A Review of Polymeric Micelles and Their Applications
Abstract
:1. Introduction
1.1. Why and When Does Self-Assembly Occur?
1.2. Determination of CMC
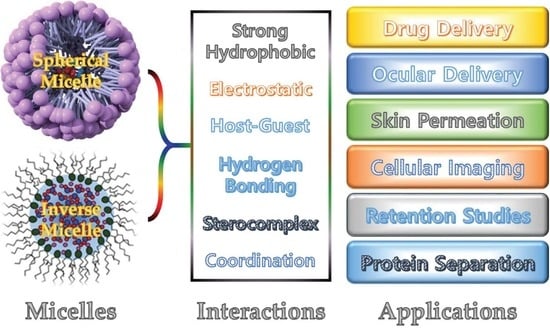
2. Polymeric Micelles
2.1. Polymeric Spherical Micelles
2.2. Polymeric Inverse Micelle
2.3. Different Types of Polymeric Micelles
3. Application of Polymeric Micelles
3.1. Cancer Drug Delivery
3.2. Another Application
4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riess, G. Micellization of block copolymers. Prog. Polym. Sci. 2003, 28, 1107–1170. [Google Scholar] [CrossRef] [Green Version]
- Discher, D.E.; Eisenberg, A. Polymer vesicles. Science 2002, 297, 967–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Kesselman, E.; Talmon, Y.; Hillmyer, M.A.; Lodge, T.P. Multicompartment micelles from ABC miktoarm stars in water. Science 2004, 306, 98–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marras, A.E.; Ting, J.M.; Stevens, K.C.; Tirrell, M.V. Advances in the structural design of polyelectrolyte complex micelles. J. Phys. Chem. B 2021, 125, 7076–7089. [Google Scholar] [CrossRef] [PubMed]
- Dewald, I.; Fery, A. Polymeric micelles and vesicles in polyelectrolyte multilayers: Introducing hierarchy and compartmentalization. Adv. Mater. Interfaces 2017, 4, 1600317. [Google Scholar] [CrossRef]
- Kim, J.H.; Ramasamy, T.; Tran, T.H.; Choi, J.Y.; Cho, H.J.; Yong, C.S.; Kim, J.O. Polyelectrolyte complex micelles by self-assembly of polypeptide-based triblock copolymer for doxorubicin delivery. Asian J. Pharm. Sci. 2014, 9, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Esparza, K.; Jayawardena, D.; Onyuksel, H. Phospholipid micelles for peptide drug delivery. Methods Mol. Biol. 2019, 2000, 43–57. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Penttila, P.A.; Vierros, S.; Utriainen, K.; Carl, N.; Rautkari, L.; Sammalkorpi, M.; Sterberg, M.O. Phospholipid-based reverse micelle structures in vegetable oil modified by water content, free fatty acid, and temperature. Langmuir 2019, 35, 8373–8382. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Liang, F.; Hu, D.; Li, H.; Yang, W.; Zhu, Q. Determining the critical micelle concentration of surfactants by a simple and fast titration method. Anal. Chem. 2020, 92, 4259–4265. [Google Scholar] [CrossRef]
- Su, H.; Wang, F.; Ran, W.; Zhang, W.; Dai, W.; Wang, H.; Anderson, C.F.; Wang, Z.; Zheng, C.; Zhang, P.; et al. The role of critical micellization concentration in efficacy and toxicity of supramolecular polymers. Proc. Natl. Acad. Sci. USA 2020, 117, 4518–4526. [Google Scholar] [CrossRef]
- Trujillo, M.; Schramm, M.P. Measuring critical micelle concentration as a function of cavitand additives using surface tension and dye micellization. Ronald E McNair Postbac Achiev. Program. 2010, 14, 155–168. [Google Scholar]
- Qazi, M.J.; Schlegel, S.J.; Backus, E.H.G.; Bonn, M.; Bonn, D.; Shahidzadeh, N. Dynamic surface tension of surfactants in the presence of high salt concentrations. Langmuir 2020, 36, 7956–7964. [Google Scholar] [CrossRef]
- Chakraborty, T.; Chakraborty, I.; Ghosh, S. The methods of determination of critical micellar concentrations of the amphiphilic systems in aqueous medium. Arab. J. Chem. 2011, 4, 265–270. [Google Scholar] [CrossRef] [Green Version]
- Mohajeri, E.; Noudeh, G.D. Effect of temperature on the critical micelle concentration and micellization thermodynamic of nonionic surfactants: Polyoxyethylene sorbitan fatty acid esters. E-J. Chem. 2012, 9, 961739. [Google Scholar] [CrossRef]
- Bilia, A.R.; Bergonzi, M.C.; Guccione, C.; Manconi, M.; Fadda, A.M.; Sinico, C. Vesicles and micelles: Two versatile vectors for the delivery of natural products. J. Drug Deliv. Sci. Technol. 2016, 32, 241–255. [Google Scholar] [CrossRef]
- Brinkhuis, R.P.; Rutjes, F.P.J.T.; van Hest, J.C.M. Polymeric vesicles in biomedical applications. Polym. Chem. 2011, 2, 1449–1462. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Yang, B.; Chen, S.; Du, J. Polymer vesicles: Mechanism, preparation, application, and responsive behavior. Prog. Polym. Sci. 2017, 64, 1–22. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Zhao, X.; Yang, Y.; Li, H.; Zhou, X.; Yuan, W. Asymmetrical polymer vesicles for drug delivery and other applications. Front. Pharmacol. 2017, 8, 374. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Wang, Y.; Song, J. Polymer vesicles for antimicrobial applications. Polymers 2021, 13, 2903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, Q.; Wang, F.; Sun, H.; Xiao, J.; Cornel, E.J.; Zhu, Y.; Du, J. Giant polymer vesicles with a latticelike membrane. ACS Macro Lett. 2021, 10, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.; Voit, B.; Gaitzsch, J. The chemistry of cross-linked polymeric vesicles and their functionalization towards biocatalytic nanoreactors. Colloid Polym. Sci. 2021, 299, 309–324. [Google Scholar] [CrossRef]
- Karimi, M.A.; Mozaheb, M.A.; Hatefi-Mehrjardi, A.; Tavallali, H.; Attaran, A.M.; Shamsi, R. A new simple method for determining the critical micelle concentration of surfactants using surface plasmon resonance of silver nanoparticles. J. Anal. Sci. Technol. 2015, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Scholz, N.; Behnke, T.; Resch-Genger, U. Determination of the critical micelle concentration of neutral and ionic surfactants with fluorometry, conductometry, and surface tension—a method comparison. J. Fluoresc. 2018, 28, 465–476. [Google Scholar] [CrossRef]
- Topel, Ö.; Çakır, B.A.; Budama, L.; Hoda, N. Determination of critical micelle concentration of polybutadiene-block-poly(ethyleneoxide) diblock copolymer by fluorescence spectroscopy and dynamic light scattering. J. Mol. Liq. 2013, 177, 40–43. [Google Scholar] [CrossRef]
- Gadelha, G.; Nawaz, M.S.; Hankins, N.P.; Khan, S.J.; Wang, R.; Tang, C.Y. Assessment of micellar solutions as draw solutions for forward osmosis. Desalination 2014, 354, 97–106. [Google Scholar] [CrossRef]
- Amos, D.A.; Markels, J.H.; Lynn, S.; Radke, C.J. Osmotic pressure and interparticle interactions in ionic micellar surfactant solutions. J. Phys. Chem. B 1998, 102, 2739–2753. [Google Scholar] [CrossRef]
- Ghosh, S.; Krishnan, A.; Das, P.K.; Ramakrishnan, S. Determination of critical micelle concentration by hyper-rayleigh scattering. J. Am. Chem. Soc. 2003, 125, 1602–1606. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, M.; Prieto, G.; Rega, C.; Varela, L.M.; Sarmiento, F.; Mosquera, V. A Comparative study of the determination of the critical micelle concentration by conductivity and dielectric constant measurements. Langmuir 1998, 14, 4422–4426. [Google Scholar] [CrossRef]
- López Fontán, J.L.; Costa, J.; Ruso, J.M.; Prieto, G.; Sarmiento, F. electrical conductivities and critical micelle concentrations (determined by the local polynomial regression method) of imipramine and clomipramine hydrochlorides from (283 to 313) K. J. Chem. Eng. Data 2004, 49, 1008–1012. [Google Scholar] [CrossRef]
- Bowden, S.T. The double-capillary method of surface tension measurement. J. Phys. Chem. 1930, 34, 1866–1868. [Google Scholar] [CrossRef]
- Zhang, H. 2—Surface characterization techniques for polyurethane biomaterials. In Advances in Polyurethane Biomaterials; Cooper, S.L., Guan, J., Eds.; Woodhead Publishing: Kidlington, UK, 2016; pp. 23–73. [Google Scholar]
- Tiab, D.; Donaldson, E.C. Chapter 6—Wettability. In Petrophysics, 4th ed.; Tiab, D., Donaldson, E.C., Eds.; Gulf Professional Publishing: Boston, MA, USA, 2016; pp. 319–357. [Google Scholar]
- Chen, F.; Ji, Z.; Qi, Q. Effect of liquid surface tension on the filtration performance of coalescing filters. Sep. Purif. Technol. 2019, 209, 881–891. [Google Scholar] [CrossRef]
- Ritacco, H.; Kovensky, J.; Fernández-Cirelli, A.; Castro, M.J.L. A simplified method for the determination of critical micelle concentration. J. Chem. Educ. 2001, 78, 347. [Google Scholar] [CrossRef]
- Salem, J.K.; El-Nahhal, I.M.; Salama, S.F. Determination of the critical micelle concentration by absorbance and fluorescence techniques using fluorescein probe. Chem. Phys. Lett. 2019, 730, 445–450. [Google Scholar] [CrossRef]
- Hamley, I.W. Self-assembly of amphiphilic peptides. Soft Matter 2011, 7, 4122–4138. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Cao, C.; Wei, P.; Xu, M.; Liu, Z.; Liu, L.; Zhong, Y.; Li, R.; Zhou, Y.; Yi, T. Self-Assembly of amphiphilic peptides for recognizing high furin-expressing cancer cells. ACS Appl. Mater. Interfaces 2019, 11, 12327–12334. [Google Scholar] [CrossRef]
- Cao, M.; Cao, C.; Zhou, P.; Wang, N.; Wang, D.; Wang, J.; Xia, D.; Xu, H. Self-assembly of amphiphilic peptides: Effects of the single-chain-to-gemini structural transition and the side chain groups. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 263–270. [Google Scholar] [CrossRef]
- Soleimani Zohr Shiri, M.; Henderson, W.; Mucalo, M.R. A review of the lesser-studied microemulsion-based synthesis methodologies used for preparing nanoparticle systems of the noble metals, Os, Re, Ir and Rh. Materials 2019, 12, 1896. [Google Scholar] [CrossRef] [Green Version]
- Webber, S.E. Polymer micelles: An example of self-assembling polymers. J. Phys. Chem. B 1998, 102, 2618–2626. [Google Scholar] [CrossRef]
- Hanafy, N.A.N.; El-Kemary, M.; Leporatti, S. Micelles structure development as a strategy to improve smart cancer therapy. Cancers 2018, 10, 238. [Google Scholar] [CrossRef] [Green Version]
- Kulthe, S.S.; Choudhari, Y.M.; Inamdar, N.N.; Mourya, V. Polymeric micelles: Authoritative aspects for drug delivery. Des. Monomers Polym. 2012, 15, 465–521. [Google Scholar] [CrossRef]
- Torchilin, V.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Control Release 2001, 73, 137–172. [Google Scholar] [CrossRef]
- Liu, J.; Lee, H.; Allen, C. Formulation of drugs in block copolymer micelles: Drug loading and release. Curr. Pharm. Des. 2006, 12, 4685–4701. [Google Scholar] [CrossRef]
- Fournier, E.; Dufresne, M.H.; Smith, D.C.; Ranger, M.; Leroux, J.C. A novel one-step drug-loading procedure for water-soluble amphiphilic nanocarriers. Pharm. Res. 2004, 21, 962–968. [Google Scholar] [CrossRef]
- Teagarden, D.L.; Baker, D.S. Practical aspects of lyophilization using non-aqueous co-solvent systems. Eur. J. Pharm. Sci. 2002, 15, 115–133. [Google Scholar] [CrossRef]
- Letchford, K.; Zastre, J.; Liggins, R.; Burt, H. Synthesis and micellar characterization of short block length methoxy poly(ethylene glycol)-block-poly(caprolactone) diblock copolymers. Colloids Surf B Biointerfaces 2004, 35, 81–91. [Google Scholar] [CrossRef]
- Liu, J.; Zeng, F.; Allen, C. Influence of serum protein on polycarbonate-based copolymer micelles as a delivery system for a hydrophobic anti-cancer agent. J. Control Release 2005, 103, 481–497. [Google Scholar] [CrossRef]
- Hibino, M.; Tanaka, K.; Ouchi, M.; Terashima, T. Amphiphilic random-block copolymer micelles in water: Precise and dynamic self-assembly controlled by random copolymer association. Macromolecules 2022, 55, 178–189. [Google Scholar] [CrossRef]
- Yokoyama, M.; Satoh, A.; Sakurai, Y.; Okano, T.; Matsumura, Y.; Kakizoe, T.; Kataoka, K. Incorporation of water-insoluble anticancer drug into polymeric micelles and control of their particle size. J. Control. Release 1998, 55, 219–229. [Google Scholar] [CrossRef]
- Le Garrec, D.; Gori, S.; Luo, L.; Lessard, D.; Smith, D.C.; Yessine, M.A.; Ranger, M.; Leroux, J.C. Poly(N-vinylpyrrolidone)-block-poly(D,L-lactide) as a new polymeric solubilizer for hydrophobic anticancer drugs: In vitro and in vivo evaluation. J. Control Release 2004, 99, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Minatti, E.; Viville, P.; Borsali, R.; Schappacher, M.; Deffieux, A.; Lazzaroni, R. Micellar morphological changes promoted by cyclization of PS-b-PI copolymer: DLS and AFM experiments. Macromolecules 2003, 36, 4125–4133. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Fan, Y.; Zhou, Y.; Wang, X.; Fan, C.; Liu, Y.; Zhang, Q. Preparation and evaluation of novel mixed micelles as nanocarriers for intravenous delivery of propofol. Nanoscale Res. Lett. 2011, 6, 275. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Li, W.; Duan, X.; Zhu, L.; Fan, L.; Qiao, Y.; Wu, H. Preparation of two types of polymeric micelles based on poly(β-L-Malic Acid) for antitumor drug delivery. PLoS ONE 2016, 11, e0162607. [Google Scholar] [CrossRef]
- Salimi, A.; Sharif Makhmal Zadeh, B.; Kazemi, M. Preparation and optimization of polymeric micelles as an oral drug delivery system for deferoxamine mesylate: In vitro and ex vivo studies. Res. Pharm. Sci. 2019, 14, 293–307. [Google Scholar] [CrossRef]
- Patra, A.; Satpathy, S.; Shenoy, A.K.; Bush, J.A.; Kazi, M.; Hussain, M.D. Formulation and evaluation of mixed polymeric micelles of quercetin for treatment of breast, ovarian, and multidrug resistant cancers. Int. J. Nanomed. 2018, 13, 2869–2881. [Google Scholar] [CrossRef] [Green Version]
- Bae, Y.; Nishiyama, N.; Fukushima, S.; Koyama, H.; Yasuhiro, M.; Kataoka, K. Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: Tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconjugate Chem. 2005, 16, 122–130. [Google Scholar] [CrossRef]
- Bailly, N.; Thomas, M.; Klumperman, B. Poly(N-vinylpyrrolidone)-block-poly(vinyl acetate) as a drug delivery vehicle for hydrophobic drugs. Biomacromolecules 2012, 13, 4109–4117. [Google Scholar] [CrossRef]
- Discher, B.M.; Won, Y.-Y.; Ege, D.S.; Lee, J.C.-M.; Bates, F.S.; Discher, D.E.; Hammer, D.A. Polymersomes: Tough vesicles made from diblock copolymers. Science 1999, 284, 1143–1146. [Google Scholar] [CrossRef] [Green Version]
- Tsai, Y.-M.; Jan, W.-C.; Chien, C.-F.; Lee, W.-C.; Lin, L.-C.; Tsai, T.-H. Optimised nano-formulation on the bioavailability of hydrophobic polyphenol, curcumin, in freely-moving rats. Food Chem. 2011, 127, 918–925. [Google Scholar] [CrossRef]
- Lombardo, D.; Munaò, G.; Calandra, P.; Pasqua, L.; Caccamo, M.T. Evidence of pre-micellar aggregates in aqueous solution of amphiphilic PDMS–PEO block copolymer. Phys. Chem. Chem. Phys. 2019, 21, 11983–11991. [Google Scholar] [CrossRef]
- Li, C.; Tho, C.C.; Galaktionova, D.; Chen, X.; Král, P.; Mirsaidov, U. Dynamics of amphiphilic block copolymers in an aqueous solution: Direct imaging of micelle formation and nanoparticle encapsulation. Nanoscale 2019, 11, 2299–2305. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Horechyy, A.; Bittrich, E.; Nandan, B.; Uhlmann, P.; Fery, A. Amphiphilic Block Copolymer Micelles in Selective Solvents: The Effect of Solvent Selectivity on Micelle Formation. Polymers 2019, 11, 1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grillo, I.; Morfin, I.; Prévost, S. Structural characterization of pluronic micelles swollen with perfume molecules. Langmuir 2018, 34, 13395–13408. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, T.-L.; Chakroun, R.; Janoszka, N.; Chen, C.; Klein, K.; Wong, C.K.; Gröschel, A.H. pH-Controlled hierarchical assembly/disassembly of multicompartment micelles in water. Macromol. Rapid Commun. 2020, 41, 2000301. [Google Scholar] [CrossRef]
- Sill, K.N.; Sullivan, B.; Carie, A.; Semple, J.E. Synthesis and characterization of micelle-forming PEG-poly(amino acid) copolymers with iron-hydroxamate cross-linkable blocks for encapsulation and release of hydrophobic drugs. Biomacromolecules 2017, 18, 1874–1884. [Google Scholar] [CrossRef] [Green Version]
- Rios-Doria, J.; Carie, A.; Costich, T.; Burke, B.; Skaff, H.; Panicucci, R.; Sill, K. A versatile polymer micelle drug delivery system for encapsulation and in vivo stabilization of hydrophobic anticancer drugs. J. Drug Deliv. 2012, 2012, 951741. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.X.; Banana, K.; Liu, H.Y.; Krause, M.; Yang, M. Cross-linked porous polymer resins with reverse micellar imprints: Factors affecting the porosity of the polymers. Macromolecules 1999, 32, 277–281. [Google Scholar] [CrossRef]
- Dhawan, S.; Singh, H.; Ghosh, S.; Khokhar, V.; Pandey, S.; Banerjee, M.; Haridas, V. Unprecedented formation of reverse micellar vesicles from psuedopeptidic bottlebrush polymers. Chem. Commun. 2020, 56, 12005–12008. [Google Scholar] [CrossRef]
- Jones, M.-C.; Leroux, J.-C. Reverse micelles from amphiphilic branched polymers. Soft Matter 2010, 6, 5850–5859. [Google Scholar] [CrossRef]
- Arsene, M.-L.; Răut, I.; Călin, M.; Jecu, M.-L.; Doni, M.; Gurban, A.-M. Versatility of reverse micelles: From biomimetic models to nano (bio)sensor design. Processes 2021, 9, 345. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Yao, Y.; Zhang, S.; Gu, Z. Reverse micelle-based water-soluble nanoparticles for simultaneous bioimaging and drug delivery. Org. Biomol. Chem. 2017, 15, 3232–3238. [Google Scholar] [CrossRef]
- Zhang, Y.; Pearce, S.; Eloi, J.-C.; Harniman, R.L.; Tian, J.; Cordoba, C.; Kang, Y.; Fukui, T.; Qiu, H.; Blackburn, A.; et al. Dendritic micelles with controlled branching and sensor applications. J. Am. Chem. Soc. 2021, 143, 5805–5814. [Google Scholar] [CrossRef]
- Kosakowska, K.A.; Casey, B.K.; Albert, J.N.L.; Wang, Y.; Ashbaugh, H.S.; Grayson, S.M. Synthesis and self-assembly of amphiphilic star/linear–dendritic polymers: Effect of core versus peripheral branching on reverse micelle aggregation. Biomacromolecules 2018, 19, 3177–3189. [Google Scholar] [CrossRef]
- Gao, H.; Jones, M.-C.; Tewari, P.; Ranger, M.; Leroux, J.-C. Star-shaped alkylated poly(glycerol methacrylate) reverse micelles: Synthesis and evaluation of their solubilizing properties in dichloromethane. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 2425–2435. [Google Scholar] [CrossRef]
- Saha, A.; Ramakrishnan, S. Unimolecular micelles and reverse micelles based on hyperbranched polyethers—Comparative study of AB2 + A-R and A2 + B3 + A-R type strategies. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 80–91. [Google Scholar] [CrossRef]
- Luo, S.; Hu, X.; Zhang, Y.; Ling, C.; Liu, X.; Chen, S. Synthesis of thermoresponsive unimolecular polymeric micelles with a hydrophilic hyperbranched poly(glycidol) core. Polym. J. 2011, 43, 41–50. [Google Scholar] [CrossRef] [Green Version]
- He, K.; Zou, Z.; Hu, Y.; Yang, Y.; Xiao, Y.; Gao, P.; Li, X.; Ye, X. Purification of α-glucosidase from mouse intestine by countercurrent chromatography coupled with a reverse micelle solvent system. J. Sep. Sci. 2016, 39, 703–708. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, Y.; Zhang, F.; Luo, G. Protein extraction from grape seeds by reverse micelles: Optimization of the forward extraction. OALib 2017, 04, 1–12. [Google Scholar] [CrossRef]
- Chatzidaki, M.D.; Papavasileiou, K.D.; Papadopoulos, M.G.; Xenakis, A. Reverse micelles as antioxidant carriers: An experimental and molecular dynamics study. Langmuir 2017, 33, 5077–5085. [Google Scholar] [CrossRef]
- Harris, C.; Gaster, C.; Gelabert, M.C. Reverse micelles as templates for the fabrication of size-controlled nanoparticles: A physical chemistry experiment. J. Chem. Educ. 2019, 96, 565–570. [Google Scholar] [CrossRef]
- Nguyen, T.B.T.; Li, S.; Deratani, A. Reverse micelles prepared from amphiphilic polylactide-b-poly(ethylene glycol) block copolymers for controlled release of hydrophilic drugs. Int. J. Pharm. 2015, 495, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.-C.; Gao, H.; Leroux, J.-C. Reverse polymeric micelles for pharmaceutical applications. J. Control. Release 2008, 132, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, X.; Yin, Z. Synthesis and evaluation of cationic polymeric micelles as carriers of lumbrokinase for targeted thrombolysis. Asian J. Pharm. Sci. 2019, 14, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Jiang, Y.; Yang, C.; Lu, X.; Chen, H.; Mao, S.; Liu, M.; Yuan, H.; Luo, P.; Du, Y. Mechanism of the mixed surfactant micelle formation. J. Phys. Chem. B 2010, 114, 7808–7816. [Google Scholar] [CrossRef]
- Dar, A.A.; Rather, G.M.; Das, A.R. Mixed micelle formation and solubilization behavior toward polycyclic aromatic hydrocarbons of binary and ternary cationic−nonionic surfactant mixtures. J. Phys. Chem. B 2007, 111, 3122–3132. [Google Scholar] [CrossRef]
- Sobczyński, J.; Chudzik-Rząd, B. Chapter 9—Mixed micelles as drug delivery nanocarriers. In Design and Development of New Nanocarriers; Grumezescu, A.M., Ed.; William Andrew Publishing: Lublin, Poland, 2018; pp. 331–364. [Google Scholar]
- Ebrahim Attia, A.B.; Ong, Z.Y.; Hedrick, J.L.; Lee, P.P.; Ee, P.L.R.; Hammond, P.T.; Yang, Y.-Y. Mixed micelles self-assembled from block copolymers for drug delivery. Curr. Opin. Colloid Interface Sci. 2011, 16, 182–194. [Google Scholar] [CrossRef]
- Hespel, L.; Asmar, A.E.; Morandi, G.; Lecamp, L.; Picton, L.; Burel, F. Synthesis of dual-sensitive core cross-linked mixed micelles through thiol–ene addition and subsequent drug release behavior. Macromol. Chem. Phys. 2017, 218, 1700016. [Google Scholar] [CrossRef]
- Sun, F.; Jaspers, T.C.; van Hasselt, P.M.; Hennink, W.E.; van Nostrum, C.F. A mixed micelle formulation for oral delivery of vitamin K. Pharm. Res. 2016, 33, 2168–2179. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Qian, Y.; Hu, X.; Ge, Z.; Liu, S. Mixed polymeric micelles as multifunctional scaffold for combined magnetic resonance imaging contrast enhancement and targeted chemotherapeutic drug delivery. J. Mater. Chem. 2012, 22, 5020–5030. [Google Scholar] [CrossRef]
- Yu, S.C.; Chia-En, C.; Hua-Jing, J.; Ming-Thau, S.; Hsiu-O, H. Self-assembled mixed micelle as carriers for efficient delivery of hydrophobic chemotherapeutic agent. Front. Bioeng. Biotechnol. 2016, 4. [Google Scholar]
- Schulz, A.; Jaksch, S.; Schubel, R.; Wegener, E.; Di, Z.; Han, Y.; Meister, A.; Kressler, J.; Kabanov, A.V.; Luxenhofer, R.; et al. Drug-induced morphology switch in drug delivery systems based on poly(2-oxazoline)s. ACS Nano 2014, 8, 2686–2696. [Google Scholar] [CrossRef]
- Nazemi, A.; Boott, C.E.; Lunn, D.J.; Gwyther, J.; Hayward, D.W.; Richardson, R.M.; Winnik, M.A.; Manners, I. Monodisperse cylindrical micelles and block comicelles of controlled length in aqueous media. J. Am. Chem. Soc. 2016, 138, 4484–4493. [Google Scholar] [CrossRef] [Green Version]
- Brendel, J.C.; Catrouillet, S.; Sanchis, J.; Jolliffe, K.A.; Perrier, S. Shaping block copolymer micelles by supramolecular polymerization: Making ‘tubisomes’. Polym. Chem. 2019, 10, 2616–2625. [Google Scholar] [CrossRef]
- Catrouillet, S.; Brendel, J.C.; Larnaudie, S.; Barlow, T.; Jolliffe, K.A.; Perrier, S. Tunable length of cyclic peptide–polymer conjugate self-assemblies in water. ACS Macro Lett. 2016, 5, 1119–1123. [Google Scholar] [CrossRef] [Green Version]
- Chapman, R.; Warr, G.G.; Perrier, S.; Jolliffe, K.A. Water-soluble and pH-responsive polymeric nanotubes from cyclic peptide templates. Chem. A Eur. J. 2013, 19, 1955–1961. [Google Scholar] [CrossRef] [PubMed]
- Sivokhin, A.P.; Orekhov, D.V.; Kazantsev, O.A.; Gubanova, O.V.; Kamorin, D.M.; Zarubina, I.S.; Bolshakova, E.A.; Zaitsev, S.D. Amphiphilic thermoresponsive copolymer bottlebrushes: Synthesis, characterization, and study of their self-assembly into flower-like micelles. Polym. J. 2021, 53, 655–665. [Google Scholar] [CrossRef]
- Carie, A.; Sullivan, B.; Ellis, T.; Semple, J.E.; Buley, T.; Costich, T.L.; Crouse, R.; Bakewell, S.; Sill, K. Stabilized polymer micelles for the development of IT-147, an epothilone D drug-loaded formulation. J. Drug Deliv. 2016, 2016, 8046739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakewell, S.J.; Carie, A.; Costich, T.L.; Sethuraman, J.; Semple, J.E.; Sullivan, B.; Martinez, G.V.; Dominguez-Viqueira, W.; Sill, K.N. Imaging the delivery of drug-loaded, iron-stabilized micelles. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1353–1362. [Google Scholar] [CrossRef]
- Sun, X.; Bandara, N. Applications of reverse micelles technique in food science: A comprehensive review. Trends Food Sci. Technol. 2019, 91, 106–115. [Google Scholar] [CrossRef]
- Burt, H.M.; Zhang, X.; Toleikis, P.; Embree, L.; Hunter, W.L. Development of copolymers of poly(d,l-lactide) and methoxypolyethylene glycol as micellar carriers of paclitaxel. Colloids Surf. B Biointerfaces 1999, 16, 161–171. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, M.; Lu, C.; Liu, D. Polypeptide-based amphiphilic brush copolymers as unimolecular micelles: Synthesis, characterisation, and encapsulation study. Micro Nano Lett. 2018, 13, 1329–1334. [Google Scholar] [CrossRef]
- Civiale, C.; Licciardi, M.; Cavallaro, G.; Giammona, G.; Mazzone, M.G. Polyhydroxyethylaspartamide-based micelles for ocular drug delivery. Int. J. Pharm. 2009, 378, 177–186. [Google Scholar] [CrossRef]
- Lee, R.-S.; Lin, C.-H.; Aljuffali, I.A.; Hu, K.-Y.; Fang, J.-Y. Passive targeting of thermosensitive diblock copolymer micelles to the lungs: Synthesis and characterization of poly(N-isopropylacrylamide)-block-poly(ε-caprolactone). J. Nanobiotechnol. 2015, 13, 42. [Google Scholar] [CrossRef] [Green Version]
- Uji, H.; Watabe, N.; Komi, T.; Sakaguchi, T.; Akamatsu, R.; Mihara, K.; Kimura, S. Downsizing to 25-nm reverse polymeric micelle composed of AB3-type polydepsipeptide with comprising siRNA. Chem. Lett. 2022, 51, 235–238. [Google Scholar] [CrossRef]
- Sharif, B.; Bah, M. The reduction in iodine absorption through rat skin by polymeric micelles in comparison with Povidone-Iodine: An ex-vivo study. Ars Pharm. 2020, 62, 105–112. [Google Scholar] [CrossRef]
- Sung, M.; Shin, D.H.; Lee, H.J.; Jang, K.H.; Shin, K.; Kim, J.W. Enhancing skin permeation of nanoemulsions through associative polymeric micelles-mediated drop-to-skin dipolar interactions. J. Mol. Liq. 2021, 344, 117741. [Google Scholar] [CrossRef]
- Niu, J.; Yuan, M.; Chen, C.; Wang, L.; Tang, Z.; Fan, Y.; Liu, X.; Ma, Y.J.; Gan, Y. Berberine-loaded thiolated pluronic F127 polymeric micelles for improving skin permeation and retention. Int. J. Nanomed. 2020, 15, 9987–10005. [Google Scholar] [CrossRef]













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perumal, S.; Atchudan, R.; Lee, W. A Review of Polymeric Micelles and Their Applications. Polymers 2022, 14, 2510. https://doi.org/10.3390/polym14122510
Perumal S, Atchudan R, Lee W. A Review of Polymeric Micelles and Their Applications. Polymers. 2022; 14(12):2510. https://doi.org/10.3390/polym14122510
Chicago/Turabian StylePerumal, Suguna, Raji Atchudan, and Wonmok Lee. 2022. "A Review of Polymeric Micelles and Their Applications" Polymers 14, no. 12: 2510. https://doi.org/10.3390/polym14122510
APA StylePerumal, S., Atchudan, R., & Lee, W. (2022). A Review of Polymeric Micelles and Their Applications. Polymers, 14(12), 2510. https://doi.org/10.3390/polym14122510