Chitosan Membrane Containing Copaiba Oil (Copaifera spp.) for Skin Wound Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
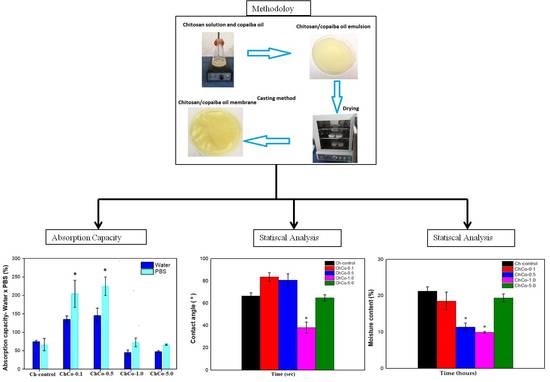
2.2. Membrane Production by Casting Method
2.3. Characterization of Membranes
2.3.1. Macroscopic Analysis
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Absorption Capacity/Degree of Intumescence
2.3.4. Moisture Determination
2.3.5. Contact Angle Measurement
2.3.6. X-ray Diffractometry (XRD) Analysis
2.3.7. Statistical Analysis
3. Results and Discussion
3.1. Macroscopic Analysis of Membranes
3.2. SEM
3.3. Absorption Capacity/Degree of Intumescences
3.4. Contact Angle and Moisture Content Determination
3.5. X-ray Diffractometry (XRD) Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Hack, M.E.A.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; El-Hakim, Y.M.A.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of Appropriate Wound Dressing for Various Wounds. Front. Bioeng. Biotechnol. 2020, 8, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. Biomedicine 2015, 5, 1–5. [Google Scholar] [CrossRef]
- Gobi, R.; Ravichandiran, P.; Babu, R.; Yoo, D. Biopolymer and Synthetic Polymer-Based Nanocomposites in Wound Dressing Applications: A Review. Polymers 2021, 13, 1962. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Chen, X.; Shen, Z.; Chen, H. Marine Polysaccharides for Wound Dressings Application: An Overview. Pharmaceutics 2021, 13, 1666. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, G.S. Chitosan-Based Copaiba Oil Carrier Polymeric Systems: Obtaining, Characterization and Antifungal Activity In Vitro; Monography, Federal University of Rio Grande do Norte: Rio Grande do Norte, Brazil, 2020; pp. 1–24. [Google Scholar]
- Yoshida, C.M.P.; Pacheco, M.S.; de Moraes, M.A.; Lopes, P.S.; Severino, P.; Souto, E.B.; da Silva, C.F. Effect of Chitosan and Aloe Vera Extract Concentrations on the Physicochemical Properties of Chitosan Biofilms. Polymers 2021, 13, 1187. [Google Scholar] [CrossRef] [PubMed]
- Brás, T.; Rosa, D.; Gonçalves, A.C.; Gomes, A.C.; Alves, V.D.; Crespo, J.G.; Duarte, M.F.; Neves, L.A. Development of bioactive films based on chitosan and Cynara cardunculus leaves extracts for wound dressings. Int. J. Biol. Macromol. 2020, 163, 1707–1718. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Hamedi, H.; Tonelli, A.E.; King, M.W. Chitosan/Graphene Oxide Composite Films and Their Biomedical and Drug Delivery Applications: A Review. Appl. Sci. 2021, 11, 7776. [Google Scholar] [CrossRef]
- Soriente, A.; Fasolino, I.; Gomez-Sánchez, A.; Prokhorov, E.; Buonocore, G.G.; Luna-Barcenas, G.; Ambrosio, L.; Raucci, M.G. Chitosan/hydroxyapatite nanocomposite scaffolds to modulate osteogenic and inflammatory response. J. Biomed. Mater. Res. Part A 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Tamer, T.M.; Valachová, K.; Omer, A.M.; El-Shafeey, M.; Eldin, M.S.M.; Šoltés, L. Antioxidant and antibacterial polyelectrolyte wound dressing based on chitosan/hyaluronan/phosphatidylcholine dihydroquercetin. Int. J. Biol. Macromol. 2021, 166, 18–31. [Google Scholar] [CrossRef]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 1–15. [Google Scholar] [CrossRef]
- Vivcharenko, V.; Benko, A.; Palka, K.; Wojcik, M.; Przekora, A. Elastic and biodegradable chitosan/agarose film revealing slightly acidic pH for potential applications in regenerative medicine as artificial skin graft. Int. J. Biol. Macromol. 2020, 164, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Ładniak, A.; Jurak, M.; Wiącek, A.E. Wettability of DPPC Monolayers Deposited from the Titanium Dioxide–Chitosan–Hyaluronic Acid Subphases on Glass. Colloids Interfaces 2019, 3, 15. [Google Scholar] [CrossRef] [Green Version]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef]
- Ribeiro, D.M.L.; Júnior, A.R.C.; de Macedo, G.H.R.V.; Chagas, V.L.; Silva, L.D.S.; Cutrim, B.D.S.; Santos, D.M.; Soares, B.L.L.; Zagmignan, A.; Miranda, R.D.C.M.D.; et al. Polysaccharide-Based Formulations for Healing of Skin-Related Wound Infections: Lessons from Animal Models and Clinical Trials. Biomolecules 2019, 10, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamer, T.M.; Hassan, M.A.; Valachová, K.; Omer, A.M.; El-Shafeey, M.E.; Eldin, M.S.M.; Šoltés, L. Enhancement of wound healing by chitosan/hyaluronan polyelectrolyte membrane loaded with glutathione: In vitro and in vivo evaluations. J. Biotechnol. 2020, 310, 103–113. [Google Scholar] [CrossRef]
- Kenawy, E.; Omer, A.M.; Tamer, T.M.; Elmeligy, M.A.; Eldin, M.S.M. Fabrication of biodegradable gelatin/chitosan/cinnamaldehyde crosslinked membranes for antibacterial wound dressing applications. Int. J. Biol. Macromol. 2019, 139, 440–448. [Google Scholar] [CrossRef]
- Xie, H.; Chen, X.; Shen, X.; He, Y.; Chen, W.; Luo, Q.; Ge, W.; Yuan, W.; Tang, X.; Hou, D.; et al. Preparation of chitosan-collagen-alginate composite dressing and its promoting effects on wound healing. Int. J. Biol. Macromol. 2018, 107, 93–104. [Google Scholar] [CrossRef]
- Susanto, A.; Susanah, S.; Priosoeryanto, B.P.; Satari, M.H.; Komara, I. The effect of the chitosan-collagen membrane on wound healing process in rat mandibular defect. J. Indian Soc. Periodontol. 2019, 23, 113–118. [Google Scholar] [CrossRef]
- Winkelman, W.J. Aromatherapy, botanicals, and essential oils in acne. Clin. Dermatol. 2018, 36, 299–305. [Google Scholar] [CrossRef]
- Tobouti, P.L.; Martins, T.C.D.A.; Pereira, T.J.; Mussi, M.C.M. Antimicrobial activity of copaiba oil: A review and a call for further research. Biomed. Pharmacother. 2017, 94, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.B.; Silva, R.D.B.; Lameira, O.A.; Webber, L.P.; Couto, R.S.D.; Martins, M.D.; Lima, R.R. Copaiba oil-resin (Copaifera reticulata Ducke) modulates the inflammation in a model of injury to rats’ tongues. BMC Complement. Altern. Med. 2017, 17, 313. [Google Scholar] [CrossRef] [Green Version]
- Alvarenga, M.O.P.; Bittencourt, L.O.; Mendes, P.F.S.; Ribeiro, J.T.; Lameira, O.A.; Monteiro, M.C.; Barboza, C.A.G.; Martins, M.D.; Lima, R.R. Safety and Effectiveness of Copaiba Oleoresin (C. reticulata Ducke) on Inflammation and Tissue Repair of Oral Wounds in Rats. Int. J. Mol. Sci. 2020, 21, 3568. [Google Scholar] [CrossRef]
- Sibin, A.P.A.; Barizão, C.L.; Castro-Ghizoni, C.V.; Silva, F.M.S.; Sá-Nakanishi, A.B.; Bracht, L.; Bersani-Amado, C.A.; Marçal-Natali, M.R.; Bracht, A.; Comar, J.F. β-Caryophyllene, the major constituent of copaiba oil, reduces systemic inflammation and oxidative stress in arthritic rats. J. Cell. Biochem. 2018, 119, 10262–10277. [Google Scholar] [CrossRef]
- Pascoal, D.R.C.; Velozo, E.S.; Braga, M.E.M.; de Sousa, H.C.; Cabral-Albuquerque, E.C.M.; De Melo, S.A.B.V. Bioactive compounds of Copaifera sp. impregnated into three-dimensional gelatin dressings. Drug Deliv. Transl. Res. 2020, 10, 1537–1551. [Google Scholar] [CrossRef]
- Ghizoni, C.V.C.; Ames, A.P.A.; Lameira, O.A.; Amado, C.A.B.; Nakanishi, A.B.S.; Bracht, L.; Natali, M.R.M.; Peralta, R.M.; Bracht, A.; Comar, J.F. Anti-Inflammatory and Antioxidant Actions of Copaiba Oil Are Related to Liver Cell Modifications in Arthritic Rats. J. Cell. Biochem. 2017, 118, 3409–3423. [Google Scholar] [CrossRef] [PubMed]
- Becker, G.; Brusco, I.; Casoti, R.; Marchiori, M.C.L.; Cruz, L.; Trevisan, G.; Oliveira, S.M. Copaiba oleoresin has topical antinociceptive activity in a UVB radiation-induced skin-burn model in mice. J. Ethnopharmacol. 2020, 250, 112476. [Google Scholar] [CrossRef] [PubMed]
- Dias, F.G.G.; Pereira, L.F.; Furtado, R.A.; Magalhães, G.M.; Miguel, M.P.; Dias, L.G.G.G.; Jorge, A.T.; Honsho, C.S.; Ambrósio, S.R.; Bastos, J.K.; et al. Topical formulations containing Copaifera duckei Dwyer oleoresin improve cutaneous wound healing. Avicenna J. Phytomed. 2021, 11, 120–133. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8051314/pdf/AJP-11-120.pdf (accessed on 29 October 2021).
- Marangon, C.; Martins, V.; Leite, P.M.; Santos, D.; Nitschke, M.; Plepis, A. Chitosan/gelatin/copaiba oil emulsion formulation and its potential on controlling the growth of pathogenic bacteria. Ind. Crop. Prod. 2017, 99, 163–171. [Google Scholar] [CrossRef]
- Norcino, L.; Mendes, J.; Natarelli, C.; Manrich, A.; Oliveira, J.; Mattoso, L. Pectin films loaded with copaiba oil nanoemulsions for potential use as bio-based active packaging. Food Hydrocoll. 2020, 106, 105862. [Google Scholar] [CrossRef]
- Pascoal, D.; Cabral-Albuquerque, E.; Velozo, E.; de Sousa, H.; de Melo, S.V.; Braga, M. Copaiba oil-loaded commercial wound dressings using supercritical CO 2: A potential alternative topical antileishmanial treatment. J. Supercrit. Fluids 2017, 129, 106–115. [Google Scholar] [CrossRef]
- Lucca, L.G.; De Matos, S.P.; Kreutz, T.; Teixeira, H.F.; Veiga, V.F.; De Araújo, B.V.; Limberger, R.P.; Koester, L.S. Anti-inflammatory Effect from a Hydrogel Containing Nanoemulsified Copaiba oil (Copaifera multijuga Hayne). AAPS PharmSciTech 2017, 19, 522–530. [Google Scholar] [CrossRef]
- Xavier-Jr, F.; Gueutin, C.; Chacun, H.; Vauthier, C.; Egito, E. Mucoadhesive paclitaxel-loaded chitosan-poly (isobutyl cyanoacrylate) core-shell nanocapsules containing copaiba oil designed for oral drug delivery. J. Drug Deliv. Sci. Technol. 2019, 53, 1–9. [Google Scholar] [CrossRef]
- Cunha, C.S.; Castro, P.J.; Sousa, S.; Pullar, R.; Tobaldi, D.M.; Piccirillo, C.; Pintado, M.M. Films of chitosan and natural modified hydroxyapatite as effective UV-protecting, biocompatible and antibacterial wound dressings. Int. J. Biol. Macromol. 2020, 159, 1177–1185. [Google Scholar] [CrossRef]
- Escárcega-Galaz, A.A.; Sánchez-Machado, D.I.; López-Cervantes, J.; Sanches-Silva, A.; Madera-Santana, T.J.; Paseiro-Losada, P. Mechanical, structural and physical aspects of chitosan-based films as antimicrobial dressings. Int. J. Biol. Macromol. 2018, 116, 472–481. [Google Scholar] [CrossRef]
- Yassue-Cordeiro, P.H.; Zandonai, C.H.; Genesi, P.B.; Lopes, P.S.; Sanchez-Lopez, E.; Garcia, M.L.; Fernandes-Machado, N.R.C.; Severino, P.; Souto, E.B.; Da Silva, C.F. Development of Chitosan/Silver Sulfadiazine/Zeolite Composite Films for Wound Dressing. Pharmaceutics 2019, 11, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Eulálio, H.Y.C.; Vieira, M.; Fideles, T.B.; Tomás, H.; Silva, S.M.L.; Peniche, C.A.; Fook, M.V.L. Physicochemical Properties and Cell Viability of Shrimp Chitosan Films as Affected by Film Casting Solvents. I-Potential Use as Wound Dressing. Materials 2020, 13, 5005. [Google Scholar] [CrossRef]
- Akhavan-Kharazian, N.; Izadi-Vasafi, H. Preparation and characterization of chitosan/gelatin/nanocrystalline cellulose/calcium peroxide films for potential wound dressing applications. Int. J. Biol. Macromol. 2019, 133, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Debone, H.S.; Lopes, P.S.; Severino, P.; Yoshida, C.M.P.; Souto, E.B.; da Silva, C.F. Chitosan/Copaiba oleoresin films for would dressing application. Int. J. Pharm. 2019, 555, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Gozdecka, A.; Wiącek, A.E. Effect of UV radiation and chitosan coating on the adsorption-photocatalytic activity of TiO2 particles. Mater. Sci. Eng. C 2018, 93, 582–594. [Google Scholar] [CrossRef]
- Jurak, M.; Mroczka, R.; Łopucki, R. Properties of Artificial Phospholipid Membranes Containing Lauryl Gallate or Cholesterol. J. Membr. Biol. 2018, 251, 277–294. [Google Scholar] [CrossRef] [Green Version]
- Jurak, M.; Szafran, K.; Cea, P.; Martín, S. Analysis of Molecular Interactions between Components in Phospholipid-Immunosuppressant-Antioxidant Mixed Langmuir Films. Langmuir 2021, 37, 5601–5616. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Lima, K.; Bastos, G.; Oliveira, J.; Nascimento, L.D.; Costa, C.; Filho, G.; Concha, V.; Passos, M. PCL/Andiroba Oil (Carapa guianensis Aubl.) Hybrid Film for Wound Healing Applications. Polymers 2021, 13, 1591. [Google Scholar] [CrossRef]
- De Moraes, A.R.D.P.; Tavares, G.; Rocha, F.J.S.; de Paula, E.; Giorgio, S. Effects of nanoemulsions prepared with essential oils of copaiba- and andiroba against Leishmania infantum and Leishmania amazonensis infections. Exp. Parasitol. 2018, 187, 12–21. [Google Scholar] [CrossRef]
- Güneş, S.; Tıhmınlıoğlu, F. Hypericum perforatum incorporated chitosan films as potential bioactive wound dressing material. Int. J. Biol. Macromol. 2017, 102, 933–943. [Google Scholar] [CrossRef]
- Menzies, K.L.; Jones, L. The Impact of Contact Angle on the Biocompatibility of Biomaterials. Optom. Vis. Sci. 2010, 87, 387–399. [Google Scholar] [CrossRef]
- Pinheiro, J.G.D.O.; Tavares, E.D.A.; Da Silva, S.S.; Silva, J.F.; De Carvalho, Y.M.B.G.; Ferreira, M.R.A.; Araújo, A.A.D.S.; Barbosa, E.G.; Pedrosa, M.D.F.F.; Soares, L.A.L.; et al. Inclusion Complexes of Copaiba (Copaifera multijuga Hayne) Oleoresin and Cyclodextrins: Physicochemical Characterization and Anti-Inflammatory Activity. Int. J. Mol. Sci. 2017, 18, 2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tovar, C.D.G.; Castro, J.I.; Llano, C.H.V.; Porras, D.P.N.; Ospina, J.D.; Zapata, M.E.V.; Hernandez, J.H.M.; Chaur, M.N. Synthesis, Characterization, and Histological Evaluation of Chitosan-Ruta Graveolens Essential Oil Films. Molecules 2020, 25, 1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Characteristics | Units | Presentation |
|---|---|---|
| Appearance (25 °C) | -------- | Liquid, with low viscosity |
| Color | -------- | Yellowish, greenish to brown |
| Aroma | -------- | Typical wooden |
| Density | g/mL | 0.912 |
| Refraction index (20 °C) | -------- | 1.5 |
| Solubility in water | -------- | Insoluble |
| Description | Color | Homogeneity | Continuity | Handling | |
|---|---|---|---|---|---|
| Ch-control |  | Slightly yellowish | Loud | Loud | Loud |
| ChCo-0.1 |  | Slightly yellowish | Intermediate | Loud | Loud |
| ChCo-0.5 |  | Slightly yellowish | Intermediate | Loud | Loud |
| ChCo-1.0 |  | More intense yellow+ | Intermediate | Loud | Loud |
| ChCo-5.0 |  | More intense yellow++ | Low | Low | Low |
| Description | >Diameter (μm) | <Diameter (μm) |
|---|---|---|
| Ch-control | - | - |
| ChCo-0.1 | - | - |
| ChCo-0.5 | 65.20 | 33.75 |
| ChCo-1.0 | 123.78 | 30.39 |
| ChCo-5.0 | 10.38 | 2.25 |
| Origin of Variation | Sum of Squares | Degrees of Freedom (DF) | Middle Square | Value of F | p-Value |
|---|---|---|---|---|---|
| Between groups | 493,634 | 9 | 54,848.20 | 30.2 | <0.05 |
| Within two groups | 364,143 | 200 | 1820.71 | - | - |
| Total | 857,777 | 209 | 0.00001 |
| Membranes | Contact Angle (°) | Moisture Content (%) | |
|---|---|---|---|
| Control chitosan membrane | Ch-control | 66.73 ± 2.56 | 21.28 ± 1.18 |
| Chitosan membrane with 0.1% copaiba oil | ChCo-0.1 | 83.63 ± 3.78 | 18.57 ± 2.45 |
| Chitosan membrane with 0.5% copaiba oil | ChCo-0.5 | 80.90 ± 5.65 | 11.39 ± 1.13 |
| Chitosan membrane with 1% copaiba oil | ChCo-1.0 | 38.33 ± 4.97 | 9.93 ± 0.25 |
| Chitosan membrane with 5% copaiba oil | ChCo-5.0 | 65.03 ± 2.77 | 19.42 ± 1.12 |
| Origin of Variation | Sum of Squares | Degrees of Freedom (DF) | Middle Square | Value of F | p-Value |
|---|---|---|---|---|---|
| Between groups | 3886.70 | 4 | 971.68 | 56.93 | <0.05 |
| Within two groups | 170.69 | 10 | 17.07 | - | - |
| Total | 4057.39 | 14 | 0.00008 |
| Origin of Variation | Sum of Squares | Degrees of Freedom (DF) | Middle Square | Value of F | p-Value |
|---|---|---|---|---|---|
| Between groups | 312.31 | 4 | 78.0769 | 38.78 | <0.05 |
| Within two groups | 20.13 | 10 | 2.01318 | - | - |
| Total | 332.44 | 14 | 0.00027 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paranhos, S.B.; Ferreira, E.d.S.; Canelas, C.A.d.A.; da Paz, S.P.A.; Passos, M.F.; da Costa, C.E.F.; da Silva, A.C.R.; Monteiro, S.N.; Candido, V.S. Chitosan Membrane Containing Copaiba Oil (Copaifera spp.) for Skin Wound Treatment. Polymers 2022, 14, 35. https://doi.org/10.3390/polym14010035
Paranhos SB, Ferreira EdS, Canelas CAdA, da Paz SPA, Passos MF, da Costa CEF, da Silva ACR, Monteiro SN, Candido VS. Chitosan Membrane Containing Copaiba Oil (Copaifera spp.) for Skin Wound Treatment. Polymers. 2022; 14(1):35. https://doi.org/10.3390/polym14010035
Chicago/Turabian StyleParanhos, Sheila Barbosa, Elisângela da Silva Ferreira, Caio Augusto de Almeida Canelas, Simone Patrícia Aranha da Paz, Marcele Fonseca Passos, Carlos Emmerson Ferreira da Costa, Alisson Clay Rios da Silva, Sergio Neves Monteiro, and Verônica Scarpini Candido. 2022. "Chitosan Membrane Containing Copaiba Oil (Copaifera spp.) for Skin Wound Treatment" Polymers 14, no. 1: 35. https://doi.org/10.3390/polym14010035
APA StyleParanhos, S. B., Ferreira, E. d. S., Canelas, C. A. d. A., da Paz, S. P. A., Passos, M. F., da Costa, C. E. F., da Silva, A. C. R., Monteiro, S. N., & Candido, V. S. (2022). Chitosan Membrane Containing Copaiba Oil (Copaifera spp.) for Skin Wound Treatment. Polymers, 14(1), 35. https://doi.org/10.3390/polym14010035