Flexural Strength, Elastic Modulus and Remineralizing Abilities of Bioactive Resin-Based Dental Sealants
Abstract
:1. Introduction
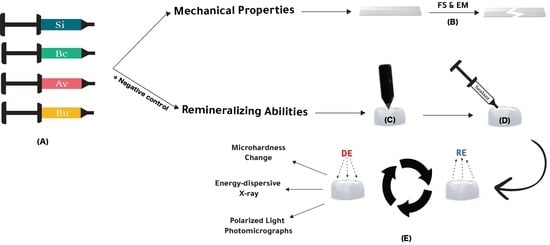
2. Materials and Methods
2.1. Study Groups
2.2. Mechanical Assessments: Flexural Strength and Elastic Modulus
2.3. Remineralization Abilities Assessments
2.3.1. Sample Preparation
2.3.2. pH-Cycling Model
2.3.3. Surface Hardness Change
2.3.4. Scanning Electron Microscopy with Energy-Dispersive X-ray Spectrometer (SEM-EDX) Analysis
2.3.5. Polarized Light Photomicrographs
2.4. Statistical Analysis
3. Results
3.1. Flexural Strength and Elastic Modulus
3.2. Surface Hardness Change
3.3. Scanning Electron Microscopy with Energy-Dispersive X-ray Spectrometer (SEM-EDX) Analysis
3.4. Polarized Light Photomicrographs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marcenes, W.; Kassebaum, N.J.; Bernabé, E.; Flaxman, A.; Naghavi, M.; Lopez, A.; Murray, C.J.L. Global burden of oral conditions in 1990–2010: A systematic analysis. J. Dent. Res. 2013, 92, 592–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Featherstone, J.D.B. Dental caries: A dynamic disease process. Aust. Dent. J. 2008, 53, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Angelillo, I.F.; Torre, I.; Nobile, C.G.A.; Villari, P. Caries and Fluorosis Prevalence in Communities with Different Concentrations of Fluoride in the Water. Caries Res. 1999, 33, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Arvin, E.; Bardow, A.; Spliid, H. Caries affected by calcium and fluoride in drinking water and family income. J. Water Health 2018, 16, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Lagerweij, M.D.; van Loveren, C. Declining Caries Trends: Are We Satisfied? Curr. Oral Health Rep. 2015, 2, 212–217. [Google Scholar] [CrossRef] [Green Version]
- Frencken, J.E.; Sharma, P.; Stenhouse, L.; Green, D.; Laverty, D.; Dietrich, T. Global epidemiology of dental caries and severe periodontitis—A comprehensive review. J. Clin. Periodontol. 2017, 44, S94–S105. [Google Scholar] [CrossRef]
- Miura, H.; Araki, Y.; Haraguchi, K.; Arai, Y.; Umenai, T. Socioeconomic factors and dental caries in developing countries: A cross-national study. Soc. Sci. Med. 1997, 44, 269–272. [Google Scholar] [CrossRef]
- Rai, N.K.; Tiwari, T. Parental factors influencing the development of early childhood caries in developing nations: A systematic review. Front. Public Health 2018, 6, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahovuo-Saloranta, A.; Forss, H.; Walsh, T.; Nordblad, A.; Mäkelä, M.; Worthington, H.V. Pit and fissure sealants for preventing dental decay in permanent teeth. Cochrane Database Syst. Rev. 2017, 7, CD001830. [Google Scholar] [CrossRef] [Green Version]
- Borges, B.C.D.; De Souza Borges, J.; Braz, R.; Montes, M.A.J.R.; De Assunção Pinheiro, I.V. Arrest of non-cavitated dentinal occlusal caries by sealing pits and fissures: A 36-month, randomised controlled clinical trial. Int. Dent. J. 2012, 62, 251–255. [Google Scholar] [CrossRef]
- Ganesh, M.; Shobha, T. Comparative evaluation of the marginal sealing ability of Fuji VII® and Concise® as pit and fissure sealants. J. Contemp. Dent. Pract. 2007, 8, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Al-Jobair, A.; Al-Hammad, N.; Alsadhan, S.; Salama, F. Retention and caries-preventive effect of glass ionomer and resin-based sealants: An 18-month-randomized clinical trial. Dent. Mater. J. 2017, 36, 654–661. [Google Scholar] [CrossRef] [Green Version]
- Ananda, S.-R.; Mythri, H. A comparative study of fluoride release from two different sealants. J. Clin. Exp. Dent. 2014, 6, e497–e501. [Google Scholar] [CrossRef] [Green Version]
- Pardi, V.; Sinhoreti, M.A.C.; Pereira, A.C.; Ambrosano, G.M.B.; Meneghim, M.D.C. In vitro evaluation of microleakage of different materials used as pit-and-fissure sealants. Braz. Dent. J. 2006, 17, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Bansal, T. A comparative evaluation of the amount of fluoride release and re-release after recharging from aesthetic restorative materials: An in vitro study. J. Clin. Diagn. Res. 2015, 9, ZC11–ZC14. [Google Scholar] [CrossRef]
- Tüzüner, T.; Dimkov, A.; Nicholson, J.W. The effect of antimicrobial additives on the properties of dental glass-ionomer cements: A review. Acta Biomater. Odontol. Scand. 2019, 5, 9–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baig, M.S.; Fleming, G.J.P. ScienceDirect Conventional glass-ionomer materials: A review of the developments in glass powder, polyacid liquid and the strategies of reinforcement. J. Dent. 2015, 43, 897–912. [Google Scholar] [CrossRef] [PubMed]
- Lohbauer, U. Dental glass ionomer cements as permanent filling materials? -Properties, limitations and future trends. Materials 2010, 3, 76–96. [Google Scholar] [CrossRef]
- De Moor, R.J.; Verbeeck, R.M.; De Maeyer, E.A. Fluoride release profiles of restorative glass ionomer formulations. Dent. Mater. 1996, 12, 88–95. [Google Scholar] [CrossRef]
- Basappa, N.; Raju, O.; Dahake, P.T. Fluoride: Is It Worth to be added in Pit and Fissure Sealants? Int. J. Clin. Pediatric Dent. 2012, 5, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.S.; AlQarni, F.D.; Al-Dulaijan, Y.A.; Weir, M.D.; Oates, T.W.; Xu, H.H.; Melo, M.A.S. Tuning nano-amorphous calcium phosphate content in novel rechargeable antibacterial dental sealant. Materials 2018, 11, 1544. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.S.; Garcia, I.M.; Vila, T.; Balhaddad, A.A.; Collares, F.M.; Weir, M.D.; Xu, H.H.; Melo, M.A.S. Multifunctional antibacterial dental sealants suppress biofilms derived from children at high risk of caries. Biomater. Sci. 2020, 8, 3472–3484. [Google Scholar] [CrossRef] [PubMed]
- AlShahrani, S.S.; AlAbbas, M.A.S.; Garcia, I.M.; AlGhannam, M.I.; AlRuwaili, M.A.; Collares, F.M.; Ibrahim, M.S. The antibacterial effects of resin-based dental sealants: A systematic review of in vitro studies. Materials 2021, 14, 413. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Piao, Y.Z.; Kim, S.M.; Lee, Y.K.; Kim, K.N.; Kim, K.M. Acid neutralizing, mechanical and physical properties of pit and fissure sealants containing melt-derived 45S5 bioactive glass. Dent. Mater. 2013, 29, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Kwon, J.S.; Kim, K.N.; Kim, K.M. Enamel Surface with Pit and Fissure Sealant Containing 45S5 Bioactive Glass. J. Dent. Res. 2016, 95, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.S.; Ibrahim, A.S.; Balhaddad, A.A.; Weir, M.D.; Lin, N.J.; Tay, F.R.; Oates, T.W.; Xu, H.H.K.; Melo, M.A.S. A novel dental sealant containing dimethylaminohexadecyl methacrylate suppresses the cariogenic pathogenicity of streptococcus mutans biofilms. Int. J. Mol. Sci. 2019, 20, 3491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, M.S.; Balhaddad, A.A.; Garcia, I.M.; Collares, F.M.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. pH-responsive calcium and phosphate-ion releasing antibacterial sealants on carious enamel lesions in vitro. J. Dent. 2020, 97, 103323. [Google Scholar] [CrossRef]
- Mousavinasab, S.M.; Khoroushi, M.; Keshani, F.; Hashemi, S. Flexural strength and morphological characteristics of resin-modified glass-ionomer containing bioactive glass. J. Contemp. Dent. Pract. 2011, 12, 41–46. [Google Scholar] [CrossRef]
- Yan, W.J.; Zheng, J.J.; Chen, X.X. Application of fluoride releasing flowable resin in pit and fissure sealant of children with early enamel caries. J. Peking Univ. Health Sci. 2018, 50, 911–914. [Google Scholar]
- Yang, S.Y.; Choi, J.W.; Kim, K.M.; Kwon, J.S. Prevention of secondary caries using resin-based pit and fissure sealants containing hydrated calcium silicate. Polymers 2020, 12, 1200. [Google Scholar] [CrossRef]
- Zawaideh, F.I.; Owais, A.I.; Kawaja, W. Ability of Pit and Fissure Sealant-containing Amorphous Calcium Phosphate to inhibit Enamel Demineralization. Int. J. Clin. Pediatric Dent. 2016, 9, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Kosior, P.; Dobrzyński, M.; Korczyński, M.; Herman, K.; Czajczyńska-Waszkiewicz, A.; Kowalczyk-Zając, M.; Piesiak-Pańczyszyn, D.; Fita, K.; Janeczek, M. Long-term release of fluoride from fissure sealants—In vitro study. J. Trace Elem. Med. Biol. 2017, 41, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli-Hojjati, S.; Atai, M.; Haghgoo, R.; Rahimian-Imam, S.; Kameli, S.; Ahmaian-Babaki, F.; Hamzeh, F.; Ahmadyar, M. Comparison of various concentrations of tricalcium phosphate nanoparticles on mechanical properties and remineralization of fissure sealants. J. Dent. 2014, 11, 379–388. [Google Scholar]
- Galo, R.; Contente, M.M.M.G.; Borsatto, M.C. Wear of two pit and fissure sealants in contact with primary teeth. Eur. J. Dent. 2014, 8, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Khalili Sadrabad, Z.; Safari, E.; Alavi, M.; Shadkar, M.M.; Hosseini Naghavi, S.H. Effect of a fluoride-releasing fissure sealant and a conventional fissure sealant on inhibition of primary carious lesions with or without exposure to fluoride-containing toothpaste. J. Dent. Res. Dent. Clin. Dent. Prospect. 2019, 13, 147–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, M.S.; Balhaddad, A.A.; Garcia, I.M.; Hefni, E.; Collares, F.M.; Martinho, F.C.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Tooth sealing formulation with bacteria-killing surface and on-demand ion release/recharge inhibits early childhood caries key pathogens. J. Biomed. Mater. Res. - Part B Appl. Biomater. 2020, 108, 3217–3227. [Google Scholar] [CrossRef]
- International Organization for Standardization. Dentistry-Polymer-Based Restorative Materials; International Organization for Standardization: Geneva, Switzerland, 2019. [Google Scholar]
- Magalhães, A.C.; Comar, L.P.; Rios, D.; Delbem, A.C.B.; Buzalaf, M.A.R. Effect of a 4% titanium tetrafluoride (TiF4) varnish on demineralisation and remineralisation of bovine enamel in vitro. J. Dent. 2008, 36, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, C.S.; Hara, A.T.; Paes Leme, A.F.; Cury, J.A. pH-Cycling models to evaluate the effect of low fluoride dentifrice on enamel De- and remineralization. Braz. Dent. J. 2008, 19, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Lobo, M.M.; Pecharki, G.D.; Tengan, C.; da Silva, D.D.; da Silva Tagliaferro, E.P.; Napimoga, M.H. Fluoride-releasing capacity and cariostatic effect provided by sealants. J. Oral Sci. 2005, 47, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azillah, M.A.; Anstice, H.M.; Pearson, G.J. Long-term flexural strength of three direct aesthetic restorative materials. J. Dent. 1998, 26, 177–182. [Google Scholar] [CrossRef]
- Dejak, B.; Młotkowski, A.; Romanowicz, M. Finite element analysis of stresses in molars during clenching and mastication. J. Prosthet. Dent. 2003, 90, 591–597. [Google Scholar] [CrossRef]
- PULPDENT Corporation. ACTIVATM KIDS. 2018. Available online: https://www.pulpdent.com/wp-content/uploads/2019/09/XP-VK-IN-03w.pdf (accessed on 14 December 2021).
- Itota, T.; Nakatsuka, T.; Tanaka, K.; Tashiro, Y.; Mccabe, J.F.; Yoshiyama, M. Neutralizing effect by resin-based materials containing silane-coated glass fillers. Dent. Mater. J. 2010, 29, 362–368. [Google Scholar] [CrossRef] [Green Version]
- Alamri, A.; Salloot, Z.; Alshaia, A.; Ibrahim, M.S. The Effect of Bioactive Glass-Enhanced Orthodontic Bonding Resins on Prevention of Demineralization: A Systematic Review. Molecules 2020, 25, 2495. [Google Scholar] [CrossRef] [PubMed]
- Yu, O.Y.; Zhao, I.S.; Mei, M.L.; Lo, E.C.-M.; Chu, C.-H. A Review of the Common Models Used in Mechanistic Studies on Demineralization-Remineralization for Cariology Research. Dent. J. 2017, 5, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zurita, G.; Luis Alberto, L.; Camargo, H.; Torres-Rodríguez, C.; Delgado-Mejía, E. Simplified chemical method of demineralization in human dental enamel. Revista Cubana de Estomatologia 2019, 56, 13–24. [Google Scholar]
- Arends, J.; Ten Bosch, J.J. Demineralization and remineralization evaluation techniques. J. Dent. Res. 1992, 71 (Suppl. 3), 924–928. [Google Scholar] [CrossRef] [PubMed]
- Langhorst, S.E.; O’Donnell, J.N.R.; Skrtic, D. In vitro remineralization of enamel by polymeric amorphous calcium phosphate composite: Quantitative microradiographic study. Dent. Mater. 2009, 25, 884–891. [Google Scholar] [CrossRef] [Green Version]
- SHOFU INC. BeautiSealant. 2021. Available online: https://www.shofu.com/wp-content/uploads/BeautiSealant-BRO-US.pdf (accessed on 14 December 2021).
- Premier® Dental Products Company. (n.d.). SAFETY DATA SHEET: BioCoatTM Bioactive Pit & Fissure Sealant. Available online: https://www.premierdentalco.com/wp-content/uploads/sds/SDS BioCoat R2.pdf (accessed on 14 December 2021).
- De Medeiros, R.C.G.; Soares, J.D.; De Sousa, F.B. Natural enamel caries in polarized light microscopy: Differences in histopathological features derived from a qualitative versus a quantitative approach to interpret enamel birefringence. J. Microsc. 2012, 246, 177–189. [Google Scholar] [CrossRef]
- Gwinnett, A.J. Normal Enamel. II. Qualitative Polarized Light Study. J. Dent. Res. 1966, 45, 261–265. [Google Scholar] [CrossRef]









| Name | Type | Composition | Manufacturer | Code |
|---|---|---|---|---|
| Blank | Negative control | - | - | B |
| Seal-it sealant | Non-bioactive sealant | Bisphenol A ethoxylate dimethacrylate of isomers (30–50%) Triethylene glycol dimethacrylate (20–30%) Camphorquinone (<0.1%) | SPIDENT, Incheon, South Korea | Si |
| ACTIVA KIDS Bioactive—restorative | Bioactive material | Mix of methacrylates and diurethane with modified polyacrylic acid (44.6%), silica, amorphous (6.7%), sodium fluoride (0.75%) | Pulpdent, Watertown, MA, USA | Av |
| BioCoat® sealant | Bioactive sealant | Triethylene glycol dimethacrylates, (1-methylethylidene)bis[4,1-phenyleneoxy(2-hydroxy-3,1 propanediyl)] bismethacrylate, fumed silica, barrium aluminoborosilicate (<60%); calcium donor (<2%); photo-initiator (<2.5) | Premier, Plymouth Meeting, PA, USA | Bc |
| BeautiSealant | Bioactive sealant | Primer: acetone, phosphoric acid monomer, carboxylic acid monomer, distilled water. Sealant: S-PRG fillers (30% by weight), micro fumed silica, UDMA, TEGDMA. | SHOFU, Kyoto, Japan | Bu |
| Material Type | Flexural Strength Mean ± SD (MPa) | Elastic Modulus Mean ± SD (GPa) | Surface Hardness Change Mean ± SD (%) |
|---|---|---|---|
| Negative Control | Not applicable | Not applicable | −70.1 ± 3.4 b |
| Si | 84.7 ± 6.6 ab | 4.6 ± 0.4 ab | −54.9 ± 7.5 b |
| Bc | 94.4 ± 9.7 a | 5.2 ± 0.5 a | 52.9 ± 24.8 a |
| Av | 79.9 ± 6.9 b | 4.4 ± 0.4 bc | −35.1 ± 7.4 b |
| Bu | 78.4 ± 9.2 b | 3.9 ± 0.6 c | 39.7 ± 18.4 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, M.S.; Alabbas, M.S.; Alsomaly, K.U.; AlMansour, A.A.; Aljouie, A.A.; Alzahrani, M.M.; Asseri, A.A.; AlHumaid, J. Flexural Strength, Elastic Modulus and Remineralizing Abilities of Bioactive Resin-Based Dental Sealants. Polymers 2022, 14, 61. https://doi.org/10.3390/polym14010061
Ibrahim MS, Alabbas MS, Alsomaly KU, AlMansour AA, Aljouie AA, Alzahrani MM, Asseri AA, AlHumaid J. Flexural Strength, Elastic Modulus and Remineralizing Abilities of Bioactive Resin-Based Dental Sealants. Polymers. 2022; 14(1):61. https://doi.org/10.3390/polym14010061
Chicago/Turabian StyleIbrahim, Maria Salem, Mana’a S. Alabbas, Khalid U. Alsomaly, Abdullah A. AlMansour, Alhareth Abdulaziz Aljouie, Majed M. Alzahrani, Ahmed A. Asseri, and Jehan AlHumaid. 2022. "Flexural Strength, Elastic Modulus and Remineralizing Abilities of Bioactive Resin-Based Dental Sealants" Polymers 14, no. 1: 61. https://doi.org/10.3390/polym14010061
APA StyleIbrahim, M. S., Alabbas, M. S., Alsomaly, K. U., AlMansour, A. A., Aljouie, A. A., Alzahrani, M. M., Asseri, A. A., & AlHumaid, J. (2022). Flexural Strength, Elastic Modulus and Remineralizing Abilities of Bioactive Resin-Based Dental Sealants. Polymers, 14(1), 61. https://doi.org/10.3390/polym14010061