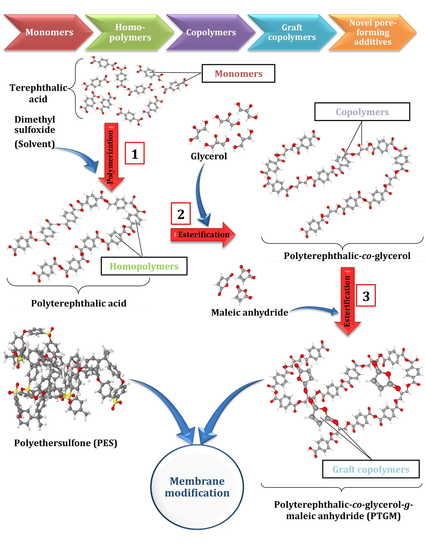
Modification of Polyethersulfone Ultrafiltration Membrane Using Poly(terephthalic acid-co-glycerol-g-maleic anhydride) as Novel Pore Former
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
2.2.1. Fabrication of PolyTerephthalic acid-co-Glycerol-g-Maleic Anhydride (PTGM) Nanoparticle
2.2.2. PTGM Characterization
2.2.3. Fabrication of Membrane
2.3. Characterization of the Membranes
2.3.1. Membrane Morphology
2.3.2. Contact Angle Measurement
2.3.3. Fourier-Transform Infrared Spectroscopy
2.3.4. Membrane Porosity Pore Size and Pore Density
2.3.5. Membrane Performance
3. Results and Discussion
3.1. PTGM Characterization
3.2. Impact of PTGM on PES Membrane
3.3. FTIR of PES/PTGM Membrane
3.4. Membrane Surface Hydrophilicity, Porosity, and Pore Size
3.5. Membrane Performance
3.6. Membrane Stability
3.7. Comparison Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al Aani, S.; Bonny, T.; Hasan, S.W.; Hilal, N. Can machine language and artificial intelligence revolutionize process automation for water treatment and desalination? Desalination 2019, 458, 84–96. [Google Scholar] [CrossRef]
- Zhong, Q.; Shi, G.; Sun, Q.; Mu, P.; Li, J. Robust PVA-GO-TiO2 composite membrane for efficient separation oil-in-water emulsions with stable high flux. J. Membr. Sci. 2021, 640, 119836. [Google Scholar] [CrossRef]
- Wu, M.; Xiang, B.; Mu, P.; Li, J. Janus nanofibrous membrane with special micro-nanostructure for highly efficient separation of oil–water emulsion. Sep. Purif. Technol. 2022, 297, 121532. [Google Scholar] [CrossRef]
- Malik, T.; Razzaq, H.; Razzaque, S.; Nawaz, H.; Siddiqa, A.; Siddiq, M.; Qaisar, S. Design and synthesis of polymeric membranes using water-soluble pore formers: An overview. Polym. Bull. 2019, 76, 4879–4901. [Google Scholar] [CrossRef]
- Ali, A.M.; Rashid, K.T.; Yahya, A.A.; Majdi, H.S.; Salih, I.K.; Yusoh, K.; Alsalhy, Q.F.; AbdulRazak, A.A.; Figoli, A. Fabrication of Gum Arabic-Graphene (GGA) Modified Polyphenylsulfone (PPSU) Mixed Matrix Membranes: A Systematic Evaluation Study for Ultrafiltration (UF) Applications. Membranes 2021, 11, 542. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, R.R.; Shabeeb, K.M.; Alzubaydi, A.B.; Figoli, A.; Criscuoli, A.; Drioli, E.; Alsalhy, Q.F. Characterization of the Efficiency of Photo-Catalytic Ultrafiltation PES Membrane Modified with Tungsten Oxide in the Removal of Tinzaparin Sodium. Eng. Technol. J. 2022, 40, 1–10. [Google Scholar] [CrossRef]
- Al-Araji, D.D.; Al-Ani, F.H.; Alsalhy, Q.F. The permeation and separation characteristics of Polymeric Membranes Incorporated with Nanoparticles for Dye Removal and Interaction Mechanisms between Polymer and Nanoparticles: A Mini Review. Eng. Technol. J. 2022, 40, 1–13. [Google Scholar] [CrossRef]
- Al-Ani, F.H.; Alsalhy, Q.F.; Raheem, R.S.; Rashid, K.T.; Figoli, A. Experimental investigation of the effect of implanting tio2-nps on pvc for long-term uf membrane performance to treat refinery wastewater. Membranes 2020, 10, 77. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; He, Y.; Zhuang, J.; Shi, H. An intelligent natural fibrous membrane anchored with ZnO for switchable oil/water separation and water purification. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 128041. [Google Scholar] [CrossRef]
- Alsalhy, Q.F.; Al-Ani, F.H.; Al-Najar, A.E. A new Sponge-GAC-Sponge membrane module for submerged membrane bioreactor use in hospital wastewater treatment. Biochem. Eng. J. 2018, 133, 130–139. [Google Scholar] [CrossRef]
- Huajaikaew, E.; Piroonpan, T.; Booncharoen, K.; Pasanphan, W. Comb-like poly (dodecyl methacrylate) modified SiO2 nanoparticles as nanohybrid coatings: Electron beam grafting and tuning superhydrophobic/water-repellent surface studies. Prog. Org. Coat. 2022, 163, 106658. [Google Scholar] [CrossRef]
- Al Timimi, D.A.H.; Alsalhy, Q.F.; AbdulRazak, A.A.; Drioli, E. Novel polyether sulfone/polyethylenimine grafted nano-silica nanocomposite membranes: Interaction mechanism and ultrafiltration performance. J. Membr. Sci. 2022, 659, 120784. [Google Scholar] [CrossRef]
- Jamed, M.J.; Alhathal Alanezi, A.; Alsalhy, Q.F. Effects of embedding functionalized multi-walled carbon nanotubes and alumina on the direct contact poly (vinylidene fluoride-co-hexafluoropropylene) membrane distillation performance. Chem. Eng. Commun. 2019, 206, 1035–1057. [Google Scholar] [CrossRef]
- Qu, H.; Xiao, X.; Han, Z.; Hu, M.; Shen, S.; Yang, L.; Jia, F.; Wang, T.; Ye, Z.; Sun, W.; et al. Graphene Oxide Nanofiltration Membrane Based on Three-Dimensional Size-Controllable Metal–Organic Frameworks for Water Treatment. ACS Appl. Nano Mater. 2022, 5, 5196–5207. [Google Scholar] [CrossRef]
- Kadhim, R.J.; Al-Ani, F.H.; Al-Shaeli, M.; Alsalhy, Q.F.; Figoli, A. Removal of dyes using graphene oxide (GO) mixed matrix membranes. Membranes 2020, 10, 366. [Google Scholar] [CrossRef]
- Jalal Sadiq, A.; Shabeeb, K.M.; Khalil, B.I.; Alsalhy, Q.F. Effect of embedding MWCNT-g-GO with PVC on the performance of PVC membranes for oily wastewater treatment. Chem. Eng. Commun. 2020, 207, 733–750. [Google Scholar] [CrossRef]
- Aljumaily, M.M.; Ali, N.S.; Mahdi, A.E.; Alayan, H.M.; AlOmar, M.; Hameed, M.M.; Ismael, B.; Alsalhy, Q.F.; Alsaadi, M.A.; Majdi, H.S.; et al. Modification of Poly (vinylidene fluoride-co-hexafluoropropylene) Membranes with DES-Functionalized Carbon Nanospheres for Removal of Methyl Orange by Membrane Distillation. Water 2022, 14, 1396. [Google Scholar] [CrossRef]
- Ghadhban, M.Y.; Majdi, H.S.; Rashid, K.T.; Alsalhy, Q.F.; Lakshmi, D.S.; Salih, I.K.; Figoli, A. Removal of dye from a leather tanning factory by flat-sheet blend ultrafiltration (UF) membrane. Membranes 2020, 10, 47. [Google Scholar] [CrossRef] [Green Version]
- Al-Ani, D.M.; Al-Ani, F.H.; Alsalhy, Q.F.; Ibrahim, S.S. Preparation and characterization of ultrafiltration membranes from PPSU-PES polymer blend for dye removal. Chem. Eng. Commun. 2021, 208, 41–59. [Google Scholar] [CrossRef]
- Plisko, T.; Karslyan, Y.; Bildyukevich, A. Effect of polyphenylsulfone and polysulfone incompatibility on the structure and performance of blend membranes for ultrafiltration. Materials 2021, 14, 5740. [Google Scholar] [CrossRef]
- Rastegar, B.; Saljoughi, E.; Mousavi, S.M.; Kiani, S. Polyphenylsulfone/polyethylene glycol hexadecyl ether blend membranes with enhanced surface hydrophilicity for high-performance nanofiltration of dye solution. Korean J. Chem. Eng. 2022, 39, 1–9. [Google Scholar] [CrossRef]
- Hussein, S.S.; Ibrahim, S.S.; Toma, M.A.; Alsalhy, Q.F.; Drioli, E. Novel chemical modification of polyvinyl chloride membrane by free radical graft copolymerization for direct contact membrane distillation (DCMD) application. J. Membr. Sci. 2020, 611, 118266. [Google Scholar] [CrossRef]
- Yoo, S.H.; Kim, J.H.; Jho, J.Y.; Won, J.; Kang, Y.S. Influence of the addition of PVP on the morphology of asymmetric polyimide phase inversion membranes: Effect of PVP molecular weight. J. Membr. Sci. 2004, 236, 203–207. [Google Scholar] [CrossRef]
- Alsalhy, Q.F.; Rashid, K.T.; Ibrahim, S.S.; Ghanim, A.H.; Van der Bruggen, B.; Luis, P.; Zablouk, M. Poly (vinylidene fluoride-co-hexafluoropropylene)(PVDF-co-HFP) hollow fiber membranes prepared from PVDF-co-HFP/PEG-600Mw/DMAC solution for membrane distillation. J. Appl. Polym. Sci. 2013, 129, 3304–3313. [Google Scholar] [CrossRef]
- Nazarova, O.; Chernova, E.; Dobrodumov, A.; Zolotova, Y.; Bezrukova, M.; Nekrasova, T.; Vlasova, E.; Panarin, E. New water-soluble copolymers of 2-methacryloyloxyethyl phosphorylcholine for surface modification. J. Appl. Polym. Sci. 2021, 138, 50272. [Google Scholar] [CrossRef]
- Rashid, K.T.; Alayan, H.M.; Mahdi, A.E.; Al-Baiati, M.N.; Majdi, H.S.; Salih, I.K.; Ali, J.M.; Alsalhy, Q.F. Novel Water-Soluble Poly (terephthalic-co-glycerol-g-fumaric acid) Copolymer Nanoparticles Harnessed as Pore Formers for Polyethersulfone Membrane Modification: Permeability–Selectivity Tradeoff Manipulation. Water 2022, 14, 1507. [Google Scholar] [CrossRef]
- Alayande, A.B.; Obaid, M.; Yu, H.W.; Kim, I.S. High-flux ultrafiltration membrane with open porous hydrophilic structure using dual pore formers. Chemosphere 2019, 227, 662–669. [Google Scholar] [CrossRef]
- Ho, C.C.; Su, J.F. Boosting permeation and separation characteristics of polyethersulfone ultrafiltration membranes by structure modification via dual-PVP pore formers. Polymer 2022, 241, 124560. [Google Scholar] [CrossRef]
- Lin, Y.C.; Zhuang, G.L.; Tasi, F.; Tseng, H.H. Removal of protein, histological dye and tetracycline from simulated bioindustrial wastewater with a dual pore size PPSU membrane. J. Hazard. Mater. 2022, 431, 128525. [Google Scholar] [CrossRef]
- Haghighat, N.; Vatanpour, V.; Sheydaei, M.; Nikjavan, Z. Preparation of a novel polyvinyl chloride (PVC) ultrafiltration membrane modified with Ag/TiO2 nanoparticle with enhanced hydrophilicity and antibacterial activities. Sep. Purif. Technol. 2020, 237, 116374. [Google Scholar] [CrossRef]
- Manawi, Y.; Kochkodan, V.; Mahmoudi, E.; Johnson, D.J.; Mohammad, A.W.; Atieh, M.A. Characterization and separation performance of a novel polyethersulfone membrane blended with acacia gum. Sci. Rep. 2017, 7, 15831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costacurta, S.; Biasetto, L.; Pippel, E.; Woltersdorf, J.; Colombo, P. Hierarchical porosity components by infiltration of a ceramic foam. J. Am. Ceram. Soc. 2007, 90, 2172–2177. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, F.; Ma, J.; Wu, M.; Zhang, J.; Gao, C. Effect of PEG additive on the morphology and performance of polysulfone ultrafiltration membranes. Desalination 2011, 272, 51–58. [Google Scholar] [CrossRef]
- Safarpour, M.; Khataee, A.; Vatanpour, V. Preparation of a novel polyvinylidene fluoride (PVDF) ultrafiltration membrane modified with reduced graphene oxide/titanium dioxide (TiO2) nanocomposite with enhanced hydrophilicity and antifouling properties. Ind. Eng. Chem. Res. 2014, 53, 13370–13382. [Google Scholar] [CrossRef]
- Leo, C.P.; Lee, W.C.; Ahmad, A.L.; Mohammad, A.W. Polysulfone membranes blended with ZnO nanoparticles for reducing fouling by oleic acid. Sep. Purif. Technol. 2012, 89, 51–56. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Atta, R.; Zolotarev, A.; Kuzminova, A.; Ermakov, S.; Penkova, A. Development of Novel Membranes Based on Polyvinyl Alcohol Modified by Pluronic F127 for Pervaporation Dehydration of Isopropanol. Sustainability 2022, 14, 3561. [Google Scholar] [CrossRef]
- Zhao, Z.P.; Wang, Z.; Wang, S.C. Formation, charged characteristic and BSA adsorption behavior of carboxymethyl chitosan/PES composite MF membrane. J. Membr. Sci. 2003, 217, 151–158. [Google Scholar] [CrossRef]
- Al Hachim, Z.S.; Ridha, A.M.; AL-Baiati, M.N.; Alsalhy, Q.F.; Majdi, H.S. Sustainable Modification of Polyethersulfone Membrane with Poly (Maleic Anhydride-Co-Glycerol) as Novel Copolymer. Water 2022, 14, 1207. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, L.; Shan, B.; Xie, C.; Liu, C.; Cui, F.; Li, G. Preparation and characterization of SLS-CNT/PES ultrafiltration membrane with antifouling and antibacterial properties. J. Membr. Sci. 2018, 548, 459–469. [Google Scholar] [CrossRef]
- Amirilargani, M.; Saljoughi, E.; Mohammadi, T. Improvement of permeation performance of polyethersulfone (PES) ultrafiltration membranes via addition of Tween-20. J. Appl. Polym. Sci. 2010, 115, 504–513. [Google Scholar] [CrossRef]
- Shi, L.; Wang, R.; Cao, Y.; Liang, D.T.; Tay, J.H. Effect of additives on the fabrication of poly (vinylidene fluoride-co-hexafluropropylene)(PVDF-HFP) asymmetric microporous hollow fiber membranes. J. Membr. Sci. 2008, 315, 195–204. [Google Scholar] [CrossRef]
- Wang, H.T.; Ao, D.; Lu, M.C.; Chang, N. Alteration of the morphology of polyvinylidene fluoride membrane by incorporating MOF-199 nanomaterials for improving water permeation with antifouling and antibacterial property. J. Chin. Chem. Soc. 2020, 67, 1807–1817. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H.; Beygzadeh, M. Novel high flux antifouling nanofiltration membranes for dye removal containing carboxymethyl chitosan coated Fe3O4 nanoparticles. Desalination 2014, 349, 145–154. [Google Scholar] [CrossRef]
- Abdallah, H.; Jamil, T.S.; Shaban, A.M.; Mansor, E.S.; Souaya, E.R. Influence of the polyacrylonitrile proportion on the fabricated UF blend membranes’ performance for humic acid removal. J. Polym. Eng. 2018, 38, 129–136. [Google Scholar] [CrossRef]
- Yan, W.; Wang, Z.; Wu, J.; Zhao, S.; Wang, J.; Wang, S. Enhancing the flux of brackish water TFC RO membrane by improving support surface porosity via a secondary pore-forming method. J. Membr. Sci. 2016, 498, 227–241. [Google Scholar] [CrossRef]











| Membrane Code | PES (wt.%) | DMAC (wt.%) | PTGM (wt.%) |
|---|---|---|---|
| a | 20 | 80 | 0 |
| b | 20 | 79 | 1 |
| c | 20 | 77 | 3 |
| d | 20 | 75 | 5 |
| e | 20 | 73 | 7 |
| f | 20 | 71 | 9 |
| g | 20 | 69 | 11 |
| Porous Membrane | Contact Angle (°) | Porosity (%) | Mean Pore Size (nm) | Pure Water Flux (L.m−2.h−1) | Rejection (%) | Process | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Polymer | Pore Former | |||||||
| 20% PES | 5% PTGM | 52.58 | 81.21 | 54.91 | 203.1 | 93.8% BSA 95.6% SA | UF | This Work |
| 22% PVDF-HFP | 5% CNS | 87 | 89.9 | NA | 35 | >99.9% MO | UF (MD) | [17] |
| 20% PES | 4% TGF | 50.4 | 73.3 | 40.59 | 300 | 96% BSA | UF | [26] |
| 15% PES | 2.5 g PMG | 42.04 | 83 | 108.28 | 908 | 98% BSA | UF | [38] |
| 15% PVC | 0.119% MWCNT-g-GO | 13.9 | 81.4 | 259 | 254 | 88.9% COD | NF | [16] |
| 19% PES | 2% SLS-CNT; 15% PVP | 57 | 74 | 64.29 | 597 | 95.6% BSA | UF | [39] |
| 17% PES | 5% PEG 400; 2% Tween-20 | NA | 35.31 | 73.2 | 36.9 | 93.3% BSA | UF | [40] |
| PVDF-HFP | 4% LiCl; 10 wt.% glycerol | 79 | NA | 7.85 | 51 | 90% Aqueous solution | UF | [41] |
| PVDF | MOF-199/PEG | 85 | 80.89 | 50 | 185.05 | 94% BSA | UF | [42] |
| 20% PES | 0.5% CC–Fe3O4; 1% PVP | 52.5 | 86.3 | 5.5 | 36 | 99% Dye | NF | [43] |
| 0% PES; 14% PAN | 4% PVP; 0% DEG | 76 | 55 | NA | 100 | 92.47% Humic acid | UF | [44] |
| 15% PSF | 0.7% SiO2 | 71.3 | 78 | 10.7 | 55 | 99.1% NaCl solutions | RO | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljanabi, A.A.A.; Mousa, N.E.; Aljumaily, M.M.; Majdi, H.S.; Yahya, A.A.; AL-Baiati, M.N.; Hashim, N.; Rashid, K.T.; Al-Saadi, S.; Alsalhy, Q.F. Modification of Polyethersulfone Ultrafiltration Membrane Using Poly(terephthalic acid-co-glycerol-g-maleic anhydride) as Novel Pore Former. Polymers 2022, 14, 3408. https://doi.org/10.3390/polym14163408
Aljanabi AAA, Mousa NE, Aljumaily MM, Majdi HS, Yahya AA, AL-Baiati MN, Hashim N, Rashid KT, Al-Saadi S, Alsalhy QF. Modification of Polyethersulfone Ultrafiltration Membrane Using Poly(terephthalic acid-co-glycerol-g-maleic anhydride) as Novel Pore Former. Polymers. 2022; 14(16):3408. https://doi.org/10.3390/polym14163408
Chicago/Turabian StyleAljanabi, Ali A. Abbas, Noor Edin Mousa, Mustafa M. Aljumaily, Hasan Sh. Majdi, Ali Amer Yahya, Mohammad N. AL-Baiati, Noor Hashim, Khaild T. Rashid, Saad Al-Saadi, and Qusay F. Alsalhy. 2022. "Modification of Polyethersulfone Ultrafiltration Membrane Using Poly(terephthalic acid-co-glycerol-g-maleic anhydride) as Novel Pore Former" Polymers 14, no. 16: 3408. https://doi.org/10.3390/polym14163408
APA StyleAljanabi, A. A. A., Mousa, N. E., Aljumaily, M. M., Majdi, H. S., Yahya, A. A., AL-Baiati, M. N., Hashim, N., Rashid, K. T., Al-Saadi, S., & Alsalhy, Q. F. (2022). Modification of Polyethersulfone Ultrafiltration Membrane Using Poly(terephthalic acid-co-glycerol-g-maleic anhydride) as Novel Pore Former. Polymers, 14(16), 3408. https://doi.org/10.3390/polym14163408