Influences of Process Parameters of Near-Field Direct-Writing Melt Electrospinning on Performances of Polycaprolactone/Nano-Hydroxyapatite Scaffolds
Abstract
:1. Introduction
2. Experimental
2.1. Experimental Materials
2.2. Preparation of PCL/nHA Scaffolds
2.3. Testing
2.3.1. Macroscopic Morphology
2.3.2. Fiber Diameter Based on SEM
2.3.3. Porosity
2.3.4. Mechanical Performance
2.3.5. Fiber Peeling Strength
2.3.6. Thermal Stability
- (1)
- Thermogravimetric (TG) analysis
- (2)
- Differential scanning calorimeter (DSC) analysis
2.3.7. Degradability
3. Results and Discussion
3.1. Influence of Process Parameters on the Morphology of PCL/nHA Scaffolds
3.1.1. Influence of Spinning Voltage on the Morphology of PCL/nHA Scaffolds
3.1.2. Influence of Receiving Distance on the Morphology of PCL/nHA Scaffolds
3.1.3. Influence of Moving Speed of the Receiving Plate on the Morphology of PCL/nHA Scaffolds
3.1.4. Influence of Melt Temperature on the Morphology of PCL/nHA Scaffolds
3.2. Fiber Diameter and Porosity of PCL/nHA Scaffolds
3.3. Mechanical Properties of PCL/nHA Scaffolds
3.4. Thermal Propreties of PCL/nHA Scaffolds
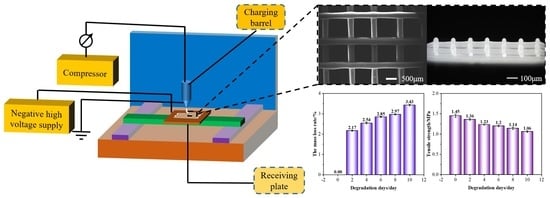
3.5. Degradability of PCL/nHA Scaffolds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chen, Z.; Hao, M.; Qian, X.; Chen, W.; Zeng, M.; Huang, J.; Li, R.; Fan, J.; Liu, Y. Characterization on Modification and Biocompatibility of PCL Scaffold Prepared with Near-field Direct-writing Melt Electrospinning. Chem. Res. Chin. Univ. 2021, 37, 578–583. [Google Scholar] [CrossRef]
- Meng, J.; Boschetto, F.; Yagi, S.; Marin, E.; Adachi, T.; Chen, X.; Pezzotti, G.; Sakurai, S.; Yamane, H.; Xu, H. Design and manufacturing of 3D high-precision micro-fibrous poly (l-lactic acid) scaffold using melt electrowriting technique for bone tissue engineering. Mater. Des. 2021, 210, 110063. [Google Scholar] [CrossRef]
- Jin, Y.; Gao, Q.; Xie, C.; Li, G.; Du, J.; Fu, J.; He, Y. Fabrication of heterogeneous scaffolds using melt electrospinning writing: Design and optimization. Mater. Des. 2020, 185, 108274. [Google Scholar] [CrossRef]
- Nazemi, M.M.; Khodabandeh, A.; Hadjizadeh, A. Near-Field Electrospinning: Crucial Parameters, Challenges, and Applications. ACS Appl. Bio Mater. 2022, 5, 394–412. [Google Scholar] [CrossRef]
- Daghrery, A.; de Souza Araújo, I.J.; Castilho, M.; Malda, J.; Bottino, M.C. Unveiling the potential of melt electrowriting in regenerative dental medicine. Acta Biomater. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.D.; Dalton, P.D.; Hutmacher, D.W. Direct Writing By Way of Melt Electrospinning. Adv. Mater. 2011, 23, 5651–5657. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, E.; Cesur, S.; Guler, E.; Topal, F.; Albayrak, D.; Guncu, M.M.; Cam, M.E.; Taskin, T.; Sasmazel, H.T.; Aksu, B.; et al. Development of Satureja cuneifolia-loaded sodium alginate/polyethylene glycol scaffolds produced by 3D-printing technology as a diabetic wound dressing material. Int. J. Biol. Macromol. 2020, 161, 1040–1054. [Google Scholar] [CrossRef]
- Mahendiran, B.; Muthusamy, S.; Sampath, S.; Jaisankar, S.N.; Popat, K.C.; Selvakumar, R.; Krishnakumar, G.S. Recent trends in natural polysaccharide based bioinks for multiscale 3D printing in tissue regeneration: A review. Int. J. Biol. Macromol. 2021, 183, 564–588. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Kim, J.H.; Jeong, Y.H. Identification of sagging in melt-electrospinning of microfiber scaffolds. Mater. Sci. Eng. C 2019, 103, 109785. [Google Scholar] [CrossRef]
- Chen, P.; Cui, L.; Chen, G.; You, T.; Li, W.; Zuo, J.; Wang, C.; Zhang, W.; Jiang, C. The application of BMP-12-overexpressing mesenchymal stem cells loaded 3D-printed PLGA scaffolds in rabbit rotator cuff repair. Int. J. Biol. Macromol. 2019, 138, 79–88. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, G.; Xu, L.; Cheng, W.; Xu, B.; Huang, Y.; Sun, D. Fabrication of nanochannels via near-field electrospinning. Appl. Phys. A 2012, 108, 825–828. [Google Scholar] [CrossRef] [Green Version]
- Rezvani, Z.; Venugopal, J.R.; Urbanska, A.M.; Mills, D.K.; Ramakrishna, S.; Mozafari, M. A bird’s eye view on the use of electrospun nanofibrous scaffolds for bone tissue engineering: Current state-of-the-art, emerging directions and future trends. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2181–2200. [Google Scholar] [CrossRef] [PubMed]
- Babaie, E.; Bhaduri, S.B. Fabrication Aspects of Porous Biomaterials in Orthopedic Applications: A Review. ACS Biomater. Sci. Eng. 2018, 4, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Adithya, S.P.; Sidharthan, D.S.; Abhinandan, R.; Balagangadharan, K.; Selvamurugan, N. Nanosheets-incorporated bio-composites containing natural and synthetic polymers/ceramics for bone tissue engineering. Int. J. Biol. Macromol. 2020, 164, 1960–1972. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Vacanti, J.P.; Langer, R. Tissue engineering: The design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 1999, 354, S32–S34. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Yadav, L.R.; Chandran, S.V.; Lavanya, K.; Selvamurugan, N. Chitosan-based 3D-printed scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2021, 183, 1925–1938. [Google Scholar] [CrossRef]
- Farrugia, B.L.; Brown, T.D.; Upton, Z.; Hutmacher, D.W.; Dalton, P.D.; Dargaville, T.R. Dermal fibroblast infiltration of poly(ε-caprolactone) scaffolds fabricated by melt electrospinning in a direct writing mode. Biofabrication 2013, 5, 025001. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Chen, S.; Xu, Z.; Wang, Q.; Yuan, P.; Zhou, Y.; Zhang, Y.; Chen, J. Heparan sulfate loaded polycaprolactone-hydroxyapatite scaffolds with 3D printing for bone defect repair. Int. J. Biol. Macromol. 2020, 148, 153–162. [Google Scholar] [CrossRef]
- Li, K.; Zhang, F.; Wang, D.; Qiu, Q.; Liu, M.; Yu, A.; Cui, Y. Silkworm-inspired electrohydrodynamic jet 3D printing of composite scaffold with ordered cell scale fibers for bone tissue engineering. Int. J. Biol. Macromol. 2021, 172, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, M.; Alvarez Primo, F.; Anil Kumar, S.; Mudloff, J.A.; Dominguez, E.; Fregoso, G.; Ortiz, N.; Weiss, W.M.; Joddar, B. Bone tissue engineering techniques, advances, and scaffolds for treatment of bone defects. Curr. Opin. Biomed. Eng. 2021, 17, 100248. [Google Scholar] [CrossRef]
- Zhao, X.; Lui, Y.S.; Choo, C.K.C.; Sow, W.T.; Huang, C.L.; Ng, K.W.; Tan, L.P.; Loo, J.S.C. Calcium phosphate coated Keratin–PCL scaffolds for potential bone tissue regeneration. Mater. Sci. Eng. C 2015, 49, 746–753. [Google Scholar] [CrossRef]
- Großhaus, C.; Bakirci, E.; Berthel, M.; Hrynevich, A.; Kade, J.C.; Hochleitner, G.; Groll, J.; Dalton, P.D. Melt Electrospinning of Nanofibers from Medical-Grade Poly(ε-Caprolactone) with a Modified Nozzle. Small 2020, 16, 2003471. [Google Scholar] [CrossRef]
- Kumar, P.; Saini, M.; Dehiya, B.S.; Umar, A.; Sindhu, A.; Mohammed, H.; Al-Hadeethi, Y.; Guo, Z. Fabrication and in-vitro biocompatibility of freeze-dried CTS-nHA and CTS-nBG scaffolds for bone regeneration applications. Int. J. Biol. Macromol. 2020, 149, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Yan, J.; Zhang, K.; Lin, F.; Xiang, L.; Deng, L.; Guan, Z.; Cui, W.; Zhang, H. Pharmaceutical electrospinning and 3D printing scaffold design for bone regeneration. Adv. Drug Deliv. Rev. 2021, 174, 504–534. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Carbone, E.J.; Lo, K.W.H.; Laurencin, C.T. Electrospinning of polymer nanofibers for tissue regeneration. Prog. Polym. Sci. 2015, 46, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Hong, Y. Enhancing cell infiltration of electrospun fibrous scaffolds in tissue regeneration. Bioact. Mater. 2016, 1, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Li, X.; He, Y.; Ma, J.; Ni, G.; Zhou, S. From nano to micro to macro: Electrospun hierarchically structured polymeric fibers for biomedical applications. Prog. Polym. Sci. 2018, 81, 80–113. [Google Scholar] [CrossRef]
- Xiao, L.; Wu, M.; Yan, F.; Xie, Y.; Liu, Z.; Huang, H.; Yang, Z.; Yao, S.; Cai, L. A radial 3D polycaprolactone nanofiber scaffold modified by biomineralization and silk fibroin coating promote bone regeneration in vivo. Int. J. Biol. Macromol. 2021, 172, 19–29. [Google Scholar] [CrossRef]
- Xie, C.; Gao, Q.; Wang, P.; Shao, L.; Yuan, H.; Fu, J.; Chen, W.; He, Y. Structure-induced cell growth by 3D printing of heterogeneous scaffolds with ultrafine fibers. Mater. Des. 2019, 181, 108092. [Google Scholar] [CrossRef]









| Sample Code | Process Parameters | |||
|---|---|---|---|---|
| Spinning Voltage (kV) | Receiving Distance (mm) | Moving Speed of Receiving Plate (mm/s) | Melt Temperature (°C) | |
| S1 | 0 | 4 | 5 | 130 |
| S2 | −2 | 4 | 5 | 130 |
| S3 | −3 | 4 | 5 | 130 |
| S4 | −4 | 4 | 5 | 130 |
| S5 | −5 | 4 | 5 | 130 |
| R1 | −4 | 3 | 5 | 130 |
| R2 | −4 | 4 | 5 | 130 |
| R3 | −4 | 5 | 5 | 130 |
| R4 | −4 | 6 | 5 | 130 |
| M1 | −4 | 4 | 3 | 130 |
| M2 | −4 | 4 | 4 | 130 |
| M3 | −4 | 4 | 5 | 130 |
| M4 | −4 | 4 | 6 | 130 |
| M5 | −4 | 4 | 7 | 130 |
| T1 | −4 | 4 | 5 | 100 |
| T2 | −4 | 4 | 5 | 110 |
| T3 | −4 | 4 | 5 | 120 |
| T4 | −4 | 4 | 5 | 130 |
| T5 | −4 | 4 | 5 | 140 |
| T6 | −4 | 4 | 5 | 150 |
| Parametric Variable | Parameter | Average of Fiber Diameter/μm | CV Value of Fiber Diameter/% | Porosity/% |
|---|---|---|---|---|
| Spinning voltage | 0 | 200 | 17.49 | 76.7 |
| −2 kV | 285 | 15.34 | 75.1 | |
| −3 kV | 215 | 6.56 | 79.8 | |
| −4 kV | 224 | 5.39 | 78.3 | |
| −5 kV | 225 | 7.67 | 80.2 | |
| Receiving distance | 3 mm | 212 | 7.07 | 76.5 |
| 4 mm | 224 | 5.39 | 78.3 | |
| 5 mm | 194 | 10.17 | 81.0 | |
| 6 mm | 197 | 12.89 | 80.5 | |
| Moving speed of receiving plate | 3 mm/s | 360 | 23.50 | 73.5 |
| 4 mm/s | 275 | 9.52 | 78.0 | |
| 5 mm/s | 224 | 5.39 | 78.3 | |
| 6 mm/s | 197 | 11.14 | 82.1 | |
| 7 mm/s | 193 | 12.36 | 80.2 | |
| Melt temperature | 100 °C | 157 | 11.79 | 84.8 |
| 110 °C | 182 | 13.62 | 82.8 | |
| 120 °C | 207 | 11.31 | 80.3 | |
| 130 °C | 224 | 5.39 | 78.3 | |
| 140 °C | 273 | 5.98 | 73.4 | |
| 150 °C | 299 | 6.48 | 66.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Liu, Y.; Huang, J.; Hao, M.; Hu, X.; Qian, X.; Fan, J.; Yang, H.; Yang, B. Influences of Process Parameters of Near-Field Direct-Writing Melt Electrospinning on Performances of Polycaprolactone/Nano-Hydroxyapatite Scaffolds. Polymers 2022, 14, 3404. https://doi.org/10.3390/polym14163404
Chen Z, Liu Y, Huang J, Hao M, Hu X, Qian X, Fan J, Yang H, Yang B. Influences of Process Parameters of Near-Field Direct-Writing Melt Electrospinning on Performances of Polycaprolactone/Nano-Hydroxyapatite Scaffolds. Polymers. 2022; 14(16):3404. https://doi.org/10.3390/polym14163404
Chicago/Turabian StyleChen, Zhijun, Yanbo Liu, Juan Huang, Ming Hao, Xiaodong Hu, Xiaoming Qian, Jintu Fan, Hongjun Yang, and Bo Yang. 2022. "Influences of Process Parameters of Near-Field Direct-Writing Melt Electrospinning on Performances of Polycaprolactone/Nano-Hydroxyapatite Scaffolds" Polymers 14, no. 16: 3404. https://doi.org/10.3390/polym14163404
APA StyleChen, Z., Liu, Y., Huang, J., Hao, M., Hu, X., Qian, X., Fan, J., Yang, H., & Yang, B. (2022). Influences of Process Parameters of Near-Field Direct-Writing Melt Electrospinning on Performances of Polycaprolactone/Nano-Hydroxyapatite Scaffolds. Polymers, 14(16), 3404. https://doi.org/10.3390/polym14163404