Construction, Physical Properties and Foaming Behavior of High-Content Lignin Reinforced Low-Density Polyethylene Biocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical Modification of Pristine Lignin
2.3. Preparation of Lignin-Reinforced LDPE Biocomposites
2.4. Foaming of Lignin-Reinforced LDPE Biocomposites
2.5. Characterization
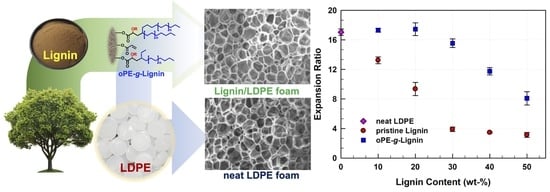
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mao, Y.; Qi, R. Preparation of polyethylene-octene elastomer foams by compression molding. J. Appl. Polym. Sci. 2008, 109, 3249–3255. [Google Scholar] [CrossRef]
- Eaves, D. Polymer Foams Trends in Use and Technology; Rapra Technology Limited: Shawbury, UK, 2001; pp. 75–94. [Google Scholar]
- Rodríguez-Pérez, M.A. Crosslinked polyolefin foams: Production, structure, properties, and applications. Adv. Polym. Sci. 2005, 184, 97–126. [Google Scholar]
- Zhao, C.; Mark, L.H.; Kim, S.; Chang, E.; Park, C.B.; Lee, P.C. Recent progress in micro-/nano-fibrillar reinforced polymeric composite foams. Polym. Eng. Sci. 2021, 61, 936–941. [Google Scholar] [CrossRef]
- Koopmans, R.J.; den Doelder, J.C.F.; Paquet, A.N. Modeling foam growth in thermoplastics. Adv. Mater. 2000, 12, 1873–1880. [Google Scholar] [CrossRef]
- Gua, P.; Xu, Y.; Lu, M.; Zhang, S. Expanded linear low-density polyethylene beads: Fabrication, melt strength, and foam morphology. Ind. Eng. Chem. Res. 2016, 55, 8104–8113. [Google Scholar] [CrossRef]
- Rochman, C.M.; Browne, M.A.; Halpern, B.S.; Hentschel, B.T.; Hoh, E.; Karapanagioti, H.K.; Rios-Mendoza, L.M.; Takada, H.; Teh, S.; Thompson, R.C. Classify plastic waste as hazardous. Nature 2013, 494, 169–170. [Google Scholar] [CrossRef]
- Zhou, A.; Zhang, Y.; Xie, S.; Chen, Y.; Li, X.; Wang, J.; Zou, J. Microplastics and their potential effects on the aquaculture systems: A critical review. Rev. Aquacult. 2020, 13, 719–733. [Google Scholar] [CrossRef]
- Claessens, M.; Cauwenberghe, L.; Vandegehuchte, M.B.; Janssen, C.R.; Janssen, C.R. New techniques for the detection of microplastics in sediments and field collected organisms. Mar. Pollut. Bull. 2013, 70, 227–233. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Luckachan, G.E.; Pillai, C.K.S. Biodegradable polymers-a review on recent trends and emerging perspectives. J. Polym. Environ. 2011, 19, 637–676. [Google Scholar]
- Sam, S.T.; Nuradibah, M.A.; Ismail, H.; Noriman, N.Z.; Ragunathan, S. Recent advances in polyolefins/natural polymer blends used for packaging application. Polym.-Plast. Technol. Mater. 2014, 53, 631–644. [Google Scholar] [CrossRef]
- Liu, X.; Gao, C.; Sangwan, P.; Yu, L.; Tong, Z. Accelerating the degradation of polyolefins through additives and blending. J. Appl. Polym. Sci. 2014, 131, 40750. [Google Scholar] [CrossRef]
- Wožniak-Braszak, A.; Knitter, M.; Markiewicz, E.; Ingramm, W.F.; Spontak, R.J. Effect of composition on the molecular dynamics of biodegradable isotactic polypropylene/thermoplastic starch blends. ACS Sustain. Chem. Eng. 2019, 7, 16050–16059. [Google Scholar] [CrossRef]
- George, A.; Sanjay, M.R.; Srisuk, R.; Parameswaranpillai, J.; Siengchin, S. A comprehensive review on chemical properties and applications of biopolymers and their composites. Int. J. Biol. Macromol. 2020, 154, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Wang, M.; Zhou, F.; Luo, H.; Xie, X.; Luo, F.; Cha, R. A review on nanocellulose as lightweight filler of polyolefin composites. Carbohydr. Polym. 2020, 243, 116466. [Google Scholar] [CrossRef]
- Jubinville, D.; Esmizadeh, E.; Saikrishnan, S.; Tzonganakis, C.; Mekonnen, T. A comprehensive review of global production and recycling methods of polyolefin (PO) based products and their post-recycling applications. Sustain. Mater. Technol. 2020, 25, e00188. [Google Scholar] [CrossRef]
- Hong, S.-H.; Hwang, S.-H. Construction and foamability of lignin-reinforced low-density polyethylene biocomposites. Mater. Today Commun. 2021, 28, 102696. [Google Scholar] [CrossRef]
- Scholten, P.B.V.; Özen, M.B.; Söyler, Z.; Thomassin, J.-M.; Whlhelm, M.; Detrembleur, C.; Meier, M.A.R. Rheological and mechanical properties of cellulose/LEDP composites using sustainable and fully renewable compatibilisers. J. Appl. Polym. Sci. 2020, 137, 48744. [Google Scholar] [CrossRef]
- Zykova, A.K.; Pantyukhov, P.V.; Mastalygina, E.E.; Chaverri-Ramos, C.; Nikolaeva, S.G.; Saavedra-Arias, J.J.; Popov, A.A.; Wortman, S.E.; Poletto, M. Biocomposites of low-density polyethylene plus wood flour or flax straw: Biodegradation kinetics across three environments. Polymers 2021, 13, 2138. [Google Scholar] [CrossRef]
- Xiong, S.-J.; Pang, B.; Zhou, S.-J.; Li, M.-K.; Yang, S.; Wang, Y.-Y.; Shi, Q.; Wang, S.-F.; Yuan, T.-Q.; Sun, R.-C. Economically competitive biodegradable PBAT/lignin composites: Effect of lignin methylation and compatibilizer. ACS Sustain. Chem. Eng. 2020, 8, 5338–5346. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, Z.; Xu, X.; Liu, X.; Liu, L.; Huang, G.; Liu, L.; Wang, H.; Song, P. Grafting lignin with bioderived polyacrylates for low-cost, ductile, and fully biobased poly(lactic acid) composites. ACS Sustain. Chem. Eng. 2020, 8, 2267–2276. [Google Scholar] [CrossRef]
- Liu, L.; Huang, G.; Song, P.; Yu, Y.; Fu, S. Converting industrial alkali lignin to biobased functional additives for improving fire behavior and smoke suppression of polybutylene succinate. ACS Sustain. Chem. Eng. 2016, 4, 4732–4742. [Google Scholar] [CrossRef]
- Borges da Silva, E.A.; Zabkova, M.; Araujo, J.D.; Cateto, C.A.; Barreiro, M.R.; Belgacem, M.N.; Rodrigues, A.E. An integrated process to produce vanillin and lignin-based polyurethanes from kraft lignin. Chem. Eng. Res. Des. 2009, 87, 1276–1292. [Google Scholar] [CrossRef]
- Chakar, F.S.; Ragauskas, A.J. Review of current and future softwood kraft lignin process chemistry. Ind. Crop. Prod. 2004, 20, 131–141. [Google Scholar] [CrossRef]
- Hong, S.-H.; Park, J.H.; Kim, O.Y.; Hwang, S.-H. Preparation of chemically modified lignin-reinforced PLA biocomposites and their 3D printing performance. Polymers 2021, 13, 667. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.-H.; Kim, D.-H.; Park, D.H.; Kim, O.Y.; Hwang, S.-H. Construction of sustainable polyurethane-based gel-coats containing poly(e-caprolactone)-grafted lignin and their coating performance. Prog. Org. Coat. 2018, 120, 234–239. [Google Scholar] [CrossRef]
- Yeo, J.-S.; Lee, J.-H.; Hwang, S.-H. Effects of lignin on the volume shrinkage and mechanical properties of a styrene/unsaturated polyester/lignin ternary composite system. Compos. Part B Eng. 2017, 130, 167–173. [Google Scholar] [CrossRef]
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A concise review of current lignin production, applications, products and their environmental impact. Ind. Crops Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Collins, M.N.; Nechifor, M.; Tanãasa, F.; Zanoaga, M.; Mcloughlin, A.; Stroózyk, M.A.; Culebras, M.; Teaca, C.-A. Valorization of lignin in polymer and composite systems for advanced engineering applications-A review. Int. J. Biol. Macromol. 2019, 131, 828–849. [Google Scholar] [CrossRef]
- Szalaty, T.J.; Klapiszewski, L.; Jesionowski, T. Recent developments in modification of lignin using ionic liquids for the fabrication of advanced materials-A review. J. Mol. Liq. 2020, 301, 112417. [Google Scholar] [CrossRef]
- Awad, S.A. Mechanical and thermal characterisations of low-density polyethylene/nanoclay composite. Polym. Polym. Compos. 2021, 29, 1325–1332. [Google Scholar] [CrossRef]
- Khanam, P.N.; AlMaadeed, M.A.A. Processing and characterization of polyethylene-based compostes. Adv. Manuf. Polym. Compos. Sci. 2015, 1, 63–79. [Google Scholar]
- Ferreira, F.V.; Trindade, G.N.; Bernardes, J.S.; Gouveia, R.F. LDPE-based composites reinforced with surface modified cellulose fibres: 3D morphological and morphometrical analyses to understand the improved mechanical performance. Eur. Polym. J. 2019, 117, 105–113. [Google Scholar] [CrossRef]
- Li, S.; Huang, A.; Chen, Y.-J.; Li, D.; Turng, L.-S. Highly filled biochar/ultra-high molecular weight polyethylene/linear low density polyethylene composites for high-performance electromagnetic interference shielding. Compos. Part B Eng. 2018, 153, 277–284. [Google Scholar] [CrossRef]
- Phiri, M.M.; Sibeko, M.A.; Phiri, M.J.; Hlangothi, S.P. Effect of free foaming and pre-curing on the thermal, morphological and physical properties of reclaimed tyre rubber foam composites. J. Clean. Prod. 2018, 218, 665–672. [Google Scholar] [CrossRef]
- Spina, R. Technological characterization of PE/EVA blends for foam injection molding. Mater. Des. 2015, 84, 64–71. [Google Scholar] [CrossRef]
- Moscoso-Sánchez, F.J.; Mendizábal, E.; Jasso-Gastinel, C.F.; Ortega-Gudiño, P.; Robledo-Ortíz, J.R.; González-Núñez, R.; Rodrigue, D. Morphological and mechanical characterization of foamed polyethylene via biaxial rotational molding. J. Cell. Plast. 2015, 51, 489–503. [Google Scholar] [CrossRef]
- Bernardo, V.; Laguan-Gutierrez, E.; Lopez-Gil, A.; Rodriguez-Perez, M.A. Highly anisotropic crosslined HDPE foams with a controlled anisotropy ratio: Production and characterization of the cellular structure and mechanical properties. Mater. Des. 2017, 114, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Laguna-Gutierrez, E.; Escudero, J.; Rodrigue-Perez, M.A. Analysis of the mechanical properties and effective diffusion coefficient under static creep loading of low-density foams based on polyethylene/clays nanocomposites. Compos. Part B Eng. 2018, 148, 156–165. [Google Scholar] [CrossRef]
- Ito, A.; Semba, T.; Ohshima, M. Effect of crosslinking points on bubble nucleation in the microcellular foaming of thermosets. Polymer 2021, 215, 123414. [Google Scholar] [CrossRef]
- Petchwattana, N.; Sirijutaratana, C. Influences of particle sizes and contents of chemical blowing agents on foaming wood plastic composite prepared from poly(vinyl chloride) and rice hull. Mater. Des. 2011, 32, 2844–2850. [Google Scholar] [CrossRef]
- Kun, D.; Pukánszky, B. Polymer/lignin blends: Interaction, properties, applications. Eur. Polym. J. 2017, 93, 618–641. [Google Scholar] [CrossRef] [Green Version]
- Roy, K.; Debnath, S.C.; Potlyaraj, P. A review on recent trends and future prospects of lignin based green rubber composites. J. Polym. Environ. 2020, 28, 367–387. [Google Scholar] [CrossRef]
- Alonso, M.V.; Rodriguez, J.J.; Oliet, M.; Rodriguez, F.; Garcia, J.; Gilarranz, M.A. Characterization and structural modification of ammonic lignosulfonate by methylation. J. Appl. Polym. Sci. 2001, 82, 2661–2668. [Google Scholar] [CrossRef]
- Gilli, E.; Schmied, F.; Diebald, S.; Horvath, A.T.; Teichert, C.; Schennach, R. Analysis of lignin precipitates on ozone treated kraft pulp by FTIR and AFM. Cellulose 2012, 19, 249–256. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, Y.; Fan, J.; Chang, J. Novel method for production of phenolics by combining lignin extraction with lignin depolymerization in aqueous ethanol. Ind. Eng. Chem. Res. 2012, 51, 103–110. [Google Scholar] [CrossRef]
- Diop, A.; Awada, H.; Zerrouki, R.; Daneault, C.; Montplaisir, D. Tosylation and characterization of lignin in water. Ind. Eng. Chem. Res. 2014, 53, 16771–16776. [Google Scholar] [CrossRef]
- Lisperguer, J.; Perez, P.; Urizar, S. Structure and thermal properties of lignins: Characterization by infrared spectroscopy and differential scanning calorimetry. J. Chil. Chem. Soc. 2009, 54, 460–463. [Google Scholar] [CrossRef] [Green Version]
- Hatekeyama, T.; Liu, Z. Handbook of Thermal Analyses; John Wiley Sons: New York, NY, USA, 1999. [Google Scholar]
- Sailaja, R.R.N.; Deepthi, M.V. Mechanical and thermal properties of compatibilized composites of polyethylene and esterified lignin. Mater. Des. 2010, 31, 4369–4379. [Google Scholar] [CrossRef]
- Diop, A.; Mijiyawa, F.; Koffi, D.; Kokta, B.V.; Montplaisir, D. Study of lignin dispersion in low-density polyethylene. J. Thermoplast. Compos. Mater. 2015, 28, 1662–1674. [Google Scholar] [CrossRef]
- Doroudiani, S.; Kortschot, M.T. Expanded wood fiber polystyrene composites: Processing-structure-mechanical properties relationships. J. Thermoplast. Compos. Mater. 2004, 17, 13–30. [Google Scholar] [CrossRef]








| Sample ID | Tm (°C) | Tc (°C) | ΔHm (J/g) | ΔHc (J/g) | Td * (°C) | |
|---|---|---|---|---|---|---|
| neat LDPE | 113.4 | 90.1 | 75.3 | 87.1 | 25.7 | 465 |
| Lignin (10 wt-%) | 113.4 | 90.2 | 70 | 87.6 | 26.5 | 489 |
| Lignin (20 wt-%) | 113.9 | 89.8 | 64.1 | 74.2 | 27.3 | 482 |
| Lignin (30 wt-%) | 114.5 | 88.4 | 53.6 | 64.3 | 26.1 | 475 |
| Lignin (40 wt-%) | 113 | 90.6 | 45.5 | 55.7 | 25.8 | 471 |
| Lignin (50 wt-%) | 114.1 | 89.4 | 37 | 46.5 | 25.3 | 457 |
| oPE-g-lignin (10 wt-%) | 113.6 | 92.1 | 80.6 | 90.3 | 30.5 | 490 |
| oPE-g-lignin (20 wt-%) | 113.1 | 93.1 | 79.9 | 82.4 | 34.1 | 483 |
| oPE-g-lignin (30 wt-%) | 113.2 | 94.9 | 75.4 | 79.6 | 36.8 | 480 |
| oPE-g-lignin (40 wt-%) | 114.3 | 95.6 | 67.8 | 73.8 | 38.6 | 477 |
| oPE-g-lignin (50 wt-%) | 114.7 | 95.4 | 58.6 | 68.7 | 40 | 469 |
| Sample ID | Apparent Density | Ave. Cell Diameter | Cell Density |
|---|---|---|---|
| (×102 g/cm3) | (μm) | (×105 Cell/cm3) | |
| neat LDPE | 5.48 ± 0.19 | 151.15 ± 27.42 | 4.2 |
| Lignin (10 wt-%) | 7.01 ± 0.33 | 81.58 ± 22.84 | 22 |
| Lignin (20 wt-%) | 10.15 ± 1.09 | 40.07 ± 15.08 | 79 |
| Lignin (30 wt-%) | 25.74 ± 2.05 | 46.69 ± 17.65 | 110 |
| Lignin (40 wt-%) | 28.87 ± 0.81 | 42.63 ± 21.46 | 131 |
| Lignin (50 wt-%) | 32.11 ± 3.33 | 20.92 ± 17.33 | 229 |
| oPE-g-lignin (10 wt-%) | 5.36 ± 0.05 | 152.54 ± 31.04 | 4.6 |
| oPE-g-lignin (20 wt-%) | 5.37 ± 0.33 | 141.56 ± 24.13 | 5.3 |
| oPE-g-lignin (30 wt-%) | 6.17 ± 0.29 | 67.60 ± 18.71 | 33 |
| oPE-g-lignin (40 wt-%) | 8.20 ± 0.61 | 72.09 ± 16.49 | 46 |
| oPE-g-lignin (50 wt-%) | 11.76 ± 1.95 | 44.86 ± 14.69 | 78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.-H.; Hwang, S.-H. Construction, Physical Properties and Foaming Behavior of High-Content Lignin Reinforced Low-Density Polyethylene Biocomposites. Polymers 2022, 14, 2688. https://doi.org/10.3390/polym14132688
Hong S-H, Hwang S-H. Construction, Physical Properties and Foaming Behavior of High-Content Lignin Reinforced Low-Density Polyethylene Biocomposites. Polymers. 2022; 14(13):2688. https://doi.org/10.3390/polym14132688
Chicago/Turabian StyleHong, Seo-Hwa, and Seok-Ho Hwang. 2022. "Construction, Physical Properties and Foaming Behavior of High-Content Lignin Reinforced Low-Density Polyethylene Biocomposites" Polymers 14, no. 13: 2688. https://doi.org/10.3390/polym14132688
APA StyleHong, S. -H., & Hwang, S. -H. (2022). Construction, Physical Properties and Foaming Behavior of High-Content Lignin Reinforced Low-Density Polyethylene Biocomposites. Polymers, 14(13), 2688. https://doi.org/10.3390/polym14132688