-cyclodextrin in Preparation of Antibacterial Material, Food Package and Preparation Method of Food Package. Patent No. CN111820219A, 27 October 2020. [Google Scholar]
- Cui, B.; Fang, Y.; Guo, L.; Yu, B.; Yuan, C.; Zou, Y. Food Packaging Films Containing Natural Antibacterial Component. U.S. Patent US20200337357A1, 29 October 2020. [Google Scholar]
- Romano, I. Packaging Material. U.S. Patent US11110694, 7 September 2021. [Google Scholar]






| Properties | α-CDs | β-CDs | γ-CDs |
|---|---|---|---|
| Number of glucose units | 6 | 7 | 8 |
| Molecular weight (g/mol) | 972 | 1135 | 1297 |
| Melting point (°C) | 275 | 280 | 275 |
| Solubility in water at 25 °C (%, w/v) | 14.5 | 1.9 | 23.2 |
| Enthalpy; ΔH (kJ/mol) | 32.1 | 34.7 | 32.3 |
| Entropy; ΔS (J/°K mol) | 57.7 | 48.9 | 61.4 |
| Cavity diameter (Å) | 4.7–5.3 | 6.0–6.5 | 7.5–8.3 |
| External diameter (Å) | 14.6 | 15.4 | 17.5 |
| Approximate volume of cavity (Å3) | 174.0 | 262.0 | 427.0 |
| Crystal forms (from water) | Hexagonal plates | Monoclinic parallelograms | Quadratic prisms |
| European trade name as food additives | E-457 | E-459 | E-458 |
| Solubility in: Tetrahydrofuran, methyl isobutyl ketone, methyl isopropyl ketone, acetone, alcohols | 0.0 | 0.0 | 0.0 |
| Solubility in propylene glycol | 0.5 | 0.5 | 0.5 |
| Solubility in pyridine | 3.5 | 3.5 | 3.5 |
| Solubility in ethyleneglycol | 7.0 | 7.0 | 7.0 |
| Solubility in N-methylpyrrolidone | 14.8 | 14.8 | 14.8 |
| Solubility in dimethylformamide | 28.3 | 28.3 | 28.3 |
| Solubility in dimethylsulfoxide | >41 | >41 | >41 |
| Origin | Types |
|---|---|
| Natural and biodegradable | Polysaccharides (starch, cellulose, chitin); proteins (gelatin, casein, silk); polyhydroxy alkanoates (PHA), polylactic acid (PLA) |
| Natural and nonbiodegradable | Polyamides; polyesteramides; unsaturated polyesters;epoxy and phenolic resins |
| Synthetic and biodegradable | Aliphatic polyesters (polyglycolic acid (PGA), polycaprolactone (PCL), polybutylene succinate (PBS)); polyvinyl alcohol (PVA); polyalkylene dicarboxylates (polyethylene succinate(PES), polybutynel adipate (PBA)); polyanhydrides |
| Polymer | Oxygen Permeation (cc.mil/m2-day-atm) | Water Vapor Permeation (g.mil/m2-day-kPa) | |
|---|---|---|---|
| Biodegradable | PHA | 8 (23 °C/85%) | 106 (23 °C/50%) |
| 85 (23 °C/0%) | 30 (25 °C/100%) | ||
| 230 (25 °C/80%) | 26 (37.8 °C/100%) | ||
| PLA | 132–590 (23 °C/50% or 0%) | 63~342 (23 °C/85%) | |
| PPC | 230 | 162 (23 °C/90%) | |
| PLA/Chitosan | 72 (25 °C/0%) | 319 (37.8 °C/95%) | |
| PBS | 208 (23 °C/50%) | 175 (25 °C) | |
| 340 (20 °C/90%) | - | ||
| PCL | 1990 (25 °C/0%) | 137 (23 °C/48%) | |
| PBAT | 2440 (23 °C/50%) | 173 (23 °C/75%) | |
| PGA | 1 (30 °C/80%) | 10 (40 °C/90%) | |
| Non Biodegradable | HDPE | 2325 (23 °C/0%) | 6 (40 °C/90%) |
| PP | 2500–3000 (23 °C/0%) | 5–10 (40 °C/90%) | |
| PET | 40 (23 °C/0%) | 15–20 (37.8 °C/90%) | |
| PVDC | ~1 (23 °C/75%) | 2 (38 °C/90%) | |
| PEF | ~18 (25 °C/50%) | ~30 (25 °C/90%) | |
| Bio-PE | 2140 (23 °C/0%) | ~3 (38 °C/90%) | |
| Nylon 6 | 40 (23 °C/0%) | 295–310 (37.8 °C/90%) | |
| Polystyrene | 4030 (23 °C/0%) | 132 (40 °C/90%) | |
| EVOH | 0.5 (23 °C/0%) | 33 (40 °C/90%) |
| Inclusion Complex | Material | Application | Reference |
|---|---|---|---|
| Mustard essential oil/β-cyclodextrin | Cellulose, sulfate film | Antimicrobial edible films, against E. coli and S. aureus. | [13] |
| Thymol/γ-cyclodextrin | Zein, nanofibrous web | Antimicrobial food packaging, inhibiting the growth of E. coli and S. aureus in meat. | [12] |
| Eucalyptus/β-cyclodextrin | Zein, ultrafine fibers | Antimicrobial, against S. aureus and L. monocytogenes. | [95] |
| Carvacrol/HP-β-cyclodextrin | Chitosan, Film | Antimicrobial packaging for chicken filet. | [96] |
| Thyme/β-CD ε-polylysine | Gelatine, nanofiber film | Antimicrobial packaging, reduction of the activity against C. jejuni in coated chicken. | [93] |
| Tea tree oil/β-cyclodextrin | Poly(ethylene oxide), nanofiber film | Antimicrobial packaging, antibacterial activity against E. coli O157:H7, tested on beef. | [97] |
| Cinnamon–oregano EO/β-cyclodextrin | Chitosan/ poly(vinyl alcohol), nanofiber film | Antifungal activity against Botrytis sp. | [98] |
| Cinnamon EO/β-cyclodextrin | Poly(vinyl alcohol), nanofiber film | Antimicrobial packaging, antibacterial activity against E. coli and S. aureus in mushrooms. | [99] |
| D-Limonene/β-cyclodextrin | Poly(butylene succinate), composite film | Antimicrobial food packaging, antibacterial properties against different bacteria straws. | [9] |
| Thyme/β-cyclodextrin | Inclusion complex extract | Natural antioxidant and antibrowning activities. | [100] |
| Curcumin, carvacrol/β-cyclodextrin | Cellulose nanocrystals, film | Antimicrobial food packaging, microbial activity against B. subtilis. | [87] |
| Palmarosa EO/β-cyclodextrin | Polyethylene terephthalate (PET) | Antifungal packaging, extends apple shelf life by slowing P. expansum growth. | [101] |
| Galangal root oil/β-cyclodextrin | Gelatin, nanofibers | Inhibitory effect against E. coli O157:H7 in beef. | [102] |
| Basil and pimenta dioica/β-cyclodextrin | Sachets | Potential to be used as food preservative against S. aureus, E. coli, L. monocytogenes, and P. aeruginosa. | [103] |
| Cinnamon EO/CD-nanosponges | α-nanosponges and β-nanosponges | Antimicrobial activity against foodborne bacteria. | [104] |
| Oregano EO/(α-CD and γ-CD) | PHBV, film | Higher antimicrobial activity against S. aureus and E. coli. | [105] |
| Litsea cubeba EO/β-cyclodextrin | Dandelion polysaccharide, nanofiber | Sustained release and long-lasting antibacterial effect against S. aureus. | [106] |
| Clove EO/β-cyclodextrin | Chitosan/ β-cyclodextrin citrate/ oxidized nanocellulose | Higher activity over Gram-negative bacteria (E. coli and P. aeruginosa). | [107] |
| Carvacrol, thymol/β-cyclodextrin | Poly(lactic acid) (PLA) | Microbial inhibition of mesophiles, yeast, molds, and coliforms. Extended the shelf life of raspberries and blackberries. | [53] |
| Carvacrol, oregano, and cinnamon EOs/β-cyclodextrin | Cardboard box | Reduction in microbial growth of mesophiles, psychrophiles, enterobacteria, yeast, and molds. Extended the shelf life of mandarins. | [108] |
| Patent Title | Application | Description | Reference |
|---|---|---|---|
| Active packaging film based on essential oil/β-cyclodextrin inclusion compound and preparation method for active packaging film | Active packaging film | Beta-cyclodextrin; essential oil with broad-spectrum antibacterial performance; by weight, essential oil/ benexate hydrochloride is 4–24% of the total amount. | [152] |
| Functional gelatin food packaging film and preparation method | Gelatin food packaging film | Using gelatin as the carboxylated beta-cyclodextrin of main raw material compound and natural active matter. | [153] |
| Cyclodextrin compositions, articles, and methods | A selectively permeable packaging material | Cyclodextrin inclusion complex and a polymer, the composition obtained with electromagnetic irradiation of a cyclodextrin composition comprising one or more radiation-polymerizable monomers and a cyclodextrin complex, the cyclodextrin complex comprising a cyclodextrin compound, and an olefinic inhibitor comprising a cyclopropane. | [154] |
| Antibacterial quality-guarantee food packaging bag and preparation method thereof | An antibacterial food packaging | Low-density polyethylene, zinc stearate, monoglyceride, polylactic resin, propylene glycol, dioctyl phthalate, ethoxylated alkylamine, porous hydroxyapatite, medical stone, beta-cyclodextrin, chitosan, lanthanum-loaded zinc oxide, 3–6 parts lanthanum-loaded titanium dioxide, and 0.5–1 part natamycin. | [155] |
| Degradable packaging film for fruit and vegetables | Packaging film | Based on a polyolefin selected from polyethylene (PE), polypropylene (PE), polystyrene (PS), and ethyl vinyl acetate (EVA) and essential oil antimicrobial agents or said essential agent, microencapsulated in an encapsulating agent selected from the group consisting of cyclodextrin (β- or γ-). | [156] |
| Clove essential oil contained sterilization plastic wrap and preparation method thereof | Clove essential oil contained sterilization plastic wrap | Clove essential oil, beta-cyclodextrin | [157] |
| Method for preparing antibacterial food packaging preservation film by doping garlic-oil-beta-cyclodextrin inclusion compound with clove oil | An antibacterial food packaging preservation film | Garlic-oil-beta-cyclodextrin inclusion compound with clove oil | [158] |
| Environmentally friendly food packaging plastic and preparation method thereof | Environmentally friendly food packaging plastic | 25–35 parts epoxy modified hyperbranched poly (beta-cyclodextrin) containing azide and vinyl groups, 8–12 parts vinyl polylactic acid, 4–6 parts (Z)-2-(2-aminothiazole 4yl) 2-pentenoic acid, 1–3 parts coupling agent, 0.5–0.9 part fullerene nano/microfibers, and 0.4–0.6 part initiator. | [159] |
| Application of hydroxypropyl-beta-cyclodextrin in preparation of antibacterial material, food packaging, and preparation method of food packaging | The food packaging includes, but is not limited to, packaging boxes and bags for packaging edible substances, and packaging bottles for packaging edible substances | Hydroxypropyl-β-cyclodextrin is prepared by enzymatically hydrolyzing starch with Bacillus, and the Bacillus expresses cyclodextrin glucosyltransferase. | [160] |
| Food packaging films containing natural antibacterial component | Edible films and, more particularly, a method for preparing a food packaging film with antibacterial activity | Perilla oil and cyclodextrin in the mixed dispersion of high-amylose corn starch and konjac glucomannan to prepare an active film. | [161] |
| Packaging material | Film or sheet for use in “active” packaging systems, capable of inhibiting the growth of microorganisms on the surface of the food product packaged therein | Encapsulated ethanol and a polymeric component selected from chitosan grafted with polyethylene glycol or cyclodextrin, a mixture of chitosan and polyethylene glycol, and a polymer or mixture of polymers for printable paint applied to the other side of the base layer. | [162] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velázquez-Contreras, F.; Zamora-Ledezma, C.; López-González, I.; Meseguer-Olmo, L.; Núñez-Delicado, E.; Gabaldón, J.A. Cyclodextrins in Polymer-Based Active Food Packaging: A Fresh Look at Nontoxic, Biodegradable, and Sustainable Technology Trends. Polymers 2022, 14, 104. https://doi.org/10.3390/polym14010104
Velázquez-Contreras F, Zamora-Ledezma C, López-González I, Meseguer-Olmo L, Núñez-Delicado E, Gabaldón JA. Cyclodextrins in Polymer-Based Active Food Packaging: A Fresh Look at Nontoxic, Biodegradable, and Sustainable Technology Trends. Polymers. 2022; 14(1):104. https://doi.org/10.3390/polym14010104
Chicago/Turabian StyleVelázquez-Contreras, Friné, Camilo Zamora-Ledezma, Iván López-González, Luis Meseguer-Olmo, Estrella Núñez-Delicado, and José Antonio Gabaldón. 2022. "Cyclodextrins in Polymer-Based Active Food Packaging: A Fresh Look at Nontoxic, Biodegradable, and Sustainable Technology Trends" Polymers 14, no. 1: 104. https://doi.org/10.3390/polym14010104
APA StyleVelázquez-Contreras, F., Zamora-Ledezma, C., López-González, I., Meseguer-Olmo, L., Núñez-Delicado, E., & Gabaldón, J. A. (2022). Cyclodextrins in Polymer-Based Active Food Packaging: A Fresh Look at Nontoxic, Biodegradable, and Sustainable Technology Trends. Polymers, 14(1), 104. https://doi.org/10.3390/polym14010104