Role of Substrate Type in the Process of Polyelectrolyte Multilayer Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Ellipsometry
2.2.2. Atomic Force Microscopy
2.2.3. Contact Angle Measurements
2.2.4. Streaming Potential Measurements
3. Results and Discussion
3.1. Substrate Characterization
3.1.1. Oxide Layer Thickness
3.1.2. Surface Morphology and Roughness
3.1.3. Zeta Potential
3.1.4. Contact Angles
3.2. Polyelectrolyte Multilayer Formation and Characterization
3.2.1. Multilayer Thickness Obtained by Ellipsometry
3.2.2. Multilayer Thickness Obtained by Atomic Force Microscopy
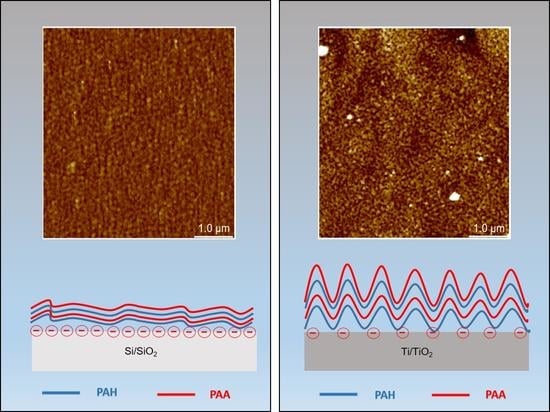
3.2.3. Morphology and Roughness of PEM Surface
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szilagyi, I.; Trefalt, G.; Tiraferri, A.; Maroni, P.; Borkovec, M. Polyelectrolyte adsorption, interparticle forces, and colloidal aggregation. Soft Matter 2014, 10, 2479–2502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaels, A.S. Polyelectrolyte Complexes. Ind. Eng. Chem. 1965, 57, 32–40. [Google Scholar] [CrossRef]
- Spruijt, E.; Leermakers, F.A.M.; Fokkink, R.; Schweins, R.; Van Well, A.A.; Cohen Stuart, M.A.; Van Der Gucht, J. Structure and Dynamics of Polyelectrolyte Complex Coacervates Studied by Scattering of Neutrons, X-rays, and Light. Macromolecules 2013, 46, 4596–4605. [Google Scholar] [CrossRef]
- Petrila, L.-M.; Bucatariu, F.; Mihai, M.; Teodosiu, C. Polyelectrolyte Multilayers: An Overview on Fabrication, Properties, and Biomedical and Environmental Applications. Materials 2021, 14, 4152. [Google Scholar] [CrossRef]
- Guzmán, E.; Rubio, R.G.; Ortega, F. A Closer Physico-Chemical Look to the Layer-by-Layer Electrostatic Self-Assembly of Polyelectrolyte Multilayers. Adv. Colloid Interface Sci. 2020, 282, 102197. [Google Scholar] [CrossRef]
- Jukić, J.; Kovačević, D.; Cindro, N.; Fink, R.; Oder, M.; Milisav, A.-M.; Požar, J. Predicting the outcomes of interpolyelectrolyte neutralization at surfaces on the basis of complexation experiments and vice versa. Soft Matter 2022, 18, 744–754. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Kolasinska, M.; Krastev, R.; Gutberlet, T.; Warszynski, P. Layer-by-Layer Deposition of Polyelectrolytes. Dipping versus Spraying. Langmuir 2009, 25, 1224–1232. [Google Scholar] [CrossRef]
- Schaaf, P.; Voegel, J.C.; Jierry, L.; Boulmedais, F. Spray-Assisted Polyelectrolyte Multilayer Buildup: From Step-by-Step to Single-Step Polyelectrolyte Film Constructions. Adv. Mater. 2012, 24, 1001–1016. [Google Scholar] [CrossRef] [Green Version]
- Kovačević, D.; Van der Burgh, S.; De Keizer, A.; Cohen Stuart, M.A. Specific Ionic Effects on Weak Polyelectrolyte Multilayer Formation. J. Phys. Chem. B 2003, 107, 7998–8002. [Google Scholar] [CrossRef]
- Borges, J.; Mano, J.F. Molecular interactions driving the layer-by-layer assembly of multilayers. Chem. Rev. 2014, 114, 8883–8942. [Google Scholar] [CrossRef] [PubMed]
- Dubas, S.T.; Schlenoff, J.B. Factors Controlling the Growth of Polyelectrolyte Multilayers. Macromolecules 1999, 32, 8153–8160. [Google Scholar] [CrossRef]
- Kovačević, D.; Van der Burgh, S.; De Keizer, A.; Cohen Stuart, M.A. Kinetics of Formation and Dissolution of Weak Polyelectrolyte Multilayers: Role of Salt and Free Polyions. Langmuir 2002, 18, 5607–5612. [Google Scholar] [CrossRef]
- Jukić, J.; Korade, K.; Milisav, A.-M.; Delač Marion, I.; Kovačević, D. Ion-specific and solvent effects on PDADMA-PSS complexation and multilayer formation. Colloids Interfaces 2021, 5, 38. [Google Scholar] [CrossRef]
- Bieker, P.; Schönhoff, M. Linear and exponential growth regimes of multilayers of weak polyelectrolytes in dependence on pH. Macromolecules 2010, 43, 5052–5059. [Google Scholar] [CrossRef]
- Yoo, D.; Shiratori, S.S.; Rubner, M.F. Controlling bilayer composition and surface wettability of sequentially adsorbed multilayers of weak polyelectrolytes. Macromolecules 1998, 31, 4309–4318. [Google Scholar] [CrossRef]
- Shiratori, S.S.; Rubner, M.F. pH-dependent thickness behavior of sequentially adsorbed layers of weak polyelectrolytes. Macromolecules 2000, 33, 4213–4219. [Google Scholar] [CrossRef]
- Barrantes, A.; Santos, O.; Sotres, J.; Arnebrant, T. Influence of pH on the build-up of poly-L-lysine/heparin multilayers. J. Colloid Interface Sci. 2012, 388, 191–200. [Google Scholar] [CrossRef]
- Volodkin, D.; von Klitzing, R. Competing mechanisms in polyelectrolyte multilayer formation and swelling: Polycation–polyanion pairing vs. polyelectrolyte–ion pairing. Curr. Opin. Colloid Interface Sci. 2014, 19, 25–31. [Google Scholar] [CrossRef]
- Bertrand, P.; Jonas, A.; Laschewsky, A.; Legras, R. Ultrathin polymer coatings by complexation of polyelectrolytes at interfaces: Suitable materials, structure and properties. Macromol. Rapid Commun. 2000, 21, 319–348. [Google Scholar] [CrossRef]
- Buron, C.C.; Filiatre, C.; Membrey, F.; Bainier, C.; Charraut, D.; Foissy, A. Effect of substrate on the adsorption of polyelectrolyte multilayers: Study by optical fixed-angle reflectometry and AFM. Colloids Surf. A 2007, 30, 105–111. [Google Scholar] [CrossRef]
- Krasemann, L.; Tieke, B. Ultrathin self-assembled polyelectrolyte membranes for pervaporation. J. Membr. Sci. 1998, 150, 23–30. [Google Scholar] [CrossRef]
- Caruso, F.; Spasova, M.; Susha, A.; Giersig, M.; Caruso, R.A. Magnetic nanocomposite particles and hollow spheres constructed by a sequential layering approach. Chem. Mater. 2001, 13, 109–116. [Google Scholar] [CrossRef]
- Schuetz, P.; Caruso, F. Copper-assisted weak polyelectrolyte multilayer formation on microspheres and subsequent film crosslinking. Adv. Funct. Mater. 2003, 13, 929–937. [Google Scholar] [CrossRef]
- Karabasz, A.; Szczepanowicz, K.; Cierniak, A.; Bereta, J.; Bzowska, M. In vitro toxicity studies of biodegradable, polyelectrolyte nanocapsules. Int. J. Nanomed. 2018, 13, 7835–7844. [Google Scholar] [CrossRef] [Green Version]
- Virga, E.; Žvab, K.; de Vos, W.M. Fouling of Nanofiltration Membranes Based on Polyelectrolyte Multilayers: The Effect of a Zwitterionic Final Layer. J. Membr. Sci. 2021, 620, 118793. [Google Scholar] [CrossRef]
- Kovačević, D.; Pratnekar, R.; Godič Torkar, K.; Salopek, J.; Dražić, G.; Abram, A.; Bohinc, K. Influence of Polyelectrolyte Multilayer Properties on Bacterial Adhesion Capacity. Polymers 2016, 8, 345. [Google Scholar] [CrossRef]
- Bohinc, K.; Bajuk, J.; Jukić, J.; Abram, A.; Oder, M.; Godič Torkar, K.; Raspor, P.; Kovačević, D. Bacterial Adhesion Capacity of Protein-Terminating Polyelectrolyte Multilayers. Int. J. Adhes. Adhes. 2020, 103, 102687. [Google Scholar] [CrossRef]
- Tan, S.Y.; Lee, S.C.; Okazaki, T.; Kuramitz, H.; Abd-Rahman, F. Detection of Mercury (II) Ions in Water by Polyelectrolyte–Gold Nanoparticles Coated Long Period Fiber Grating Sensor. Opt. Commun. 2018, 419, 18–24. [Google Scholar] [CrossRef]
- Habibi, N.; Pastorino, L.; Babolmorad, G.; Ruggiero, C.; Guda, T.; Ong, J.L. Polyelectrolyte Multilayers and Capsules: S-Layer Functionalization for Improving Stability and Biocompatibility. J. Drug Deliv. Sci. Technol. 2017, 38, 1–8. [Google Scholar] [CrossRef]
- Choi, J.; Rubner, M.F. Influence of the Degree of Ionization on Weak Polyelectrolyte Multilayer Assembly. Macromolecules 2005, 38, 116–124. [Google Scholar] [CrossRef]
- Ciddor, P.E. Refractive index of air: New equations for the visible and near infrared. Appl. Opt. 1996, 35, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Malitson, I.H. Interspecimen comparison of the refractive index of fused silica. J. Opt. Soc. Am. 1965, 55, 1205–1209. [Google Scholar] [CrossRef]
- Sarkar, S.; Gupta, V.; Kumar, M.; Schubert, J.; Probst, P.T.; Joseph, J.; König, T.A.F. Hybridized guided-mode resonances via colloidal plasmonic self-assembled grating. Appl. Mater. Interfaces 2019, 11, 13752–13760. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Jewell, C.M.; Fredin, N.J.; Lynn, D.M. Assembly of Multilayered Films Using Well-Defined, End-Labeled Poly(acrylic acid): Influence of Molecular Weight on Exponential Growth in a Synthetic Weak Polyelectrolyte System. Langmuir 2007, 23, 8452–8459. [Google Scholar] [CrossRef]
- Jellison, G.E., Jr. Optical functions of silicon determined by two-channel polarization modulation ellipsometry. Opt. Mater. 1992, 1, 41–47. [Google Scholar] [CrossRef]
- Johnson, P.B.; Christy, R.W. Optical constants of transition metals: Ti, V, Cr, Mn, Fe, Co, Ni, and Pd. Phys. Rev. B 1974, 9, 5056–5070. [Google Scholar] [CrossRef]
- Al-Bayati, A.H.; Orrman-Rossiter, K.G.; van den Berg, J.A.; Armour, D.G. Composition and structure of the native Si oxide by high depth resolution medium energy ion scattering. Surf. Sci. 1991, 241, 91–102. [Google Scholar] [CrossRef]
- Wang, G.; Li, J.; Lv, K.; Zhang, W.; Ding, X.; Yang, G.; Liu, X.; Jiang, X. Surface thermal oxidation on titanium implants to enhance osteogenic activity and in vivo osseointegration. Sci. Rep. 2016, 6, 31769. [Google Scholar] [CrossRef] [Green Version]
- Scales, P.J.; Grieser, F.; Healy, T.W.; White, L.R.; Chan, D.Y.C. Electrokinetics of the Silica-Solution Interface: A Flat Plate Streaming Potential Study. Langmuir 1992, 8, 965–974. [Google Scholar] [CrossRef]
- Kosmulski, M. Surface Charging and Points of Zero Charge; Surfactant Science Series; CRC Press: Boca Raton, FL, USA, 2009; Volume 145, pp. 1–502. [Google Scholar]
- Roessler, S.; Zimmermann, R.; Scharnweber, D.; Werner, C.; Worch, H. Characterization of oxide layers on Ti6Al4V and titanium by streaming potential and streaming current measurements. Colloids Surf. B Biointerfaces 2002, 26, 387–395. [Google Scholar] [CrossRef]
- Wasilewska, M.; Adamczyk, Z.; Sadowska, M.; Boulmedais, F.; Cieśla, M. Mechanisms of fibrinogen adsorption on silica sensors at various pHs: Experiment and theoretical modeling. Langmuir 2019, 35, 11275–11284. [Google Scholar] [CrossRef] [PubMed]
- Brkljača, Z.; Namjesnik, D.; Lützenkirchen, J.; Předota, M.; Preočanin, T. Quartz/Aqueous Electrolyte Solution Interface: Molecular Dynamic Simulation and Interfacial Potential Measurements. J. Phys. Chem. C 2018, 122, 24025–24036. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, G.; Wei, Z.; Sang, L.; Qj, M. Hemocompatibility Evaluation of Polyurethane Film with Surface-Grafted Poly(ethylene glycol) and Carboxymethyl-Chitosan. J. Appl. Polym. Sci. 2012, 127, 308–315. [Google Scholar] [CrossRef]
- Michalski, M.C.; Hardy, J.; Saramago, B.J.V. On the Surface Free Energy of PVC/EVA Polymer Blends: Comparison of Different Calculation Methods. J. Colloid Interface Sci. 1998, 208, 319–328. [Google Scholar] [CrossRef]
- Royaux, A.; El Haitamni, A.; Fichet, O.; Cantin, S. Surface free-energy determination of copper wire using a large range of model liquids. SN Appl. Sci. 2020, 2, 48. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wang, W.; Korpacz, A.N.; Dufour, C.R.; Weiland, Z.J.; Lambert, C.R.; Timko, M.T. Binary Liquid Mixture Contact-Angle Measurements for Precise Estimation of Surface Free Energy. Langmuir 2019, 35, 12317–12325. [Google Scholar] [CrossRef]
- Sui, Z.; Salloum, D.; Schlenoff, J.B. Effect of Molecular Weight on the Construction of Polyelectrolyte Multilayers: Stripping versus Sticking. Langmuir 2003, 19, 2491–2495. [Google Scholar] [CrossRef]
- Micciulla, S.; Dodoo, S.; Chevigny, C.; Laschewsky, A.; von Klitzing, R. Short versus Long Chain Polyelectrolyte Multilayers: A Direct Comparison of Self-Assembly and Structural Properties. Phys. Chem. Chem. Phys. 2014, 16, 21988–21998. [Google Scholar] [CrossRef] [Green Version]
- Salomäki, M.; Tervasmäki, P.; Areva, S.; Kankare, J. The Hofmeister Anion Effect and the Growth of Polyelectrolyte Multilayers. Langmuir 2004, 20, 3679–3683. [Google Scholar] [CrossRef]
- Yu, J.; Meharg, B.M.; Lee, I. Adsorption and Interlayer Diffusion Controlled Growth and Unique Surface Patterned Growth of Polyelectrolyte Multilayers. Polymer 2017, 109, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Trybała, A.; Szyk-Warszynska, L.; Warszynski, P. The effect of anchoring PEI layer on the build-up of polyelectrolyte multilayer films at homogeneous and heterogeneous surface. Colloids Surf. A 2009, 343, 127–132. [Google Scholar] [CrossRef]
- Li, X.; Yan, J.; Yu, T.; Zhang, B. Versatile nonfluorinated superhydrophobic coating with self-cleaning, anti-fouling, anti-corrosion and mechanical stability. Colloids Surf. A 2022, 642, 128701. [Google Scholar] [CrossRef]
- Guan, H.; Huang, S.; Ding, J.; Tian, F.; Xu, Q.; Zhao, J. Chemical environment and magnetic moment effects on point defect formations in CoCrNi-based concentrated solid-solution alloys. Acta Mater. 2020, 187, 122–134. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; Zhang, F.; Lee, C. A Novel Aluminum-Graphite Dual-Ion Battery. Adv. Energy Mater. 2016, 6, 1502588. [Google Scholar] [CrossRef] [Green Version]
- Ji, B.; Zhang, F.; Song, X.; Tang, Y. A Novel Potassium-Ion-Based Dual-Ion Battery. Adv. Mater. 2017, 29, 1700519. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, C.; Zhang, S.; Song, X.; Tang, Y.; Cheng, H. Reversible calcium alloying enables a practical room-temperature rechargeable calcium-ion battery with a high discharge voltage. Nat. Chem. 2018, 10, 667–672. [Google Scholar] [CrossRef]
- Gong, D.; Wei, C.; Xie, D.; Tang, Y. Ultrasmall antimony nanodots embedded in carbon nanowires with three-dimensional porous structure for high-performance potassium dual-ion batteries. Chem. Eng. J. 2022, 431, 133444. [Google Scholar] [CrossRef]









| Metal Substrate | (d ± SE)/nm |
|---|---|
| Si | 1.92 ± 0.02 |
| Ti | 6.70 ± 0.02 |
| Liquid | References | |||
|---|---|---|---|---|
| Deionised water | 51.0 | 21.8 | 72.8 | [45] |
| Glycerol | 26.4 | 37.0 | 63.4 | [46] |
| Toluene | 2.1 | 25.7 | 27.8 | [47] |
| Formamide | 19.0 | 39.0 | 58.0 | [48] |
| N,N-Dimethylformamide | 8.1 | 29.0 | 37.1 | [47] |
| Dimethyl sulfoxide | 8.0 | 36.0 | 44.0 | [48] |
| Metal Substrate | delips/nm | dAFM/nm |
|---|---|---|
| Si | 10.1 ± 0.1 | 9.42 ± 0.1 |
| Ti | 30.5 ± 1.0 | 27.8 ± 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesić, M.; Klačić, T.; Abram, A.; Bohinc, K.; Kovačević, D. Role of Substrate Type in the Process of Polyelectrolyte Multilayer Formation. Polymers 2022, 14, 2566. https://doi.org/10.3390/polym14132566
Mesić M, Klačić T, Abram A, Bohinc K, Kovačević D. Role of Substrate Type in the Process of Polyelectrolyte Multilayer Formation. Polymers. 2022; 14(13):2566. https://doi.org/10.3390/polym14132566
Chicago/Turabian StyleMesić, Mia, Tin Klačić, Anže Abram, Klemen Bohinc, and Davor Kovačević. 2022. "Role of Substrate Type in the Process of Polyelectrolyte Multilayer Formation" Polymers 14, no. 13: 2566. https://doi.org/10.3390/polym14132566
APA StyleMesić, M., Klačić, T., Abram, A., Bohinc, K., & Kovačević, D. (2022). Role of Substrate Type in the Process of Polyelectrolyte Multilayer Formation. Polymers, 14(13), 2566. https://doi.org/10.3390/polym14132566