Preparation and Performances of Polyether Polytriazole Elastomers Based on Click Chemistry
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
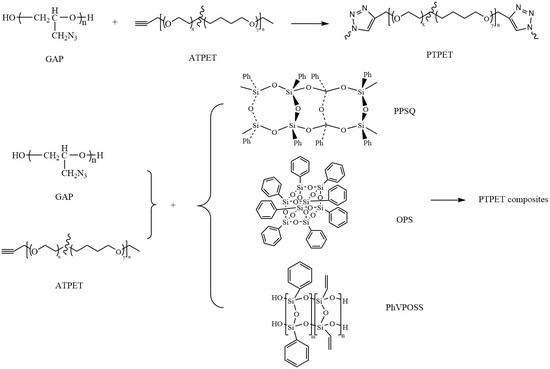
2.2. Preparation of PTPET Elastomer and Composites
2.3. Measurements and Analysis
2.3.1. FT-IR Analysis
2.3.2. Mechanical Properties Analysis
2.3.3. Differential Scanning Calorimetry (DSC) Analysis
2.3.4. Thermogravimetry (TG) Analysis
2.3.5. Fire Testing Method
3. Results and Discussion
3.1. PTPET Elastomer
3.2. PTPET Composites
3.3. FT-IR Analysis
3.4. Thermal Analysis
3.4.1. TG Analysis
3.4.2. DSC Analysis
3.5. Swelling Properties
3.6. Mechanical Properties
3.7. SEM Observation
3.8. Flame Retardance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qu, Z.Y.; Zhai, J.X.; Yang, R.J. Comparison between properties of polyether polytriazole elastomers and polyether polyurethane elastomers. Polym. Adv. Technol. 2014, 25, 314–321. [Google Scholar] [CrossRef]
- Ge, Z.; Luo, Y.; Wu, B. Study on Application of Hyperbranched Polyurethane in HTPB Propellant. Trans. Beijing Inst. Technol. 2014, 34, 437–440. [Google Scholar]
- Wu, B.; Li, G.; Luo, Y.; Xia, M.; Cai, C.P. Effect of hyperbranched polyisocyanate on properties of HTPB polyurethanes. J. Solid Rocket Technol. 2010, 33, 440–444. [Google Scholar]
- Song, C.; Peng, J.; Zhang, Y.; Wang, W. Synthesis and Characteristics of Multiblock Terpoly(Ester-Ether-Butadiene) Elastomers. Polym. Mater. Sci. Eng. 2013, 29, 21. [Google Scholar]
- Song, X.; Luo, Y.; Li, G. Synthesis and Mechanical Properties of Hyperbranched Polyether/HTPB Polyurethane Based IPN. Polym. Mater. Sci. Eng. 2009, 25, 1–4. [Google Scholar]
- Xie, F.; Yu, D.; Zhang, Y.; Zhu, C. The Factors that Influence Synthesis of Polyether-Polyoi Polyurethane Prepolymer. Paint Coat. Ind. 2006, 36, 34–37. [Google Scholar]
- Gunatillake, P.A.; Meijs, G.F.; Rizzardo, E.; Chatelier, R.C.; McCarthy, S.J.; Brandwood, A.; Schindhelm, K. Polyurethane Elastomers Based On Novel Polyether Macrodiols And Mdi—Synthesis, Mechanical-Properties, and Resistance To Hydrolysis And Oxidation. J. Appl. Polym. Sci. 1992, 46, 319–328. [Google Scholar] [CrossRef]
- Reshmi, S.K.; Vijayalakshmi, K.P.; Thomas, D.; Arunan, E.; Reghunadhan Nair, C.P. Glycidyl Azide Polymer Crosslinked Through Triazoles by Click Chemistry: Curing, Mechanical and Thermal Properties. Propellants Explos. Pyrotech. 2013, 38, 525–532. [Google Scholar] [CrossRef]
- Moses, J.E.; Moorhouse, A.D. The growing applications of click chemistry. Chem. Soc. Rev. 2007, 36, 1249–1262. [Google Scholar] [CrossRef]
- Sheng, X.; Rock, D.M.; Mauldin, T.C.; Kessler, M.R. Evaluation of different catalyst systems for bulk polymerization through “click” chemistry. Polymer 2011, 52, 4435–4441. [Google Scholar] [CrossRef]
- Li, Y.H.; Yang, R.J.; Li, J.M. Click Chemistry Reactions between Hydroxyl-Terminated or Alkynyl-Terminated Polybutadiene and Benzyl Azide. Propell. Explos. Pyrot. 2021, 46, 975–980. [Google Scholar] [CrossRef]
- Ouyang, T.; Liu, X.H.; Ouyang, H.S.; Ren, L. Recent trends in click chemistry as a promising technology for virus-related research. Virus Res. 2018, 256, 21–28. [Google Scholar] [CrossRef]
- Chen, L.; Shi, N.E.; Qian, Y.; Xie, L. Click Chemistry and Functional Organic/Polymeric Materials. Prog. Chem. 2010, 22, 406–416. [Google Scholar]
- Fuger, B.; Sklute, G.; Marek, I.; Bolm, G.Y.; Bolm, C. Synthesis of sulfoximidoyl-substituted triazoles by Huisgen 1,3-dipolar cycloaddition. Synlett 2008, 1, 116–118. [Google Scholar]
- Heravi, M.M.; Tamimi, M.; Yahyavi, H.; Hosseinnejad, T. Huisgen’s Cycloaddition Reactions: A Full Perspective. Curr. Org. Chem. 2016, 20, 1591–1647. [Google Scholar] [CrossRef]
- Chen, M.Y.; Xu, Z.; Chen, L.; Song, T.; Zheng, Z.J.; Cao, J.; Cui, Y.-M.; Xu, L.-W. Catalytic Asymmetric Huisgen Alkyne-Azide Cycloaddition of Bisalkynes by Copper(I) Nanoparticles. Chemcatchem 2018, 10, 280–286. [Google Scholar] [CrossRef]
- Candelon, N.; Lastecoueres, D.; Diallo, A.K.; Aranzaes, J.R.; Astruc, D.; Vincent, J.M. A highly active and reusable copper(I)-tren catalyst for the “click” 1,3-dipolar cycloaddition of azides and alkynes. Chem. Commun. 2008, 6, 741–743. [Google Scholar] [CrossRef]
- Totobenazara, J.; Burke, A.J. New click-chemistry methods for 1,2,3-triazoles synthesis: Recent advances and applications. Tetrahedron Lett. 2015, 56, 2853–2859. [Google Scholar] [CrossRef]
- Kumar, D.; Reddy, V.B. An Efficient, One-Pot, Regioselective Synthesis of 1,4-Diaryl-1H-1,2,3-triazoles Using Click Chemistry. Synthesis 2010, 10, 1687–1691. [Google Scholar] [CrossRef]
- Liu, F.B.; Zhang, X.L.; Jiang, W.S.; Yu, F.Y.; Deng, J.R. Study on the Curing System of Polytriazole Adhesive for Composite Solid Propellant. Propellants Explos. Pyrotech. 2018, 43, 371–378. [Google Scholar] [CrossRef]
- He, L.; Zhou, J.; Wang, Y.; Ma, Z.; Chen, C. Mechanical and Thermal Properties of Polyether Polytriazole Elastomers Formed by Click-Chemical Reaction Curing Glycidyl Azide Polymer. Molecules 2020, 25, 1988. [Google Scholar] [CrossRef]
- Hu, J.; Li, Y.; Xiao, F.; Zhang, Y.; He, J.; Yang, R. Thermal degradation and aging behavior of polytriazole polyethylene oxide-tetrahydrofuran elastomer based on click-chemistry. J. Appl. Polym. Sci. 2020, 137, 48974. [Google Scholar] [CrossRef]
- Gallos, A.; Fontaine, G.; Bourbigot, S. Reactive extrusion of intumescent stereocomplexed poly-L, D-lactide: Characterization and reaction to fire. Polym. Adv. Technol. 2013, 24, 130–133. [Google Scholar] [CrossRef]
- Fan, H.B.; Yang, R.J. Thermal decomposition of polyhedral oligomeric octaphenyl, octa(nitrophenyl), and octa(aminophenyl) silsesquioxanes. J. Therm. Anal. Calorim. 2014, 116, 349–357. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Wu, L. Preparation of poly(phenylsilsesquioxane) (PPSQ) particles with ladder structure and the thermal stability of PP/PPSQ composites. Polym. Adv. Technol. 2011, 22, 2151–2156. [Google Scholar] [CrossRef]
- Du, J.; Yang, R. Synthesis and Characterization of Polyhedral Octaphenylsilsesquioxane. Fine Chem. 2005, 22, 409–411. [Google Scholar]
- Moore, C.G.; Watson, W.F. Determination of degree of crosslinking in natural rubber vulcanizates. Part II. J. Polym. Sci. 1956, 19, 237–254. [Google Scholar]
- Qin, A.; Lam, J.W.Y.; Tang, B.Z. Click polymerization. Chem. Soc. Rev. 2010, 39, 2522–2544. [Google Scholar] [CrossRef]
- Flory, P.J., Jr. Statistical Mechanics of Cross-Linked Polymer Networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Bristow, G.M.; Watson, W.F. Cohesive energy densities of polymers. Part 2—Cohesive energy densities from viscosity measurements. Trans. Faraday Soc. 1958, 54, 1742–1747. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, S. Effect of crosslinking on the conductivity of conductive silicone rubber. J. Appl. Polym. Sci. 2003, 89, 3471–3475. [Google Scholar] [CrossRef]
















| Sample | Raw Materials Used in the Synthesis (g) | |||||
|---|---|---|---|---|---|---|
| ATPET | GAP | C2610 | PPSQ | OPS | PhVPOSS | |
| PTPET | 15 | 0.76 | 0.079 | - | - | - |
| PTPET-PPSQ | 15 | 0.76 | 0.079 | 1.6 | - | - |
| PTPET-OPS | 15 | 0.76 | 0.079 | - | 1.6 | - |
| PTPET-PhVPOSS | 15 | 0.76 | 0.079 | - | - | 1.6 |
| Sample | T5% (°C) | Tmax (°C) | Residue (%) |
|---|---|---|---|
| PTPET | 292 | 394 | 7.73 |
| PTPET-PPSQ | 347 | 396 | 8.55 |
| PTPET-OPS | 351 | 396 | 19.33 |
| PTPET-PhVPOSS | 349 | 399 | 8.29 |
| Sample | Tg (°C) |
|---|---|
| PTPET | −76 |
| PTPET-PPSQ | −72 |
| PTPET-OPS | −74 |
| PTPET-PhVPOSS | −75 |
| Samples | χ | qv | Vp | ρ (g·cm−3) | Mc (g·mol−1) | N0 (mmol·cm−3) |
|---|---|---|---|---|---|---|
| PTPET | 0.34 | 4.05 | 0.247 | 1.113 | 3776 | 0.295 |
| PTPET-PPSQ | 0.34 | 4.412 | 0.227 | 1.083 | 4457 | 0.243 |
| PTPET-OPS | 0.34 | 4.124 | 0.243 | 1.083 | 3820 | 0.284 |
| PTPET-PhVPOSS | 0.34 | 4.305 | 0.232 | 1.077 | 4192 | 0.257 |
| Sample | PTPET | PTPET-PPSQ | PTPET-OPS | PTPET-PhVPOSS |
|---|---|---|---|---|
| TTI (s) | 10 | 14 | 13 | 12 |
| p-HRR (kW/m2) | 998 | 897 | 737 | 1140 |
| THR (MJ/m2) | 101 | 124.37 | 104.71 | 112 |
| Mean CO | 0.005 | 0.015 | 0.007 | 0.006 |
| Mean CO2 | 0.555 | 1.93 | 0.836 | 0.78 |
| p-SPR (m2/s) | 0.013 | 0.039 | 0.0299 | 0.031 |
| TSR (m2/m2) | 152.7 | 519.76 | 438.5 | 421 |
| Residues (%) | 5.9 | 3.93 | 3.96 | 4.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cong, K.; Liu, Z.; Hu, F.; He, J.; Yang, R. Preparation and Performances of Polyether Polytriazole Elastomers Based on Click Chemistry. Polymers 2022, 14, 3538. https://doi.org/10.3390/polym14173538
Cong K, Liu Z, Hu F, He J, Yang R. Preparation and Performances of Polyether Polytriazole Elastomers Based on Click Chemistry. Polymers. 2022; 14(17):3538. https://doi.org/10.3390/polym14173538
Chicago/Turabian StyleCong, Kun, Zhenhui Liu, Fa Hu, Jiyu He, and Rongjie Yang. 2022. "Preparation and Performances of Polyether Polytriazole Elastomers Based on Click Chemistry" Polymers 14, no. 17: 3538. https://doi.org/10.3390/polym14173538
APA StyleCong, K., Liu, Z., Hu, F., He, J., & Yang, R. (2022). Preparation and Performances of Polyether Polytriazole Elastomers Based on Click Chemistry. Polymers, 14(17), 3538. https://doi.org/10.3390/polym14173538