A Review on the Modification of Cellulose and Its Applications
Abstract
:1. Introduction
1.1. Cellulose Source
1.2. Chemical Structure of Cellulose
1.3. Advantages of Cellulose Materials
1.4. Limitations of Traditional Cellulose Materials
1.5. Advantages of CNC to Overcome the Limitations
1.6. Recent Advantages on Cellulose Research
1.7. Cellulose Extraction
2. Different Techniques of Cellulose Modification
2.1. Cellulose Surface Modification
2.2. Reaction with Carboxylic Acid Groups
2.3. Alkyne–Azide Reaction
2.4. Diels–Alder Cycloaddition
2.5. Photo-Thiol-Ene Reaction
2.6. Isotropically Modified Cellulose
2.7. Cellulose in Ionic Liquids by Esterification and Acylation
2.8. Cellulose Modification by Etherification
2.9. Carbanilation
2.10. Cellulose Toxicity
2.11. Cellulose Modification Toxicity with Small Functional Groups
3. Grafting
4. Process and Product Toxicity
5. Design for Degradation
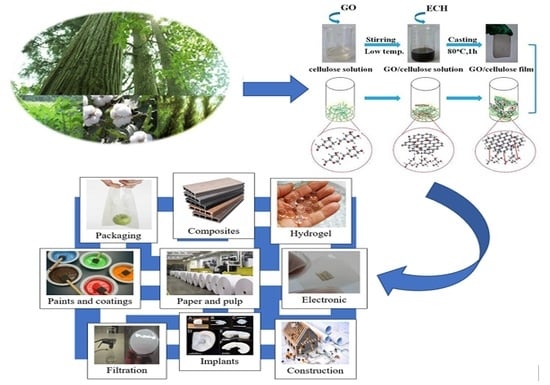
6. Applications of Cellulose
6.1. Metal Adsorption
6.2. Surface-Enhanced Raman Scattering
6.3. Antioxidant
6.4. Bio-Sensors
6.5. Enzyme Immobilization
6.6. Separation of Protein
6.7. Antimicrobial Activity
6.8. Conductivity
6.9. Other Applications
6.10. Advantages and Disadvantages of Cellulose
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, G.; Zhao, H.Q.; Liu, Q.; Fan, Z. A review on biodegradable biliary stents: Materials and future trends. Bioact. Mater. 2022, 17, 488–495. [Google Scholar] [CrossRef]
- Ahmed, R.; Siddiqui, S.I.; Al Alwan, B.; Almesfer, M.; Khanna, M.K.; Fatima, B.; Mishra, R.; Ansari, M.A.; Oh, S. Biodegradable acid based nanocomposite-CuO-ZnO-Ni(OH)2/PA: A novel material for water cleansing. J. Clean. Prod. 2022, 341, 130860. [Google Scholar] [CrossRef]
- Dalmazzo, D.; Carrión, A.J.D.B.; Tsantilis, L.; Presti, D.L.; Santagata, E. Non- petroleum- based binders for paving applications: Rheological and chemical investigation on ageing effects. In Proceedings of the 5th International Symposium on Asphalt Pavements & Environment (Ape), Padua, Italy, 11–13 September 2019; Volume 48, pp. 67–76. [Google Scholar] [CrossRef]
- Arun, R.; Shruthy, R.; Preetha, R.; Sreejit, V. Biodegradable nano composite reinforced with cellulose nano fiber from coconut industry waste for replacing synthetic plastic food packaging. Chemosphere 2021, 291, 132786. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Dong, F.; Yu, Y.; Wang, Q.; Wang, P.; Fan, X.; Yuan, J. Green modification of cellulose-based natural materials by HRP-initiated controlled “graft from” polymerization. Int. J. Biol. Macromol. 2020, 164, 1237–1245. [Google Scholar] [CrossRef]
- Garcia, A.; Gandini, A.; Belgacem, N.; Bras, J. Modification of nanocellulose with natural molecules: A green perspective for cellulose based materials with active properties. In Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 2015; Volume 249. [Google Scholar]
- Alam, I.; Kumar, J.; Sharma, C. Preparation of regenerated cellulose from rice straw lignocellulosic waste and its use for reinforced paper products. Tappi J. 2021, 20, 439–451. [Google Scholar] [CrossRef]
- Aziz, T.; Fan, H.; Haq, F.; Khan, F.U.; Numan, A.; Ullah, A.; Wazir, N. Facile modification and application of cellulose nanocrystals. Iran. Polym. J. 2019, 28, 707–724. [Google Scholar] [CrossRef]
- Aziz, T.; Fan, H.; Zhang, X.; Khan, F.U.; Fahad, S.; Ullah, A. Adhesive properties of bio-based epoxy resin reinforced by cellulose nanocrystal additives. J. Polym. Eng. 2020, 40, 314–320. [Google Scholar] [CrossRef]
- Walzl, A.; Kopacic, S.; Bauer, W.; Leitner, E. Characterization of natural polymers as functional barriers for cellulose-based packaging materials. Food Addit. Contam. Part A 2019, 36, 976–988. [Google Scholar] [CrossRef] [PubMed]
- Hubbe, M.A. Energy efficiency—A particular challenge for the cellulose-based products industries. BioResources 2021, 16. [Google Scholar] [CrossRef]
- Jusic, J.; Tamantini, S.; Romagnoli, M.; Vinciguerra, V.; Di Mattia, E.; Zikeli, F.; Cavalera, M.; Mugnozza, G.S. Improving sustainability in wood coating: Testing lignin and cellulose nanocrystals as additives to commercial acrylic wood coatings for bio-building. iForest 2021, 14, 499–507. [Google Scholar] [CrossRef]
- García-Gutiérrez, P.; Cuéllar-Franca, R.M.; Reed, D.; Dowson, G.; Styring, P.; Azapagic, A. Environmental sustainability of cellulose-supported solid ionic liquids for CO2 capture. Green Chem. 2019, 21, 4100–4114. [Google Scholar] [CrossRef] [Green Version]
- Ge, L.; Yin, J.; Yan, D.; Hong, W.; Jiao, T. Construction of Nanocrystalline Cellulose-Based Composite Fiber Films with Excellent Porosity Performances via an Electrospinning Strategy. ACS Omega 2021, 6, 4958–4967. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.P.; Godinho, M.H.; Martins, A.F.; Trindade, A.C.; Belgacem, M.N. Cellulose-Based Composite Films. Polym. Mech. 2001, 37, 257–264. [Google Scholar] [CrossRef]
- Xu, A.; Wang, Y.; Xu, X.; Xiao, Z.; Liu, R. A Clean and Sustainable Cellulose-Based Composite Film Reinforced with Waste Plastic Polyethylene Terephthalate. Adv. Mater. Sci. Eng. 2020, 2020, 7323521. [Google Scholar] [CrossRef]
- George, J.; N, S.S. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shayestehkia, M.; Khademieslam, H.; Bazyar, B.; Rangavar, H.; Taghiyari, H.R. Effects of cellulose nanocrystals as extender on physical and mechanical properties of wood cement composite panels. BioResources 2020, 15. [Google Scholar] [CrossRef]
- Aziz, T.; Zheng, J.; Jamil, M.I.; Fan, H.; Ullah, R.; Iqbal, M.; Ali, A.; Khan, F.U.; Ullah, A. Enhancement in Adhesive and Thermal Properties of Bio-based Epoxy Resin by Using Eugenol Grafted Cellulose Nanocrystals. J. Inorg. Organomet. Polym. Mater. 2021, 31, 3290–3300. [Google Scholar] [CrossRef]
- Aziz, T.; Ullah, A.; Ali, A.; Shabeer, M.; Shah, M.N.; Haq, F.; Iqbal, M.; Ullah, R.; Khan, F.U. Manufactures of bio-degradable and bio-based polymers for bio-materials in the pharmaceutical field. J. Appl. Polym. Sci. 2022, 139. [Google Scholar] [CrossRef]
- Fernandes, I.D.A.A.; Pedro, A.C.; Ribeiro, V.R.; Bortolini, D.G.; Ozaki, M.S.C.; Maciel, G.M.; Haminiuk, C.W.I. Bacterial cellulose: From production optimization to new applications. Int. J. Biol. Macromol. 2020, 164, 2598–2611. [Google Scholar] [CrossRef]
- Hu, L.; Zhong, Y.; Wu, S.; Wei, P.; Huang, J.; Xu, D.; Zhang, L.; Ye, Q.; Cai, J. Biocompatible and biodegradable super-toughness regenerated cellulose via water molecule-assisted molding. Chem. Eng. J. 2021, 417, 129229. [Google Scholar] [CrossRef]
- Jagadeeswaran, G.; Gainey, L.; Prade, R.; Mort, A.J. A family of AA9 lytic polysaccharide monooxygenases in Aspergillus nidulans is differentially regulated by multiple substrates and at least one is active on cellulose and xyloglucan. Appl. Microbiol. Biotechnol. 2016, 100, 4535–4547. [Google Scholar] [CrossRef]
- Aziz, T.; Fan, H.; Zhang, X.; Khan, F.U. Synergistic impact of cellulose nanocrystals and calcium sulfate fillers on adhesion behavior of epoxy resin. Mater. Res. Express 2019, 6, 1150b7. [Google Scholar] [CrossRef]
- Aziz, T.; Mehmood, S.; Haq, F.; Ullah, R.; Khan, F.U.; Ullah, B.; Raheel, M.; Iqbal, M.; Ullah, A. Synthesis and modification of silica-based epoxy nanocomposites with different sol–gel process enhanced thermal and mechanical properties. J. Appl. Polym. Sci. 2021, 138, 51191. [Google Scholar] [CrossRef]
- Zheng, J.; Aziz, T.; Fan, H.; Haq, F.; Khan, F.U.; Ullah, R.; Ullah, B.; Khattak, N.S.; Wei, J. Synergistic impact of cellulose nanocrystals with multiple resins on thermal and mechanical behavior. Z. Phys. Chem. 2020, 235, 1247–1262. [Google Scholar] [CrossRef]
- Haq, F.; Mehmood, S.; Haroon, M.; Kiran, M.; Waseem, K.; Aziz, T.; Farid, A. Role of Starch Based Materials as a Bio-sorbents for the Removal of Dyes and Heavy Metals from Wastewater. J. Polym. Environ. 2021, 30, 1730–1748. [Google Scholar] [CrossRef]
- Xia, G.; Zhou, Q.; Xu, Z.; Zhang, J.; Ji, X.; Zhang, J.; Nawaz, H.; Wang, J.; Peng, J. Cellulose-Based Films with Ultraviolet Shielding Performance Prepared Directly from Waste Corrugated Pulp. Polymers 2021, 13, 3359. [Google Scholar] [CrossRef]
- Wu, X.; Moon, R.J.; Martini, A. Crystalline cellulose elastic modulus predicted by atomistic models of uniform deformation and nanoscale indentation. Cellulose 2013, 20, 43–55. [Google Scholar] [CrossRef]
- Ullah, R.; Azam, A.; Aziz, T.; Rehman, H.U.; Qiao, S.; Hameed, A. Peacock Feathers Extract Use as Template for Synthesis of Ag and Au Nanoparticles and Their Biological Applications. Waste Biomass-Valorization 2021, 13, 659–666. [Google Scholar] [CrossRef]
- Aziz, T.; Fan, H.; Khan, F.U.; Ullah, R.; Haq, F.; Iqbal, M.; Ullah, A. Synthesis of Carboxymethyl Starch-Bio-Based Epoxy Resin and their Impact on Mechanical Properties. Z. Phys. Chem. 2019, 234, 1759–1769. [Google Scholar] [CrossRef]
- Nishino, T.; Takano, K.; Nakamae, K. Elastic modulus of the crystalline regions of cellulose polymorphs. J. Polym. Sci. Part B: Polym. Phys. 1995, 33, 1647–1651. [Google Scholar] [CrossRef]
- Li, C.; Fan, H.; Aziz, T.; Bittencourt, C.; Wu, L.; Wang, D.-Y.; Dubois, P. Biobased Epoxy Resin with Low Electrical Permissivity and Flame Retardancy: From Environmental Friendly High-Throughput Synthesis to Properties. ACS Sustain. Chem. Eng. 2018, 6, 8856–8867. [Google Scholar] [CrossRef]
- Khattak, N.S.; Ahmad, A.S.; Shah, L.A.; Ara, L.; Farooq, M.; Sohail, M.; Kadir, S.I. Thermal and Rheological Study of Nanocomposites, Reinforced with Bi-Phase Ceramic Nanoparticles. Z. Phys. Chem. 2018, 233, 1233–1246. [Google Scholar] [CrossRef]
- Baussanne, I.; Bras, J.; Watbled, B.; Demeunynck, M. The advantages and challenges raised by the chemistry of aldehydic cellulose nanofibers in medicinal chemistry. Futur. Med. Chem. 2018, 10, 2679–2683. [Google Scholar] [CrossRef]
- Santamala, H.; Livingston, R.; Sixta, H.; Hummel, M.; Skrifvars, M.; Saarela, O. Advantages of regenerated cellulose fibres as compared to flax fibres in the processability and mechanical performance of thermoset composites. Compos. Part A Appl. Sci. Manuf. 2016, 84, 377–385. [Google Scholar] [CrossRef]
- Scerrino, G.; Paladino, N.C.; Di Paola, V.; Morfino, G.; Amodio, E.; Gulotta, G.; Bonventre, S. The use of haemostatic agents in thyroid surgery: Efficacy and further advantages. Collagen-Fibrinogen-Thrombin Patch (CFTP) versus Cellulose Gauze. Ann Ital. Chir. 2013, 84, 545–550. [Google Scholar] [PubMed]
- Asif, M.; Ahmed, D.; Ahmad, N.; Qamar, M.T.; Alruwaili, N.K.; Bukhari, S.N.A. Extraction and Characterization of Microcrystalline Cellulose from Lagenaria siceraria Fruit Pedicles. Polymers 2022, 14, 1867. [Google Scholar] [CrossRef]
- Nagarajan, K.J.; Sanjay, M.R.; Basha, S.K.; Raghav, G.R.; Kumar, A.R.; Siengchin, S.; Rajan, S.B.; Nath, S.P.; Anish, K. Extraction of cellulose nanocrystals from red banana peduncle agro-waste and application in environmentally friendly biocomposite film. Polym. Compos. 2022. [Google Scholar] [CrossRef]
- Nasution, H.; Yahya, E.B.; Khalil, H.P.S.A.; Shaah, M.A.; Suriani, A.B.; Mohamed, A.; Alfatah, T.; Abdullah, C.K. Extraction and Isolation of Cellulose Nanofibers from Carpet Wastes Using Supercritical Carbon Dioxide Approach. Polymers 2022, 14, 326. [Google Scholar] [CrossRef]
- Lou, C.; Zhou, Y.; Yan, A.; Liu, Y. Extraction cellulose from corn-stalk taking advantage of pretreatment technology with immobilized enzyme. RSC Adv. 2021, 12, 1208–1215. [Google Scholar] [CrossRef]
- Khan, R.; Jolly, R.; Fatima, T.; Shakir, M. Extraction processes for deriving cellulose: A comprehensive review on green approaches. Polym. Adv. Technol. 2022, 33, 2069–2090. [Google Scholar] [CrossRef]
- Gopakumar, D.; Malaysia, U.; Thomas, M.; Thomas, M.; Khalil, A.; Folahan Abdul-Wahab Taiwo, O. Nanocellulose Based Aerogels for Varying Engineering Applications. Renew. Sustain. Mater. 2020, 2, 155–165. [Google Scholar] [CrossRef]
- Culica, M.E.; Rotaru, R.; Bejan, D.; Coroaba, A.; Mohan, T.; Coseri, S. Cellulose surface modification for improved attachment of carbon nanotubes. Cellulose 2022, 29, 6057–6076. [Google Scholar] [CrossRef]
- Zhu, H.; Han, Z.; Cheng, J.-H.; Sun, D.-W. Modification of cellulose from sugarcane (Saccharum officinarum) bagasse pulp by cold plasma: Dissolution, structure and surface chemistry analysis. Food Chem. 2021, 374, 131675. [Google Scholar] [CrossRef]
- Zhang, T.; Lang, J.; Liu, L.; Liu, L.; Li, H.; Gu, Y.; Yan, X.; Ding, X. Effect of carboxylic acid groups on the supercapacitive performance of functional carbon frameworks derived from bacterial cellulose. Chin. Chem. Lett. 2017, 28, 2212–2218. [Google Scholar] [CrossRef]
- Isogai, A. TEMPO-catalyzed oxidation of polysaccharides. Polym. J. 2021, 54, 387–402. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, S.P.; Zaman, M.; Mohammed, N.; Brinatti, C.; Batmaz, R.; Berry, R.; Loh, W.; Tam, K.C. Synthesis of amine functionalized cellulose nanocrystals: Optimization and characterization. Carbohydr. Res. 2015, 409, 48–55. [Google Scholar] [CrossRef]
- Selim, A.; Neethu, K.M.; Gowri, V.; Sartaliya, S.; Kaur, S.; Jayamurugan, G. Thiol-Functionalized Cellulose Wrapped Copperoxide as a Green Nano Catalyst for Regiospecific Azide-Alkyne Cycloaddition Reaction: Application in Rufinamide Synthesis. Asian J. Org. Chem. 2021, 10, 3428–3433. [Google Scholar] [CrossRef]
- Bahsis, L.; Ben El Ayouchia, H.; Anane, H.; Benhamou, K.; Kaddami, H.; Julve, M.; Stiriba, S.-E. Cellulose-copper as bio-supported recyclable catalyst for the clickable azide-alkyne [3 + 2] cycloaddition reaction in water. Int. J. Biol. Macromol. 2018, 119, 849–856. [Google Scholar] [CrossRef]
- Goldmann, A.S.; Tischer, T.; Barner, L.; Bruns, M.; Barner-Kowollik, C. Mild and Modular Surface Modification of Cellulose via Hetero Diels−Alder (HDA) Cycloaddition. Biomacromolecules 2011, 12, 1137–1145. [Google Scholar] [CrossRef]
- Hufendiek, A.; Trouillet, V.; Meier, M.A.R.; Barner-Kowollik, C. Temperature Responsive Cellulose-graft-Copolymers via Cellulose Functionalization in an Ionic Liquid and RAFT Polymerization. Biomacromolecules 2014, 15, 2563–2572. [Google Scholar] [CrossRef] [PubMed]
- Schenzel, A.; Hufendiek, A.; Barner-Kowollik, C.; Meier, M.A.R. Catalytic transesterification of cellulose in ionic liquids: Sustainable access to cellulose esters. Green Chem. 2014, 16, 3266–3271. [Google Scholar] [CrossRef]
- Ali, A.; Nadeem, M.; Lu, J.; Moradian, J.M.; Rasheed, T.; Aziz, T.; Maouche, C.; Guo, Y.; Awais, M.; Zhiqiang, F.; et al. Rapid kinetic evaluation of homogeneous single-site metallocene catalysts and cyclic diene: How do the catalytic activity, molecular weight, and diene incorporation rate of olefins affect each other? RSC Adv. 2021, 11, 31817–31826. [Google Scholar] [CrossRef] [PubMed]
- Daneshfar, Z.; Rostami, A. Cellulose sulfonic acid as a green, efficient, and reusable catalyst for Nazarov cyclization of unactivated dienones and pyrazoline synthesis. RSC Adv. 2015, 5, 104695–104707. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Xiao, H.; James, T.D. Fluorescence Sensing with Cellulose-Based Materials. ChemistryOpen 2017, 6, 685–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Q.; Han, W.; Li, X.; Jiang, Y.; Zhao, C. New insights into the autofluorescence properties of cellulose/nanocellulose. Sci. Rep. 2020, 10, 21387. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Y.; Zhang, L.; Zhong, Y.; Xu, H.; Mao, Z.; Wang, B.; Sui, X. Thiol–ene click reaction on cellulose sponge and its application for oil/water separation. RSC Adv. 2017, 7, 20147–20151. [Google Scholar] [CrossRef] [Green Version]
- Çiğil, A.B.; Urucu, O.A.; Birtane, H.; Kahraman, M.V. Cellulose/cysteine based thiol-ene UV cured adsorbent: Removal of silver (I) ions from aqueous solution. Cellulose 2021, 28, 6439–6448. [Google Scholar] [CrossRef]
- Yang, Q.; Pan, X.; Huang, F.; Li, K. Synthesis and characterization of cellulose fibers grafted with hyperbranched poly(3-methyl-3-oxetanemethanol). Cellulose 2011, 18, 1611–1621. [Google Scholar] [CrossRef]
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic Liquids and Cellulose: Dissolution, Chemical Modification and Preparation of New Cellulosic Materials. Int. J. Mol. Sci. 2014, 15, 11922–11940. [Google Scholar] [CrossRef]
- Barthel, S.; Heinze, T. Acylation and carbanilation of cellulose in ionic liquids. Green Chem. 2005, 8, 301–306. [Google Scholar] [CrossRef]
- Liu, C.F.; Zhang, A.P.; Li, W.Y.; Sun, R.C. Modification of cellulose in ionic liquid with NBS as a catalyst. In Proceedings of the Second International Papermaking and Environment Conference, Tianjin, China, 14–16 May 2008; pp. 250–254. [Google Scholar]
- Kakuchi, R.; Ito, R.; Nomura, S.; Abroshan, H.; Ninomiya, K.; Ikai, T.; Maeda, K.; Kim, H.J.; Takahashi, K. A mechanistic insight into the organocatalytic properties of imidazolium-based ionic liquids and a positive co-solvent effect on cellulose modification reactions in an ionic liquid. RSC Adv. 2017, 7, 9423–9430. [Google Scholar] [CrossRef] [Green Version]
- Olaru, N.; Ciolacu, D.; Tampu, D.; Olaru, L. Structural modifications of cellulose in heterogeneous acetylation process. J. Optoelectron. Adv. Mater. 2007, 9, 3917–3920. [Google Scholar]
- Gan, L.; Liao, J.; Lin, N.; Hu, C.; Wang, H.; Huang, J. Focus on Gradientwise Control of the Surface Acetylation of Cellulose Nanocrystals to Optimize Mechanical Reinforcement for Hydrophobic Polyester-Based Nanocomposites. ACS Omega 2017, 2, 4725–4736. [Google Scholar] [CrossRef] [PubMed]
- Kowhakul, W.; Shibahara, H.; Masamoto, H.; Shigematsu, M. Dust explosion characteristics of cellulose ethers and cellulose acetates with various degrees of acetylation. J. Loss Prev. Process Ind. 2016, 44, 544–550. [Google Scholar] [CrossRef]
- Larina, V.N.; Ur’Yash, V.F.; Kushch, D.S. Thermochemical characteristics of cellulose acetates with different degrees of acetylation. Russ. J. Phys. Chem. A 2012, 86, 1776–1778. [Google Scholar] [CrossRef]
- Abushammala, H.; Mao, J. A Review of the Surface Modification of Cellulose and Nanocellulose Using Aliphatic and Aromatic Mono- and Di-Isocyanates. Molecules 2019, 24, 2782. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wen, X.; Zhang, X.; Liu, C.-F. Acetylation of Microcrystalline Cellulose by Transesterification in AmimCl/DMSO Cosolvent System. Molecules 2017, 22, 1419. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zhang, J.; Zhang, H.; He, J.; Ren, Q.; Guo, M. Homogeneous Acetylation of Cellulose in a New Ionic Liquid. Biomacromolecules 2004, 5, 266–268. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Zhang, J.; He, J.S. Homogeneous acetylation and regioselectivity of cellulose in a new ionic liquid. Chem. J. Chin. Univ.-Chin. 2006, 27, 592–594. [Google Scholar]
- Raza, A.; Farrukh, S.; Hussain, A.; Khan, I.; Othman, M.; Ahsan, M. Performance Analysis of Blended Membranes of Cellulose Acetate with Variable Degree of Acetylation for CO2/CH4 Separation. Membranes 2021, 11, 245. [Google Scholar] [CrossRef] [PubMed]
- Lease, J.; Kawano, T.; Andou, Y. Esterification of Cellulose with Long Fatty Acid Chain through Mechanochemical Method. Polymers 2021, 13, 4397. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.; Pham, Q.T. A theoretical and experimental study on etherification of primary alcohols with the hydroxyl groups of cellulose chain (n = 1–3) in acidic condition. J. Mol. Struct. 2021, 1236, 130314. [Google Scholar] [CrossRef]
- Fox, S.C.; Li, B.; Xu, D.; Edgar, K.J. Regioselective Esterification and Etherification of Cellulose: A Review. Biomacromolecules 2011, 12, 1956–1972. [Google Scholar] [CrossRef]
- Hu, H.; You, J.; Gan, W.; Zhou, J.; Zhang, L. Synthesis of allyl cellulose in NaOH/urea aqueous solutions and its thiol–ene click reactions. Polym. Chem. 2015, 6, 3543–3548. [Google Scholar] [CrossRef]
- Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Cellulose-Based Hydrogels as Sustained Drug-Delivery Systems. Materials 2020, 13, 5270. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Aderibigbe, B.A. Chitosan and Cellulose-Based Hydrogels for Wound Management. Int. J. Mol. Sci. 2020, 21, 9656. [Google Scholar] [CrossRef] [PubMed]
- Srirachya, N.; Boonkerd, K.; Kobayashi, T. Effective elongation properties of cellulose–natural rubber composite hydrogels having interconnected domain. J. Elastomers Plast. 2019, 52, 337–355. [Google Scholar] [CrossRef]
- Sannino, A.; Esposito, A.; Nicolais, L.; Del Nobile, M.A.; Giovane, A.; Balestrieri, C.; Agresti, M.; Esposito, R. Cellulose-based hydrogels as body water retainers. J. Mater. Sci. Mater. Electron. 2000, 11, 247–253. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, Q.; Zhang, Z.; Wang, Z.; Huang, M. Chiral separations with crosslinked cellulose derivatives attached onto hybrid silica monolith particles via the thiol–ene click reaction. Anal. Methods 2020, 12, 2727–2734. [Google Scholar] [CrossRef]
- Huang, J.-L.; Li, C.-J.; Gray, D.G. Functionalization of cellulose nanocrystal films via “thiol–ene” click reaction. RSC Adv. 2014, 4, 6965–6969. [Google Scholar] [CrossRef]
- Mortha, G.; Marlin, N.; Das, S.; Dallerac, D.; Lachenal, D.; Berrima, B.; Elaloui, L.; Balaguer, S. Novel investigations on cellulose heterogeneous carbanilation using Sec/Ri/Ls/Viscometry. In Proceedings of the 16th International Symposium on Wood, Fiber and Pulping Chemistry, Tianjin, China, 8–10 June 2011; Volumes I & II, pp. 384–390. [Google Scholar]
- Zhang, Y.J.; Dong, C.X.; Chen, J.; Liu, R.Q. Cellulose Tris(3,5-Dimethylphenylcarbamate) Regioselectively Bonded to Small Pore Silica Gel as Chiral Stationary Phase for HPLC. Appl. Mech. Mater. 2011, 117–119, 1361–1364. [Google Scholar] [CrossRef]
- Buchanan, C.; Guzman-Morales, E.; Wang, B. Regioselectively substituted cellulose benzoate propionates for compensation film in optical displays. Carbohydr. Polym. 2020, 252, 117146. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Yamamoto, M.; Kasai, W.; Morita, M. Chapter 13: Synthesis and properties of regioselectively substituted cellulose cinnamates. In Polysaccharide Materials: Performance by Design; American Chemical Society: Washington, DC, USA, 2009; Volume 1017, pp. 231–241. [Google Scholar]
- Xu, M.; Li, T.; Zhang, S.; Li, W.; He, J.; Yin, C. Preparation and characterization of cellulose carbamate membrane with high strength and transparency. J. Appl. Polym. Sci. 2020, 138. [Google Scholar] [CrossRef]
- Tabaght, F.E.; El Idrissi, A.; Aqil, M.; Benahemad, A.; El Barkany, S.; Bellaouch, R.; Asehraou, A. Synthesis and characterization of (thio)carbamates based on cellulose and cellulose acetate: Biodegradation and solubility studies. Cellul. Chem. Technol. 2020, 54, 207–223. [Google Scholar] [CrossRef]
- Zheng, K.; Zhou, S.; Cheng, Z.; Huang, G. Thin-film composite forward osmosis membrane prepared from polyvinyl chloride/cellulose carbamate substrate and its potential application in brackish water desalination. J. Appl. Polym. Sci. 2020, 138. [Google Scholar] [CrossRef]
- Henniges, U.; Kloser, E.; Patel, A.; Potthast, A.; Kosma, P.; Fischer, M.; Fischer, K.; Rosenau, T. Studies on DMSO-containing carbanilation mixtures: Chemistry, oxidations and cellulose integrity. Cellulose 2007, 14, 497–511. [Google Scholar] [CrossRef]
- Simões, A.M.; Venâncio, C.; Alves, L.; Antunes, F.E.; Lopes, I. Hydrophobic modifications of hydroxyethyl cellulose polymers: Their influence on the acute toxicity to aquatic biota. J. Hazard. Mater. 2020, 409, 124966. [Google Scholar] [CrossRef]
- Ventura, C.; Pinto, F.; Lourenço, A.F.; Ferreira, P.; Louro, H.; Silva, M.J. On the toxicity of cellulose nanocrystals and nanofibrils in animal and cellular models. Cellulose 2020, 27, 5509–5544. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, L.; Zheng, S.; Chai, S.; Wei, J.; Zhong, L.; He, Y.; Xue, J. Bacteriostatic activity and cytotoxicity of bacterial cellulose-chitosan film loaded with in-situ synthesized silver nanoparticles. Carbohydr. Polym. 2021, 281, 119017. [Google Scholar] [CrossRef]
- Alexandrescu, L.; Syverud, K.; Gatti, A.; Chinga-Carrasco, G. Cytotoxicity tests of cellulose nanofibril-based structures. Cellulose 2013, 20, 1765–1775. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, J.; Wen, L.; Yang, C.; Zhang, L. A multi-functional-group modified cellulose for enhanced heavy metal cadmium adsorption: Performance and quantum chemical mechanism. Chemosphere 2019, 224, 509–518. [Google Scholar] [CrossRef]
- Colacino, K.R.; Arena, C.B.; Dong, S.; Roman, M.; Davalos, R.V.; Lee, Y.W. Folate Conjugated Cellulose Nanocrystals Potentiate Irreversible Electroporation-induced Cytotoxicity for the Selective Treatment of Cancer Cells. Technol. Cancer Res. Treat. 2014, 14, 757–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajpai, S.; Chand, N.; Ahuja, S.; Roy, M. Curcumin/cellulose micro crystals/chitosan films: Water absorption behavior and in vitro cytotoxicity. Int. J. Biol. Macromol. 2015, 75, 239–247. [Google Scholar] [CrossRef]
- Kumar, V.; Kang, J.; Hohl, R.J. Improved Dissolution and Cytotoxicity of Camptothecin Incorporated into Oxidized-Cellulose Microspheres Prepared by Spray Drying. Pharm. Dev. Technol. 2001, 6, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Nima, J.; Divya, P.L. Synthesis, characterization and in vitro cytotoxicity analysis of a novel cellulose based drug carrier for the controlled delivery of 5-fluorouracil, an anticancer drug. Appl. Surf. Sci. 2015, 355, 64–73. [Google Scholar] [CrossRef]
- Wang, H.; Sun, F.; Qu, Z.; Wang, K.; Wang, L.; Pi, X.; Gao, J.; Zhao, G. Oxygen Functional Group Modification of Cellulose-Derived Hard Carbon for Enhanced Sodium Ion Storage. ACS Sustain. Chem. Eng. 2019, 7, 18554–18565. [Google Scholar] [CrossRef]
- Vieyra, H.; Juárez, E.; López, U.F.; Morales, A.G.; Torres, M. Cytotoxicity and biocompatibility of biomaterials based in polyhydroxybutyrate reinforced with cellulose nanowhiskers determined in human peripheral leukocytes. Biomed. Mater. 2018, 13, 045011. [Google Scholar] [CrossRef]
- Liyanage, S.; Acharya, S.; Parajuli, P.; Shamshina, J.L.; Abidi, N. Production and Surface Modification of Cellulose Bioproducts. Polymers 2021, 13, 3433. [Google Scholar] [CrossRef]
- Sezer, U.A.; Sahin, I.; Aru, B.; Olmez, H.; Demirel, G.Y.; Sezer, S. Cytotoxicity, bactericidal and hemostatic evaluation of oxidized cellulose microparticles: Structure and oxidation degree approach. Carbohydr. Polym. 2019, 219, 87–94. [Google Scholar] [CrossRef]
- Kumari, P.; Pathak, G.; Gupta, R.; Sharma, D.; Meena, A. Cellulose nanofibers from lignocellulosic biomass of lemongrass using enzymatic hydrolysis: Characterization and cytotoxicity assessment. DARU J. Pharm. Sci. 2019, 27, 683–693. [Google Scholar] [CrossRef]
- Chen, Y.M.; Xi, T.F.; Zheng, Y.D.; Wan, Y.Z. In Vitro Cytotoxicity Study of the Nano-Hydroxyapatite/Bacterial Cellulose Nanocomposites. Mater. Sci. Forum 2009, 610–613, 1011–1016. [Google Scholar] [CrossRef]
- Velayati, M.; Hassani, H.; Darroudi, M. Green synthesis of Se-Nanorods using Poly Anionic Cellulose (PAC) and examination of their photocatalytic and cytotoxicity effects. Inorg. Chem. Commun. 2021, 133, 108935. [Google Scholar] [CrossRef]
- Neves, R.M.; Ornaghi, H.L.; Duchemin, B.; Zattera, A.J.; Amico, S.C. Grafting amount and structural characteristics of microcrystalline cellulose functionalized with different aminosilane contents. Cellulose 2022, 29, 3209–3224. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Qi, Y.-P.; Shen, Y.-F.; Li, H. Application of carboxymethyl cellulose-acrylate-OVPSS graft copolymer emulsion in paper reinforcement and protection. Nord. Pulp Pap. Res. J. 2022, 37, 300–310. [Google Scholar] [CrossRef]
- Lu, S.; Li, J.; Liu, F.; Chen, M.; Na, H.; Zhu, J. Impact of DBU on the synthesis of cellulose-graft-poly(l-lactide) copolymer in CO2 switchable solvent with different grafting strategies. Polymer 2021, 229, 124020. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Y.; Cui, B.; Wang, W. Biocomposites of Polylactic Acid Reinforced by DL-Lactic Acid-Grafted Microfibrillated Cellulose. J. Renew. Mater. 2022, 10, 1–12. [Google Scholar] [CrossRef]
- Luo, R.; Dong, J.; Li, X.; Luo, Y. Coassembly behavior and kinetics of cellulose nanocrystals and pH-responsive diblock copolymers PMMA-b-PDEAEMA at oil/water interfaces and applied on the liquid tubule formation. Colloid Polym. Sci. 2020, 298, 419–433. [Google Scholar] [CrossRef]
- Mohammadbagheri, Z.; Rahmati, A.; Hoshyarmanesh, P. Synthesis of a novel superabsorbent with slow-release urea fertilizer using modified cellulose as a grafting agent and flexible copolymer. Int. J. Biol. Macromol. 2021, 182, 1893–1905. [Google Scholar] [CrossRef]
- Wohlhauser, S.; Rader, C.; Weder, C. Facile Method to Determine the Molecular Weight of Polymer Grafts Grown from Cellulose Nanocrystals. Biomacromolecules 2022, 23, 699–707. [Google Scholar] [CrossRef]
- Tufail, A.; Price, W.E.; Mohseni, M.; Pramanik, B.K.; Hai, F.I. A critical review of advanced oxidation processes for emerging trace organic contaminant degradation: Mechanisms, factors, degradation products, and effluent toxicity. J. Water Process Eng. 2020, 40, 101778. [Google Scholar] [CrossRef]
- Soeiro, V.S.; Silva-Carvalho, R.; Martins, D.; Parpot, P.; Grotto, D.; Chaud, M.V.; da Gama, F.M.P.; Jozala, A.F. Alginate-amphotericin B nanocomplexes covered by nanocrystals from bacterial cellulose: Physico-chemical characterization and in vitro toxicity. Sci. Rep. 2021, 11, 23944. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.L.; Su, Y.-F.; Dill, J.A.; Turnier, J.C.; Westerberg, R.B.; Smith, C.S. Chemical and physical characteristics of cellulose insulation particulates, and evaluation of potential acute pulmonary toxicity. Am. J. Ind. Med. 2004, 46, 554–569. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Xie, Y.; Zeng, G.; Tang, L.; Liang, J.; He, Q.; Zhao, F.; Zeng, Y.; Wu, Y. The dual effects of carboxymethyl cellulose on the colloidal stability and toxicity of nanoscale zero-valent iron. Chemosphere 2015, 144, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, L.; De France, K.J.; Milz, C.I.; Siqueira, G.; Zimmermann, T.; Nyström, G. Sustainable Cellulose Nanofiber Films from Carrot Pomace as Sprayable Coatings for Food Packaging Applications. ACS Sustain. Chem. Eng. 2021, 10, 342–352. [Google Scholar] [CrossRef]
- Dirpan, A.; Djalal, M.; Kamaruddin, I. Application of an Intelligent Sensor and Active Packaging System Based on the Bacterial Cellulose of Acetobacter xylinum to Meat Products. Sensors 2022, 22, 544. [Google Scholar] [CrossRef]
- Du, L.; Arnholt, K.; Ripp, S.; Sayler, G.; Wang, S.; Liang, C.; Wang, J.; Zhuang, J. Biological toxicity of cellulose nanocrystals (CNCs) against the luxCDABE-based bioluminescent bioreporter Escherichia coli 652T7. Ecotoxicology 2015, 24, 2049–2053. [Google Scholar] [CrossRef]
- Xu, A.; Wang, Y.; Gao, J.; Wang, J. Facile fabrication of a homogeneous cellulose/polylactic acid composite film with improved biocompatibility, biodegradability and mechanical properties. Green Chem. 2019, 21, 4449–4456. [Google Scholar] [CrossRef]
- Bin Li, Y.; Zhang, Q.; Sun, Y. Improving Biogas Production and Biodegradability of Acid Hydrolytic Cotton Stalk with Addition of Nutritive Salts and Complex Anaerobic Cellulose Decomposing Bacteria. Adv. Mater. Res. 2013, 724–725, 383–390. [Google Scholar] [CrossRef]
- Torlopov, M.A.; Drozd, N.N.; Paderin, N.M.; Tarabukin, D.V.; Udoratina, E.V. Hemocompatibility, biodegradability and acute toxicity of acetylated cellulose nanocrystals of different types in comparison. Carbohydr. Polym. 2021, 269, 118307. [Google Scholar] [CrossRef]
- Celik, S.; Demirag, A.D.; Ozel, A.E.; Akyuz, S. Molecular Structure, Molecular Docking and Absorption, Distribution, Metabolism, Excretion and Toxicity study of cellulose II. J. Chin. Chem. Soc. 2021, 68, 1250–1262. [Google Scholar] [CrossRef]
- Zhao, X.F.; Ye, J.; Xiong, J.A. CELL 42-Cellulose dissolution in [C4mim]Cl. In Abstracts of Papers of The American Chemical Society; American Chemical Society: Washington, DC, USA, 2007; Volume 233, pp. 655–656. [Google Scholar]
- Pedersen, J.N.; Pérez, B.; Guo, Z. Stability of cellulase in ionic liquids: Correlations between enzyme activity and COSMO-RS descriptors. Sci. Rep. 2019, 9, 17479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seabra, A.B.; Bernardes, J.S.; Fávaro, W.J.; Paula, A.J.; Durán, N. Cellulose nanocrystals as carriers in medicine and their toxicities: A review. Carbohydr. Polym. 2018, 181, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Chen, J.; Guo, W.; Ma, F.; Sun, S.; Zhou, Q. Anti-solvents tuning cellulose nanoparticles through two competitive regeneration routes. Cellulose 2018, 25, 4513–4523. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, W.; Kong, F.; Lin, M.; Mustapha, A. Cellulose nanofibril/silver nanoparticle composite as an active food packaging system and its toxicity to human colon cells. Int. J. Biol. Macromol. 2019, 129, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Al Hokayem, K.; El Hage, R.; Svecova, L.; Otazaghine, B.; Le Moigne, N.; Sonnier, R. Flame Retardant-Functionalized Cotton Cellulose Using Phosphonate-Based Ionic Liquids. Molecules 2020, 25, 1629. [Google Scholar] [CrossRef] [Green Version]
- Hirosawa, K.; Fujii, K.; Hashimoto, K.; Shibayama, M. Solvated Structure of Cellulose in a Phosphonate-Based Ionic Liquid. Macromolecules 2017, 50, 6509–6517. [Google Scholar] [CrossRef]
- Egea-Corbacho, A.; Martín-García, A.P.; Franco, A.A.; Albendín, G.; Arellano, J.M.; Rodríguez, R.; Quiroga, J.M.; Coello, M.D. A method to remove cellulose from rich organic samples to analyse microplastics. J. Clean. Prod. 2021, 334, 130248. [Google Scholar] [CrossRef]
- Martínez, M.G.; Couce, A.A.; Dupont, C.; Perez, D.D.S.; Thiéry, S.; Meyer, X.-M.; Gourdon, C. Torrefaction of cellulose, hemicelluloses and lignin extracted from woody and agricultural biomass in TGA-GC/MS: Linking production profiles of volatile species to biomass type and macromolecular composition. Ind. Crop. Prod. 2021, 176, 114350. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, W.; Blasiak, W. Modeling Study of Woody Biomass: Interactions of Cellulose, Hemicellulose, and Lignin. Energy Fuels 2011, 25, 4786–4795. [Google Scholar] [CrossRef]
- Matson, T.D.; Barta, K.; Iretskii, A.V.; Ford, P.C. One-Pot Catalytic Conversion of Cellulose and of Woody Biomass Solids to Liquid Fuels. J. Am. Chem. Soc. 2011, 133, 14090–14097. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.X.; Duan, C.; Li, J.G.; Liu, Y.S.; Ma, X.J.; Zheng, L.Q.; Stavik, J.; Ni, Y.H. Cellulose (Dissolving Pulp) Manufacturing Processes and Properties: A Mini-Review. Bioresources 2016, 11, 5553–5564. [Google Scholar] [CrossRef]
- Morgan, D.L.; Dill, J.A.; Su, Y.; Westerberg, B.; Price, H.C.; Shines, C.J.; Smith, C.S. Evaluation of the chemical and physical properties of cellulose insulation aerosols and the potential acute pulmonary toxicity. Toxicol. Sci. 2003, 72, 44. [Google Scholar]
- Seraji, H.R.; Karimi, M.; Mahmoudi, L. In situ monitoring the change of mechanical response induced by the diffusion of saline water in glassy cellulose acetate. Desalination 2017, 420, 191–207. [Google Scholar] [CrossRef]
- Timko, M.; Tompsett, G. In situ Raman Microscopy to monitor changes in cellulose crystallinity during acid pretreatment. In Proceedings of the 2017 AIChE Annual Meeting, Minneapolis, MN, USA, 29 October–3 November 2017; Volume 253. [Google Scholar]
- Khazanov, N.; Iline-Vul, T.; Noy, E.; Goobes, G.; Senderowitz, H. Design of Compact Biomimetic Cellulose Binding Peptides as Carriers for Cellulose Catalytic Degradation. J. Phys. Chem. B 2016, 120, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, H.; Ikeuchi, A.; Kim, D.-M.; Ishigaki, Y.; Asano, H.; Kouda, K.; Kumagai, I.; Umetsu, M. Biomass-binding peptides designed by molecular evolution for efficient degradation of cellulose in biomass by cellulase. Green Chem. 2013, 15, 365–369. [Google Scholar] [CrossRef]
- Toja, F.; Toniolo, L.; Nevin, A.; Comelli, D.; Lazzari, M. The degradation of cellulose acetate: Advanced analytical tools for non-destructive study of design objects. In Science and Technology for the Conservation of Cultural Heritage; CRC Press: Boca Raton, FL, USA, 2013; pp. 103–107. [Google Scholar]
- Tavker, N.; Sharma, M. Designing of waste fruit peels extracted cellulose supported molybdenum sulfide nanostructures for photocatalytic degradation of RhB dye and industrial effluent. J. Environ. Manag. 2019, 255, 109906. [Google Scholar] [CrossRef] [PubMed]
- Bã©Guin, P.; Aubert, J.-P. The biological degradation of cellulose. FEMS Microbiol. Rev. 1994, 13, 25–58. [Google Scholar] [CrossRef]
- Chauvigne-Hines, L.M.; Lewis, M.P.; Wilkins, M.J.; Hofstad, B.A.; Shutthanandan, J.; Callister, S.J.; Culley, D.E.; Magnuson, J.K.; Smith, R.D.; Wright, A.T. Characterization of functionally active cellulose degrading enzymes in aerobic and anaerobic organisms and complex microbial communities using chemical proteome profiling. In Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 2011; p. 242. [Google Scholar]
- Velicka, R.; Rimkeviciene, M.; Kriauciuniene, Z.; Pupaliene, R.; Salina, O. The Effect of Cellulose-Degrading Micro-Organisms on the Biodestruction of Crop Residues in the Soil. Zemdirbyste 2009, 96, 113–126. [Google Scholar]
- Deshmukh, K.; Ahamed, M.B.; Deshmukh, R.; Pasha, S.K.K.; Sadasivuni, K.K.; Polu, A.R.; Ponnamma, D.; AlMaadeed, M.A.-A.; Chidambaram, K. Newly developed biodegradable polymer nanocomposites of cellulose acetate and Al2O3 nanoparticles with enhanced dielectric performance for embedded passive applications. J. Mater. Sci. Mater. Electron. 2016, 28, 973–986. [Google Scholar] [CrossRef]
- Sato, H.; Suttiwijitpukdee, N.; Hashimoto, T.; Ozaki, Y. Simultaneous Synchrotron SAXS/WAXD Study of Composition Fluctuations, Cold-Crystallization, and Melting in Biodegradable Polymer Blends of Cellulose Acetate Butyrate and Poly(3-hydroxybutyrate). Macromolecules 2012, 45, 2783–2795. [Google Scholar] [CrossRef]
- Suttiwijitpukdee, N.; Sato, H.; Zhang, J.; Hashimoto, T.; Ozaki, Y. Intermolecular interactions and crystallization behaviors of biodegradable polymer blends between poly (3-hydroxybutyrate) and cellulose acetate butyrate studied by DSC, FT-IR, and WAXD. Polymer 2011, 52, 461–471. [Google Scholar] [CrossRef]
- Selvakumar, M.; Bhat, D.K. LiClO4doped cellulose acetate as biodegradable polymer electrolyte for supercapacitors. J. Appl. Polym. Sci. 2008, 110, 594–602. [Google Scholar] [CrossRef]
- Chen, C.-C.; Wang, C.-C. Crosslinking of cotton cellulose with succinic acid in the presence of titanium dioxide nano-catalyst under UV irradiation. J. Sol-Gel Sci. Technol. 2006, 40, 31–38. [Google Scholar] [CrossRef]
- De Campos, E.A.; de Campos, S.D.; Roos, A.A.; de Souza, B.V.C.; Schneider, J.M.; Uliana, M.B.; de Oliveira, R.C. Titanium Dioxide Dispersed on Cellulose Acetate and its Application in Methylene Blue Photodegradation. Polym. Polym. Compos. 2013, 21, 423–430. [Google Scholar] [CrossRef]
- Gao, C.; Wan, Y.; He, F.; Liang, H.; Luo, H.; Han, J. Mechanical, moisture absorption, and photodegradation behaviors of bacterial cellulose nanofiber- reinforced unsaturated polyester composites. Adv. Polym. Technol. 2011, 30, 249–256. [Google Scholar] [CrossRef]
- Lin, T.; Liao, Y.; Lee, K.; Chang, Y.; Hsu, K.; Hsu, J.; Wu, M. Organic Solvent Resistant Nanocomposite Films Made from Self-precipitated Ag/TiO 2 Nanofibers and Cellulose Nanofiber for Harmful Volatile Organic Compounds Photodegradation. Adv. Mater. Interfaces 2021, 8, 2101467. [Google Scholar] [CrossRef]
- Puls, J.; Wilson, S.A.; Hölter, D. Degradation of Cellulose Acetate-Based Materials: A Review. J. Polym. Environ. 2010, 19, 152–165. [Google Scholar] [CrossRef] [Green Version]
- Rohani, M.; Bamoharram, F.F.; Khosravi, M.; Baharara, J.; Heravi, M.M. Preparation and characterisation of Preyssler heteropolyacid-cellulose acetate hybrid nanofibers: A new, green and recyclable nanocatalyst for photodegradation of methyl orange as the model dye. J. Exp. Nanosci. 2016, 12, 1–13. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Ferreira, L.F.V.; Da Silva, J.P.; Moreira, J.C. Surface photochemistry: Photodegradation study of pyrene adsorbed onto microcrystalline cellulose and silica. Int. J. Photoenergy 2004, 6, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Filho, J.A.F.; Rosolen, R.R.; Almeida, D.A.; de Azevedo, P.H.C.; Motta, M.L.L.; Aono, A.H.; dos Santos, C.A.; Horta, M.A.C.; de Souza, A.P. Trends in biological data integration for the selection of enzymes and transcription factors related to cellulose and hemicellulose degradation in fungi. 3 Biotech 2021, 11, 475. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.G.; Crouch, L.; Labourel, A.; Forsberg, Z.; Bukhman, Y.; Vaaje-Kolstad, G.; Gilbert, H.J.; Keating, D.H. Systems biology defines the biological significance of redox-active proteins during cellulose degradation in an aerobic bacterium. Mol. Microbiol. 2014, 94, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Asiri, A.M.; Pugliese, V.; Petrosino, F.; Khan, S.B.; Alamry, K.A.; Alfifi, S.Y.; Marwani, H.M.; Alotaibi, M.M.; Mukherjee, D.; Chakraborty, S. Photocatalytic Degradation of Textile Dye on Blended Cellulose Acetate Membranes. Polymers 2022, 14, 636. [Google Scholar] [CrossRef]
- Bierhalz, A.C.K. Cellulose Nanomaterials in Textile Applications. Cellul. Chem. Technol. 2021, 55, 725–741. [Google Scholar] [CrossRef]
- Darabi, S.; Hummel, M.; Rantasalo, S.; Rissanen, M.; Månsson, I.; Hilke, H.; Hwang, B.; Skrifvars, M.; Hamedi, M.M.; Sixta, H.; et al. Green Conducting Cellulose Yarns for Machine-Sewn Electronic Textiles. ACS Appl. Mater. Interfaces 2020, 12, 56403–56412. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Endo, T. Deacetylation behavior of binary blend films of cellulose acetate and various polymers. J. Appl. Polym. Sci. 2006, 100, 1816–1823. [Google Scholar] [CrossRef]
- Homem, N.C.; Amorim, M.T.P. Synthesis of cellulose acetate using as raw material textile wastes. Mater. Today: Proc. 2020, 31, S315–S317. [Google Scholar] [CrossRef]
- Felgueiras, C.; Azoia, N.G.; Gonçalves, C.; Gama, M.; Dourado, F. Trends on the Cellulose-Based Textiles: Raw Materials and Technologies. Front. Bioeng. Biotechnol. 2021, 9, 608826. [Google Scholar] [CrossRef]
- Moreira, A.C.G.; Manrique, Y.A.; Martins, I.M.; Simões, M.G.; Carreira, A.S.; Simões, P.N.; Rodrigues, A.E.; Lopes, J.C.B.; Dias, M.M. Continuous production of cellulose acetate microspheres for textile impregnation using a mesostructured reactor. Cellulose 2022, 29, 3595–3612. [Google Scholar] [CrossRef]
- Wan, J.; Hu, R.; Li, J.; Mi, S.; Xian, J.; Xiao, Z.; Liu, Z.; Mei, A.; Xu, S.; Fan, M.; et al. A universal construction of robust interface between 2D conductive polymer and cellulose for textile supercapacitor. Carbohydr. Polym. 2022, 284, 119230. [Google Scholar] [CrossRef]
- Fan, T.T.; Liu, S.Y.; Wang, C.W.; Long, T. Optimization of Producing Enzyme Conditions for Facultative Anaerobic Cellulose Degrading Bacteria in Aerobic and Anaerobic Environments. Adv. Intel. Soft Comput. 2012, 125, 485–492. [Google Scholar]
- Wang, Y.; Xia, Y.; Ju, F.; Zhang, T. Metagenome approaches revealed a biological prospect for improvement on mesophilic cellulose degradation. Appl. Microbiol. Biotechnol. 2015, 99, 10871–10879. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Lin, X.; White, K.L.; Lin, S.; Wu, H.; Cao, S.; Huang, L.; Chen, L. Effect of the degree of substitution on the hydrophobicity of acetylated cellulose for production of liquid marbles. Cellulose 2016, 23, 811–821. [Google Scholar] [CrossRef]
- Fei, P.; Liao, L.; Cheng, B.; Song, J. Quantitative analysis of cellulose acetate with a high degree of substitution by FTIR and its application. Anal. Methods 2017, 9, 6194–6201. [Google Scholar] [CrossRef]
- Kim, M.I.; Lee, Y.-S. Deacetylation of cellulose acetate nanofibers by fluorination for carbon nanofibers. Mater. Lett. 2016, 181, 236–239. [Google Scholar] [CrossRef]
- Zhang, M.; Du, H.; Liu, K.; Nie, S.; Xu, T.; Zhang, X.; Si, C. Fabrication and applications of cellulose-based nanogenerators. Adv. Compos. Hybrid Mater. 2021, 4, 865–884. [Google Scholar] [CrossRef]
- She, J.; Tian, C.; Wu, Y.; Li, X.; Luo, S.; Qing, Y.; Jiang, Z. Cellulose Nanofibrils Aerogel Cross-Linked by Poly(vinyl alcohol) and Acrylic Acid for Efficient and Recycled Adsorption with Heavy Metal Ions. J. Nanosci. Nanotechnol. 2018, 18, 4167–4175. [Google Scholar] [CrossRef]
- Hu, T.; Hu, X.; Tang, C.; Liu, D. Adsorbent grafted on cellulose by in situ synthesis of EDTA-like groups and its properties of metal ion adsorption from aqueous solution. Cellulose 2021, 29, 941–952. [Google Scholar] [CrossRef]
- Ampiaw, R.E.; Lee, W. Persimmon tannins as biosorbents for precious and heavy metal adsorption in wastewater: A review. Int. J. Environ. Sci. Technol. 2020, 17, 3835–3846. [Google Scholar] [CrossRef]
- Chandra, S.; Mahto, T.K.; Chowdhuri, A.R.; Das, B.; Sahu, S.K. One step synthesis of functionalized carbon dots for the ultrasensitive detection of Escherichia coli and iron (III). Sensors Actuators B: Chem. 2017, 245, 835–844. [Google Scholar] [CrossRef]
- Astrini, N.; Anah, L.; Haryadi, H.R. Adsorption of Heavy Metal Ion from Aqueous Solution by Using Cellulose Based Hydrogel Composite. Macromol. Symp. 2015, 353, 191–197. [Google Scholar] [CrossRef]
- Gurung, M.; Adhikari, B.B.; Alam, S.; Kawakita, H.; Ohto, K.; Inoue, K. Persimmon tannin-based new sorption material for resource recycling and recovery of precious metals. Chem. Eng. J. 2013, 228, 405–414. [Google Scholar] [CrossRef]
- Kumar, M.; Gehlot, P.S.; Parihar, D.; Surolia, P.K.; Prasad, G. Application of grafted cellulosic material as bioadsorbent for segregating of non-desirable content from waste water—A review. Mater. Today: Proc. 2021, 43, 2903–2908. [Google Scholar] [CrossRef]
- Huang, C.; Cai, B.; Zhang, L.; Zhang, C.; Pan, H. Preparation of iron-based metal-organic framework @cellulose aerogel by in situ growth method and its application to dye adsorption. J. Solid State Chem. 2021, 297, 122030. [Google Scholar] [CrossRef]
- Palmieri, S.; Cipolletta, G.; Pastore, C.; Giosuè, C.; Akyol, Ç.; Eusebi, A.L.; Frison, N.; Tittarelli, F.; Fatone, F. Pilot scale cellulose recovery from sewage sludge and reuse in building and construction material. Waste Manag. 2019, 100, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Ramdzan, N.S.M.; Fen, Y.W.; Omar, N.A.S.; Anas, N.A.A.; Liew, J.Y.C.; Daniyal, W.M.E.M.M.; Hashim, H.S. Detection of mercury ion using surface plasmon resonance spectroscopy based on nanocrystalline cellulose/poly(3,4-ethylenedioxythiophene) thin film. Measurement 2021, 182, 109728. [Google Scholar] [CrossRef]
- Diez-Gil, C.; Caballero, A.; Martinez, R.; Ratera, I.; Tarraga, A.; Molina, P.; Veciana, J. Cellulose-based optical sensor for the selective and quantitative detection of mercury ions in aqueous media. In Proceedings of the Transducers ‘07 & Eurosensors XXI, Lyon, France, 10–14 June 2007; Digest of Technical Papers. Volumes 1 and 2. [Google Scholar]
- Bothra, S.; Upadhyay, Y.; Kumar, R.; Kumar, S.A.; Sahoo, S.K. Chemically modified cellulose strips with pyridoxal conjugated red fluorescent gold nanoclusters for nanomolar detection of mercuric ions. Biosens. Bioelectron. 2017, 90, 329–335. [Google Scholar] [CrossRef]
- Ma, W.; Fang, Y. Experimental (SERS) and theoretical (DFT) studies on the adsorption of p-, m-, and o-nitroaniline on gold nanoparticles. J. Colloid Interface Sci. 2006, 303, 1–8. [Google Scholar] [CrossRef]
- Wei, H.; Rodriguez, K.; Renneckar, S.; Leng, W.; Vikesland, P.J. Correction: Preparation and evaluation of nanocellulose–gold nanoparticle nanocomposites for SERS applications. Analyst 2016, 141, 2072. [Google Scholar] [CrossRef] [Green Version]
- Furlan, D.M.; Morgado, D.L.; de Oliveira, A.J.; Faceto, Â.D.; de Moraes, D.A.; Varanda, L.C.; Frollini, E. Sisal cellulose and magnetite nanoparticles: Formation and properties of magnetic hybrid films. J. Mater. Res. Technol. 2019, 8, 2170–2179. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, J.; Mi, Y.; Li, Q.; Guo, Z. Synthesis and characterization of α-lipoic acid grafted chitosan derivatives with antioxidant activity. React. Funct. Polym. 2022, 172, 105205. [Google Scholar] [CrossRef]
- Zheng, G.; Polavarapu, L.; Liz-Marzán, L.M.; Pastoriza-Santos, I.; Pérez-Juste, J. Gold nanoparticle-loaded filter paper: A recyclable dip-catalyst for real-time reaction monitoring by surface enhanced Raman scattering. Chem. Commun. 2014, 51, 4572–4575. [Google Scholar] [CrossRef]
- Zheng, G.; Pastoriza-Santos, I.; Pérez-Juste, J.; Liz-Marzán, L.M. Plasmonic metal-organic frameworks. SmartMat 2021, 2, 446–465. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, C.; Huang, L.; Guo, W.; Li, D.; Bian, J.; Ma, M. Stretchable, Antifreezing, Non-Drying, and Fast-Response Sensors Based on Cellulose Nanocomposite Hydrogels for Signal Detection. Macromol. Mater. Eng. 2021, 306, 2100549. [Google Scholar] [CrossRef]
- Auvinen, V.-V.; Merivaara, A.; Kiiskinen, J.; Paukkonen, H.; Laurén, P.; Hakkarainen, T.; Koivuniemi, R.; Sarkanen, R.; Ylikomi, T.; Laaksonen, T.; et al. Effects of nanofibrillated cellulose hydrogels on adipose tissue extract and hepatocellular carcinoma cell spheroids in freeze-drying. Cryobiology 2019, 91, 137–145. [Google Scholar] [CrossRef]
- Punitha, S.; Uvarani, R.; Panneerselvam, A. Effect of pH in aqueous (Hydroxy Propyl Methyl Cellulose) polymer solution. Results Mater. 2020, 7, 100120. [Google Scholar] [CrossRef]
- Kamei, J.; Yabu, H. One step fabrication of mesh-reinforced hierarchic perforated microporous honeycomb films with tunable filtering property. Soft Matter 2017, 13, 7834–7839. [Google Scholar] [CrossRef]
- Luo, Z.; Fang, Y. SERS of C60/C70 on gold-coated filter paper or filter film influenced by the gold thickness. J. Colloid Interface Sci. 2005, 283, 459–463. [Google Scholar] [CrossRef]
- Mosier-Boss, P.A. Review of SERS Substrates for Chemical Sensing. Nanomaterials 2017, 7, 142. [Google Scholar] [CrossRef] [Green Version]
- Park, M.; Chang, H.; Jeong, D.H.; Hyun, J. Spatial deformation of nanocellulose hydrogel enhances SERS. BioChip J. 2013, 7, 234–241. [Google Scholar] [CrossRef]
- Park, M.; Lee, D.; Hyun, J. Nanocellulose-alginate hydrogel for cell encapsulation. Carbohydr. Polym. 2015, 116, 223–228. [Google Scholar] [CrossRef]
- Esteves, C.V.; Sevastyanova, O.; Östlund, S.; Brännvall, E. Differences and similarities between kraft and oxygen delignification of softwood fibers: Effects on chemical and physical properties. Cellulose 2021, 28, 3149–3167. [Google Scholar] [CrossRef]
- Ayoub, A.; Treasure, T.; Hansen, L.; Nypelö, T.; Jameel, H.; Khan, S.; Chang, H.-M.; Hubbe, M.A.; Venditti, R.A. Effect of plasticizers and polymer blends for processing softwood kraft lignin as carbon fiber precursors. Cellulose 2020, 28, 1039–1053. [Google Scholar] [CrossRef]
- Johnston, J.H.; Nilsson, T.W. Nanogold and nanosilver composites with lignin-containing cellulose fibres. J. Mater. Sci. 2011, 47, 1103–1112. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Choi, J.; Park, I.; Ko, S. Facile Biosynthesis and Antioxidant Property of Nanogold-Cellulose Fiber Composite. J. Nanomater. 2015, 16, 195. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Takahashi, K.; Ito, Y.; Aita, T. Preparation of oriented gold plate/cellulose nanofiber composite films by using TEMPO-oxidized cellulose nanofiber as a reducing agent. Microsyst. Technol. 2018, 27, 1039–1049. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, B.; Wang, T.; Yang, L.; Xu, X.; Chen, C.; Wei, F.; Lv, W.; Zhang, L.; Sun, D. Synthesis of cellulose–silica nanocomposites by in situ biomineralization during fermentation. Cellulose 2019, 27, 703–712. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, T.-J.; Zhang, D.-W.; Li, H.-Y.; Ma, Y.-R.; Qi, L.-M.; Zhou, Y.-L.; Zhang, X.-X. Amperometric hydrogen peroxide biosensor based on the immobilization of heme proteins on gold nanoparticles–bacteria cellulose nanofibers nanocomposite. Talanta 2011, 84, 71–77. [Google Scholar] [CrossRef]
- Henderson, W.A.; Xiang, L.; Fourie, N.H.; Abey, S.K.; Ferguson, E.G.; Diallo, A.F.; Kenea, N.D.; Kim, C.H. Simple lateral flow assays for microbial detection in stool. Anal. Methods 2018, 10, 5358–5363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Fang, F.; Sun, B.; Yin, C.; Tan, J.; Wan, Y.; Zhang, J.; Sun, P.; Fan, Q.; Wang, P.; et al. Near-infrared small molecule coupled with rigidness and flexibility for high-performance multimodal imaging-guided photodynamic and photothermal synergistic therapy. Nanoscale Horiz. 2020, 6, 177–185. [Google Scholar] [CrossRef]
- Li, G.; Sun, K.; Li, D.; Lv, P.; Wang, Q.; Huang, F.; Wei, Q. Biosensor based on bacterial cellulose-Au nanoparticles electrode modified with laccase for hydroquinone detection. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 408–414. [Google Scholar] [CrossRef]
- Hamad, W.Y. Photonic and Semiconductor Materials Based on Cellulose Nanocrystals. Adv. Polym. Sci. 2015, 287–328. [Google Scholar] [CrossRef]
- Schlesinger, M.; Giese, M.; Blusch, L.K.; Hamad, W.Y.; MacLachlan, M.J. Chiral nematic cellulose–gold nanoparticle composites from mesoporous photonic cellulose. Chem. Commun. 2014, 51, 530–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlesinger, M.; Hamad, W.Y.; MacLachlan, M.J. Optically tunable chiral nematic mesoporous cellulose films. Soft Matter 2015, 11, 4686–4694. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, W.; Huang, L.; Hu, N.; Liu, W.; Liu, Y.; Li, S.; Yang, C.; Suo, Y.; Wang, J. Fluorometric determination of dopamine by using molybdenum disulfide quantum dots. Mikrochim. Acta 2018, 185, 234. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Bai, X.; Wang, Y.; Jin, W.; Zhang, X.; Hu, S. Inkjet Printing of Nanoporous Gold Electrode Arrays on Cellulose Membranes for High-Sensitive Paper-Like Electrochemical Oxygen Sensors Using Ionic Liquid Electrolytes. Anal. Chem. 2012, 84, 3745–3750. [Google Scholar] [CrossRef]
- Wei, X.; Qi, L.; Tan, J.; Liu, R.; Wang, F. A colorimetric sensor for determination of cysteine by carboxymethyl cellulose-functionalized gold nanoparticles. Anal. Chim. Acta 2010, 671, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yan, X.; Feng, Q.; Zhao, P.; Xu, X.; Ng, D.H.L.; Bian, L. Citric Acid/Cysteine-Modified Cellulose-Based Materials: Green Preparation and Their Applications in Anticounterfeiting, Chemical Sensing, and UV Shielding. ACS Sustain. Chem. Eng. 2017, 5, 11387–11394. [Google Scholar] [CrossRef]
- Yang, X.; Pan, Q.; Ao, Y.; Du, J.; Dong, Z.; Zhai, M.; Zhao, L. Facile preparation of L-cysteine–modified cellulose microspheres as a low-cost adsorbent for selective and efficient adsorption of Au(III) from the aqueous solution. Environ. Sci. Pollut. Res. 2020, 27, 38334–38343. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Hu, H.; Huang, J. Colorimetric detection of cysteine by surface functionalization of natural cellulose substance. Sens. Actuators B Chem. 2012, 171–172, 878–885. [Google Scholar] [CrossRef]
- Yu, Z.; Hu, C.; Guan, L.; Zhang, W.; Gu, J. Green Synthesis of Cellulose Nanofibrils Decorated with Ag Nanoparticles and Their Application in Colorimetric Detection of l-Cysteine. ACS Sustain. Chem. Eng. 2020, 8, 12713–12721. [Google Scholar] [CrossRef]
- Rajnish, K.N.; Samuel, M.S.; Datta, S.; Chandrasekar, N.; Balaji, R.; Jose, S.; Selvarajan, E. Immobilization of cellulase enzymes on nano and micro-materials for breakdown of cellulose for biofuel production-a narrative review. Int. J. Biol. Macromol. 2021, 182, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Drozd, R.; Szymańska, M.; Przygrodzka, K.; Hoppe, J.; Leniec, G.; Kowalska, U. The Simple Method of Preparation of Highly Carboxylated Bacterial Cellulose with Ni- and Mg-Ferrite-Based Versatile Magnetic Carrier for Enzyme Immobilization. Int. J. Mol. Sci. 2021, 22, 8563. [Google Scholar] [CrossRef]
- Jusner, P.; Aoki, M.; Potthast, A.; Rosenau, T. A cautionary note on “exothermic events” upon contact of carbodiimide coupling agents and the cellulose solvent N-methylmorpholine-N-oxide. Cellulose 2020, 27, 7349–7359. [Google Scholar] [CrossRef]
- Mahmoud, K.A.; Male, K.B.; Hrapovic, S.; Luong, J.H.T. Cellulose Nanocrystal/Gold Nanoparticle Composite as a Matrix for Enzyme Immobilization. ACS Appl. Mater. Interfaces 2009, 1, 1383–1386. [Google Scholar] [CrossRef]
- Tang, J.; Li, Y.; Song, Y.; Wu, X.; Yu, G.; Tam, K.C. Carbodiimide coupling versus click chemistry for nanoparticle surface functionalization: A comparative study for the encapsulation of sodium cholate by cellulose nanocrystals modified with β-cyclodextrin. Carbohydr. Polym. 2020, 244, 116512. [Google Scholar] [CrossRef]
- Verma, N.; Sisodiya, L.; Gahlaut, A.; Hooda, V.; Hooda, V. Novel approach using activated cellulose film for efficient immobilization of purified diamine oxidase to enhance enzyme performance and stability. Prep. Biochem. Biotechnol. 2020, 50, 468–476. [Google Scholar] [CrossRef]
- Nagendran, A.; Mohan, D. Protein separation by cellulose acetate/sulfonated poly(ether imide) blend ultrafiltration membranes. J. Appl. Polym. Sci. 2008, 110, 2047–2057. [Google Scholar] [CrossRef]
- Qiao, L.; Li, S.; Du, K. Fabrication and characterization of porous cellulose beads with high strength and specific surface area via preliminary chemical cross-linking reaction for protein separation. Biochem. Eng. J. 2019, 153, 107412. [Google Scholar] [CrossRef]
- Adar, F.; Atalla, R. Analysis of Lignin and Cellulose in Biological Energy Sources by Raman Microscopy. Spectroscopy 2010, 25, 18. [Google Scholar]
- Ji, G.; Wu, Y.; Wang, C. Analysis of microbial characterization in an upflow anaerobic sludge bed/biological aerated filter system for treating microcrystalline cellulose wastewater. Bioresour. Technol. 2012, 120, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Ullah, A.; Fan, H.; Jamil, M.I.; Khan, F.U.; Ullah, R.; Iqbal, M.; Ali, A.; Ullah, B. Recent Progress in Silane Coupling Agent with Its Emerging Applications. J. Polym. Environ. 2021, 29, 3427–3443. [Google Scholar] [CrossRef]
- Golonka, I.; Greber, K.; Oleksy-Wawrzyniak, M.; Paleczny, J.; Dryś, A.; Junka, A.; Sawicki, W.; Musiał, W. Antimicrobial and Antioxidative Activity of Newly Synthesized Peptides Absorbed into Bacterial Cellulose Carrier against Acne vulgaris. Int. J. Mol. Sci. 2021, 22, 7466. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; He, M.; Li, T.; Zhang, L. Preparation and properties of cellulose/silver nanocomposite fibers. Carbohydr. Polym. 2014, 115, 269–275. [Google Scholar] [CrossRef]
- Dacrory, S. Antimicrobial Activity, DFT Calculations, and Molecular Docking of Dialdehyde Cellulose/Graphene Oxide Film Against Covid-19. J. Polym. Environ. 2021, 29, 2248–2260. [Google Scholar] [CrossRef]
- Turyanska, L.; Makarovsky, O.; Patanè, A.; Kozlova, N.V.; Liu, Z.; Li, M.; Mann, S. High magnetic field quantum transport in Au nanoparticle–cellulose films. Nanotechnology 2012, 23, 045702. [Google Scholar] [CrossRef]
- Liu, Z.; Li, M.; Turyanska, L.; Makarovsky, O.; Patanè, A.; Wu, W.; Mann, S. Self-Assembly of Electrically Conducting Biopolymer Thin Films by Cellulose Regeneration in Gold Nanoparticle Aqueous Dispersions. Chem. Mater. 2010, 22, 2675–2680. [Google Scholar] [CrossRef]
- Aziz, T.; Fan, H.; Haq, F.; Khan, F.U.; Numan, A.; Iqbal, M.; Raheel, M.; Kiran, M.; Wazir, N. Adhesive properties of poly (methyl silsesquioxanes)/bio-based epoxy nanocomposites. Iran. Polym. J. 2020, 29, 911–918. [Google Scholar] [CrossRef]
- Beuriot, A.; Eichel, C.A.; Dilanian, G.; Louault, F.; Melgari, D.; Doisne, N.; Coulombe, A.; Hatem, S.N.; Balse, E. Distinct calcium/calmodulin-dependent serine protein kinase domains control cardiac sodium channel membrane expression and focal adhesion anchoring. Hear. Rhythm 2020, 17, 786–794. [Google Scholar] [CrossRef]
- Ali, A.; Muhammad, N.; Hussain, S.; Jamil, M.; Uddin, A.; Aziz, T.; Tufail, M.; Guo, Y.; Wei, T.; Rasool, G.; et al. Kinetic and Thermal Study of Ethylene and Propylene Homo Polymerization Catalyzed by ansa-Zirconocene Activated with Alkylaluminum/Borate: Effects of Alkylaluminum on Polymerization Kinetics and Polymer Structure. Polymers 2021, 13, 268. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Li, M.; Wu, W. Tunnelling conductive hybrid films of gold nanoparticles and cellulose and their applications as electrochemical electrodes. Nanotechnology 2015, 26, 465708. [Google Scholar] [CrossRef] [PubMed]
- Amirmahani, N.; Rashidi, M.; Mahmoodi, N.O. Synthetic application of gold complexes on magnetic supports. Appl. Organomet. Chem. 2020, 34, e5626. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Tian, B.; Xiao, H.; Chen, W.; Wu, H.; Jia, J. Immobilization of bismuth oxychloride on cellulose nanocrystal for photocatalytic sulfonylation of arylacetylenic acids with sodium arylsulfinates under visible light. Arab. J. Chem. 2022, 15, 103708. [Google Scholar] [CrossRef]
- Miikkulainen, V.; Leskela, M.; Ritala, M.; Puurunen, R.L. Crystallinity of inorganic films grown by atomic layer deposition: Overview and general trends. J. Appl. Phys. 2013, 113, 021301. [Google Scholar] [CrossRef]







| Cellulose (mg) | Time (h) | Remaining Solid (mg) | Conversion (%) |
|---|---|---|---|
| 200 | 0.5 | 180 | 60 |
| 200 | 2 | 95 | >99 |
| 200 | 4 | 102 | >99 |
| 200 | 8 | 101 | >99 |
| 100 | 8 | 99 | >99 |
| 400 | 8 | 99 | >99 |
| 600 | 8 | 100 | >99 |
| 200 | 8 | 98 | >99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R.; et al. A Review on the Modification of Cellulose and Its Applications. Polymers 2022, 14, 3206. https://doi.org/10.3390/polym14153206
Aziz T, Farid A, Haq F, Kiran M, Ullah A, Zhang K, Li C, Ghazanfar S, Sun H, Ullah R, et al. A Review on the Modification of Cellulose and Its Applications. Polymers. 2022; 14(15):3206. https://doi.org/10.3390/polym14153206
Chicago/Turabian StyleAziz, Tariq, Arshad Farid, Fazal Haq, Mehwish Kiran, Asmat Ullah, Kechun Zhang, Cheng Li, Shakira Ghazanfar, Hongyue Sun, Roh Ullah, and et al. 2022. "A Review on the Modification of Cellulose and Its Applications" Polymers 14, no. 15: 3206. https://doi.org/10.3390/polym14153206
APA StyleAziz, T., Farid, A., Haq, F., Kiran, M., Ullah, A., Zhang, K., Li, C., Ghazanfar, S., Sun, H., Ullah, R., Ali, A., Muzammal, M., Shah, M., Akhtar, N., Selim, S., Hagagy, N., Samy, M., & Al Jaouni, S. K. (2022). A Review on the Modification of Cellulose and Its Applications. Polymers, 14(15), 3206. https://doi.org/10.3390/polym14153206