Bio-Based Rigid Polyurethane Foams Modified with C-MOF/MWCNTs and TBPBP as Building Insulation Materials: Synergistic Effect and Corresponding Mechanism for Enhancing Fire and Smoke Safety
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
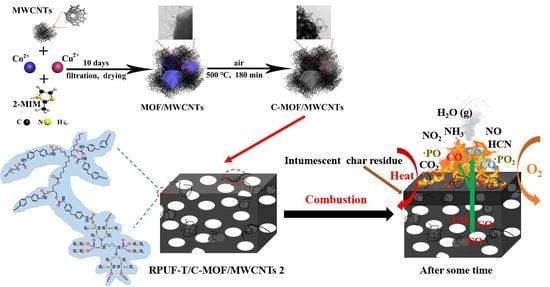
2.2. Preparation of MOF/MWCNTs and C-MOF/MWCNTs
2.3. Preparation of Bio-Based Rigid Polyurethane Foams (RPUFs)
2.4. Characterization
3. Results and Discussion
3.1. Characterization of C-MOF/MWCNTs
3.2. Characterization of RPUFs
3.3. Mechanism for Enhancing Fire and Smoke Safety
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vahabi, H.; Rastin, H.; Movahedifar, E.; Antoun, K.; Brosse, N.; Saeb, M.R. Flame Retardancy of Bio-Based Polyurethanes: Opportunities and Challenges. Polymers 2020, 12, 1234. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Salmeia, K.A.; Rentsch, D.; Hao, J.; Gaan, S. Thermal decomposition and flammability of rigid PU foams containing some DOPO derivatives and other phosphorus compounds. J. Anal. Appl. Pyrolysis 2017, 124, 219–229. [Google Scholar] [CrossRef]
- Akdogan, E.; Erdem, M.; Ureyen, M.E.; Kaya, M. Synergistic effects of expandable graphite and ammonium pentaborate octahydrate on the flame-retardant, thermal insulation, and mechanical properties of rigid polyurethane foam. Polym. Compos. 2020, 41, 1749–1762. [Google Scholar] [CrossRef]
- Sienkiewicz, A.; Czub, P. Flame Retardancy of Biobased Composites—Research Development. Materials 2020, 13, 5253. [Google Scholar] [CrossRef]
- Zhang, C.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R. Recent advances in vegetable oil-based polymers and their composites. Prog. Polym. Sci. 2017, 71, 91–143. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Wu, Y.; Li, W.; Chen, C. Functionalized graphene with Co-ZIF adsorbed borate ions as an effective flame retardant and smoke suppression agent for epoxy resin. J. Hazard. Mater. 2018, 363, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Hu, W.; Gui, Z.; Hu, Y. A novel Co(II)–based metal-organic framework with phosphorus-containing structure: Build for enhancing fire safety of epoxy. Compos. Sci. Technol. 2017, 152, 231–242. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Ge, X.; Ma, J.; Zhang, Z.; Li, Q.; Wang, C.; Yin, L. Reduced graphene oxide wrapped MOFs-derived cobalt-doped porous carbon polyhedrons as sulfur immobilizers as cathodes for high performance lithium sulfur batteries. Nano Energy 2016, 23, 15–26. [Google Scholar] [CrossRef]
- Hermes, S.; Schröter, M.-K.; Schmid, R.; Khodeir, L.; Muhler, M.; Tissler, A.; Fischer, R.W.; Fischer, R.A. Metal@MOF: Loading of Highly Porous Coordination Polymers Host Lattices by Metal Organic Chemical Vapor Deposition. Angew. Chem. Int. Ed. 2005, 44, 6237–6241. [Google Scholar] [CrossRef]
- Xu, W.; Wang, G.; Xu, J.; Liu, Y.; Chen, R.; Yan, H. Modification of diatomite with melamine coated zeolitic imidazolate framework-8 as an effective flame retardant to enhance flame retardancy and smoke suppression of rigid polyurethane foam. J. Hazard. Mater. 2019, 379, 120819. [Google Scholar] [CrossRef]
- Bo, G.; Xu, X.; Tian, X.; Wu, J.; Yan, Y. Enhancing the Fire Safety and Smoke Safety of Bio–Based Rigid Polyurethane Foam via Inserting a Reactive Flame Retardant Containing P@N and Blending Silica Aerogel Powder. Polymers 2021, 13, 2140. [Google Scholar] [CrossRef] [PubMed]
- Nethravathi, C.; Sen, S.; Ravishankar, N.; Rajamathi, M.; Pietzonka, C.; Harbrecht, B. Ferrimagnetic Nanogranular Co3O4 through Solvothermal Decomposition of Colloidally Dispersed Monolayers of α-Cobalt Hydroxide. J. Phys. Chem. B 2005, 109, 11468–11472. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.-H.; Tu, J.-P.; Zhang, Y.-Q.; Mai, Y.-J.; Wang, X.-L.; Gu, C.-D.; Zhao, X.-B. Freestanding Co3O4 nanowire array for high performance supercapacitors. RSC Adv. 2012, 2, 1835–1841. [Google Scholar] [CrossRef]
- Zhang, E.; Xie, Y.; Ci, S.; Jia, J.; Wen, Z. Porous Co3O4 hollow nanododecahedra for nonenzymatic glucose biosensor and biofuel cell. Biosens. Bioelectron. 2016, 81, 46–53. [Google Scholar] [CrossRef]
- Xu, W.; Chen, X.; Chen, J.; Jia, H. Bimetal oxide CuO/Co3O4 derived from Cu ions partly-substituted framework of ZIF-67 for toluene catalytic oxidation. J. Hazard. Mater. 2021, 403, 123869. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, H.; Guo, J.; Sun, J.; Li, H.; Zhang, S.; Gu, X. Preparation of cobalt-based metal organic framework and its application as synergistic flame retardant in thermoplastic polyurethane (TPU). Compos. Part B Eng. 2020, 182, 107498. [Google Scholar] [CrossRef]
- Li, X.; Gao, X.; Ai, L.; Jiang, J. Mechanistic insight into the interaction and adsorption of Cr(VI) with zeolitic imidazolate framework-67 microcrystals from aqueous solution. Chem. Eng. J. 2015, 274, 238–246. [Google Scholar] [CrossRef]
- Yao, J.; Chen, R.; Wang, K.; Wang, H. Direct synthesis of zeolitic imidazolate framework-8/chitosan composites in chitosan hydrogels. Micropor. Mesopor. Mat. 2013, 165, 200–204. [Google Scholar] [CrossRef]
- Hu, Y.; Kazemian, H.; Rohani, S.; Huang, Y.; Song, Y. In situ high pressure study of ZIF-8 by FTIR spectroscopy. Chem. Commun. 2011, 47, 12694. [Google Scholar] [CrossRef]
- Gross, A.F.; Sherman, E.; Vajo, J.J. Aqueous room temperature synthesis of cobalt and zinc sodalite zeolitic imidizolate frameworks. Dalton Trans. 2012, 41, 5458. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Li, M.; Wang, S.; Han, L.; Yuan, W.; Cheng, J.; Zhang, A.; Zhao, H.; Wang, Y. Hierarchically porous SiO2/polyurethane foam composites towards excellent thermal insulating, flame-retardant and smoke-suppressant performances. J. Hazard. Mater. 2019, 375, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Tai, Q.; Hu, Y.; Yuen, R.K.K.; Song, L.; Lu, H. Synthesis, structure–property relationships of polyphosphoramides with high char residues. J. Mater. Chem. 2011, 21, 6621. [Google Scholar] [CrossRef]
- Wang, P.; Xia, L.; Jian, R.; Ai, Y.; Zheng, X.; Chen, G.; Wang, J. Flame-retarding epoxy resin with an efficient P/N/S-containing flame retardant: Preparation, thermal stability, and flame retardance. Polym. Degrad. Stab. 2018, 149, 69–77. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Cai, S.; Chen, J.; Jia, H. Two-step pyrolytic engineering of carbon-doped Co3O4 with rich defects for efficient low-temperature CO oxidation. J. Mater. Chem. A 2020, 8, 6619–6630. [Google Scholar] [CrossRef]
- Koo, W.-T.; Yu, S.; Choi, S.-J.; Jang, J.-S.; Cheong, J.Y.; Kim, I.-D. Nanoscale PdO Catalyst Functionalized Co3O4 Hollow Nanocages Using MOF Templates for Selective Detection of Acetone Molecules in Exhaled Breath. ACS Appl. Mater. Interfaces 2017, 9, 8201–8210. [Google Scholar] [CrossRef]
- Khaleel, M.; Soykan, U.; Çetin, S. Influences of turkey feather fiber loading on significant characteristics of rigid polyurethane foam: Thermal degradation, heat insulation, acoustic performance, air permeability and cellular structure. Constr. Build. Mater. 2021, 308, 125014. [Google Scholar] [CrossRef]
- Cao, Z.-J.; Liao, W.; Wang, S.-X.; Zhao, H.-B.; Wang, Y.-Z. Polyurethane foams with functionalized graphene towards high fire-resistance, low smoke release, superior thermal insulation. Chem. Eng. J. 2019, 361, 1245–1254. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Nando, G.; Naik, Y.; Singha, N.K. Halogen-free flame retardant PUF: Effect of melamine compounds on mechanical, thermal and flame retardant properties. Polym. Degrad. Stab. 2010, 95, 1138–1145. [Google Scholar] [CrossRef]
- Wang, S.; Wang, S.; Shen, M.; Xu, X.; Liu, H.; Wang, D.; Wang, H.; Shang, S. Biobased Phosphorus Siloxane-Containing Polyurethane Foam with Flame-Retardant and Smoke-Suppressant Performances. ACS Sustain. Chem. Eng. 2021, 9, 8623–8634. [Google Scholar] [CrossRef]
- Qian, L.; Li, L.; Chen, Y.; Xu, B.; Qiu, Y. Quickly self-extinguishing flame retardant behavior of rigid polyurethane foams linked with phosphaphenanthrene groups. Compos. Part B Eng. 2019, 175, 107186. [Google Scholar] [CrossRef]
- Hamidov, M.; Çakmakçi, E.; Kahraman, M.V. Autocatalytic reactive flame retardants for rigid polyurethane foams. Mater. Chem. Phys. 2021, 267, 124636. [Google Scholar] [CrossRef]
- Zhao, C.; Yan, Y.; Hu, Z.; Li, L.; Fan, X. Preparation and characterization of granular silica aerogel/polyisocyanurate rigid foam composites. Constr. Build. Mater. 2015, 93, 309–316. [Google Scholar] [CrossRef]
- Gama, N.; Costa, L.C.; Amaral, V.; Ferreira, A.; Barros-Timmons, A. Insights into the physical properties of biobased polyurethane/expanded graphite composite foams. Compos. Sci. Technol. 2017, 138, 24–31. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, W.; Xiao, Y.; Yuen, A.C.Y.; Mao, L.; Pan, H.; Yu, B.; Hu, Y. Surface modification of multi-scale cuprous oxide with tunable catalytic activity towards toxic fumes and smoke suppression of rigid polyurethane foam. Appl. Surf. Sci. 2021, 556, 149792. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z.; Zhu, M. Flame retardant, mechanical and thermal insulating properties of rigid polyurethane foam modified by nano zirconium amino-tris-(methylenephosphonate) and expandable graphite. Polym. Degrad. Stab. 2019, 170, 108997. [Google Scholar] [CrossRef]
- Andersons, J.; Kirpluks, M.; Cabulis, P.; Kalnins, K. Bio-based rigid high-density polyurethane foams as a structural thermal break material. Constr. Build. Mater. 2020, 260, 120471. [Google Scholar] [CrossRef]
- Duan, H.-J.; Kang, H.-Q.; Zhang, W.-Q.; Ji, X.; Li, Z.-M.; Tang, J.-H. Core-shell structure design of pulverized expandable graphite particles and their application in flame-retardant rigid polyurethane foams. Polym. Int. 2014, 63, 72–83. [Google Scholar] [CrossRef]
- Blomfeldt, T.O.J.; Nilsson, F.; Holgate, T.; Xu, J.; Johansson, E.; Hedenqvist, M.S. Thermal Conductivity and Combustion Properties of Wheat Gluten Foams. ACS Appl. Mater. Interfaces 2012, 4, 1629–1635. [Google Scholar] [CrossRef]
- Perez, M.A.R.; Alonso, O.; Souto, J.; de Saja, J. Thermal conductivity of physically crosslinked closed cell polyolefin foams. Polym. Test. 1997, 16, 287–298. [Google Scholar] [CrossRef]
- Septevani, A.A.; Evans, D.A.; Annamalai, P.K.; Martin, D. The use of cellulose nanocrystals to enhance the thermal insulation properties and sustainability of rigid polyurethane foam. Ind. Crop. Prod. 2017, 107, 114–121. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Strzelec, K.; Kairytė, A.; Kremensas, A. Melamine, silica, and ionic liquid as a novel flame retardant for rigid polyurethane foams with enhanced flame retardancy and mechanical properties. Polym. Test. 2020, 87, 106511. [Google Scholar] [CrossRef]
- Choe, H.; Choi, Y.; Kim, J.H. Threshold cell diameter for high thermal insulation of water-blown rigid polyurethane foams. J. Ind. Eng. Chem. 2019, 73, 344–350. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, S.-A. Synthesis and properties of rigid polyurethane foams synthesized from modified urea-formaldehyde resin. Constr. Build. Mater. 2019, 202, 718–726. [Google Scholar] [CrossRef]
- Członka, S.; Kerche, E.F.; Neves, R.M.; Strąkowska, A.; Strzelec, K. Bio-Based Rigid Polyurethane Foam Composites Reinforced with Bleached Curauá Fiber. Int. J. Mol. Sci. 2021, 22, 11203. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Qian, X.; Liu, Q.; Zhang, L.; Li, H.; Liu, J.; Yan, S. Synthesis of reactive DOPO-based flame retardant and its application in rigid polyisocyanurate-polyurethane foam. Polym. Degrad. Stab. 2022, 197, 109852. [Google Scholar] [CrossRef]
- Jiao, L.; Xiao, H.; Wang, Q.; Sun, J. Thermal degradation characteristics of rigid polyurethane foam and the volatile products analysis with TG-FTIR-MS. Polym. Degrad. Stab. 2013, 98, 2687–2696. [Google Scholar] [CrossRef]
- Ding, H.; Huang, K.; Li, S.; Xu, L.; Xia, J.; Li, M. Synthesis of a novel phosphorus and nitrogen-containing bio-based polyol and its application in flame retardant polyurethane foam. J. Anal. Appl. Pyrolysis 2017, 128, 102–113. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Essawy, H.; Pizzi, A.; Fredon, E.; Gerardin, C.; Du, G.; Zhou, X. Flame-retardant and thermally-insulating tannin and soybean protein isolate (SPI) based foams for potential applications in building materials. Constr. Build. Mater. 2022, 315, 125711. [Google Scholar] [CrossRef]
- Xi, W.; Qian, L.; Chen, Y.; Wang, J.; Liu, X. Addition flame-retardant behaviors of expandable graphite and [bis(2-hydroxyethyl)amino]-methyl-phosphonic acid dimethyl ester in rigid polyurethane foams. Polym. Degrad. Stab. 2015, 122, 36–43. [Google Scholar] [CrossRef]
- Gama, N.V.; Silva, R.; Mohseni, F.; Davarpanah, A.; Amaral, V.; Ferreira, A.; Barros-Timmons, A. Enhancement of physical and reaction to fire properties of crude glycerol polyurethane foams filled with expanded graphite. Polym. Test. 2018, 69, 199–207. [Google Scholar] [CrossRef]
- Lorenzetti, A.; Dittrich, B.; Schartel, B.; Roso, M.; Modesti, M. Expandable graphite in polyurethane foams: The effect of expansion volume and intercalants on flame retardancy. J. Appl. Polym. Sci. 2017, 134, 45173. [Google Scholar] [CrossRef]
- Modesti, M.; Lorenzetti, A.; Besco, S.; Hrelja, D.; Semenzato, S.; Bertani, R.; Michelin, R. Synergism between flame retardant and modified layered silicate on thermal stability and fire behaviour of polyurethane nanocomposite foams. Polym. Degrad. Stab. 2008, 93, 2166–2171. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, P.; Shi, Y.; Rao, X.; Cai, S.; Fu, L.; Feng, Y.; Wang, L.; Zheng, X.; Yang, W. Enhanced Fire Safety of Rigid Polyurethane Foam via Synergistic Effect of Phosphorus/Nitrogen Compounds and Expandable Graphite. Molecules 2020, 25, 4741. [Google Scholar] [CrossRef]
- Wu, N.; Niu, F.; Lang, W.; Yu, J.; Fu, G. Synthesis of reactive phenylphosphoryl glycol ether oligomer and improved flame retardancy and mechanical property of modified rigid polyurethane foams. Mater. Des. 2019, 181, 107929. [Google Scholar] [CrossRef]
- Qian, L.; Feng, F.; Tang, S. Bi-phase flame-retardant effect of hexa-phenoxy-cyclotriphosphazene on rigid polyurethane foams containing expandable graphite. Polymer 2014, 55, 95–101. [Google Scholar] [CrossRef]
- Jansson, J.; Palmqvist, A.E.; Fridell, E.; Skoglundh, M.; Österlund, L.; Thormählen, P.; Langer, V. On the Catalytic Activity of Co3O4 in Low-Temperature CO Oxidation. J. Catal. 2002, 211, 387–397. [Google Scholar] [CrossRef]















| Samples | MCOP | PEG-200 | TBPBP | Deionized Water | DMP-30 | Silicone Oil | MOF /MWCNTs | C-MOF /MWCNTs | pMDI |
|---|---|---|---|---|---|---|---|---|---|
| neat RPUF [11] | 80 | 20 | 0 | 4 | 2 | 4 | 0 | 0 | 146.79 |
| RPUF-MOF/MWCNTs 1 | 80 | 20 | 0 | 4 | 2 | 4 | 5 | 0 | 146.79 |
| RPUF-MOF/MWCNTs 2 | 80 | 20 | 0 | 4 | 2 | 4 | 10 | 0 | 146.79 |
| RPUF-MOF/MWCNTs 3 | 80 | 20 | 0 | 4 | 2 | 4 | 15 | 0 | 146.79 |
| RPUF-T [11] | 35 | 20 | 45 | 4 | 2 | 4 | 0 | 0 | 154.91 |
| RPUF-T/MOF/MWCNTs 2 | 35 | 20 | 45 | 4 | 2 | 4 | 10 | 0 | 154.91 |
| RPUF-MOF/C-MWCNTs 2 | 80 | 20 | 0 | 4 | 2 | 4 | 0 | 10 | 146.79 |
| RPUF-T/C-MOF/MWCNTs 2 | 35 | 20 | 45 | 4 | 2 | 4 | 0 | 10 | 154.91 |
| Sample | Density (kg/m3) | Compressive Strength (kPa) | Thermal Conductivity Coefficient W/(mK) |
|---|---|---|---|
| neat RPUF [11] | 36.7 ± 0.9 | 236 ± 29 | 31.67 × 10−3 |
| RPUF-MOF/MWCNTs 1 | 37.6 ± 0.5 | 301 ± 26 | 33.26 × 10−3 |
| RPUF-MOF/MWCNTs 2 | 38.5 ± 0.6 | 358 ± 17 | 34.01 × 10−3 |
| RPUF-MOF/MWCNTs 3 | 39.0 ± 0.8 | 379 ± 28 | 34.29 × 10−3 |
| RPUF-T [11] | 38.2 ± 0.7 | 422 ± 30 | 34.65 × 10−3 |
| RPUF-T/MOF/MWCNTs 2 | 40.9 ± 1.1 | 453 ± 39 | 35.53 × 10−3 |
| RPUF-C-MOF/MWCNTs 2 | 38.7 ± 0.9 | 367 ± 23 | 33.88 × 10−3 |
| RPUF-T/C-MOF/MWCNTs 2 | 40.6±1.3 | 486 ± 25 | 34.93 × 10−3 |
| Samples | LOI (%) | UL-94 |
|---|---|---|
| neat RPUF | 19.2 | N.R. |
| RPUF-MOF/MWCNTs 1 | 19.6 | N.R. |
| RPUF-MOF/MWCNTs 2 | 20.1 | N.R. |
| RPUF-MOF/MWCNTs 3 | 20.4 | N.R. |
| RPUF-T | 25.6 | V-0 |
| RPUF-T/MOF/MWCNTs 2 | 27.4 | V-0 |
| RPUF-C-MOF/MWCNTs 2 | 20.3 | N.R. |
| RPUF-T/C-MOF/MWCNTs 2 | 28.1 | V-0 |
| Samples | TTI | pHHR | THR | pSPR | TSP | Av-EHC | Residue |
|---|---|---|---|---|---|---|---|
| s | kW/m2 | MJ/m2 | m2/s | m2 | MJ/kg | wt% | |
| neat RPUF | 0 | 315.56 | 30.54 | 0.096 | 7.69 | 21.36 | 3.17 |
| RPUF-MOF/MWCNTs 2 | 0 | 261.76 | 28.78 | 0.078 | 7.64 | 20.23 | 4.06 |
| RPUF-T | 3 | 287.69 | 20.67 | 0.119 | 5.07 | 15.75 | 9.29 |
| RPUF-T/MOF/MWCNTs 2 | 3 | 246.15 | 19.84 | 0.092 | 4.56 | 14.97 | 11.83 |
| RPUF-C-MOF/MWCNTs 2 | 0 | 242.94 | 26.79 | 0.071 | 6.79 | 22.04 | 5.26 |
| RPUF-T/C-MOF/MWCNTs 2 | 4 | 165.55 | 15.89 | 0.059 | 3.81 | 13.26 | 14.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bo, G.; Xu, X.; Tian, X.; Yan, J.; Su, X.; Yan, Y. Bio-Based Rigid Polyurethane Foams Modified with C-MOF/MWCNTs and TBPBP as Building Insulation Materials: Synergistic Effect and Corresponding Mechanism for Enhancing Fire and Smoke Safety. Polymers 2022, 14, 3630. https://doi.org/10.3390/polym14173630
Bo G, Xu X, Tian X, Yan J, Su X, Yan Y. Bio-Based Rigid Polyurethane Foams Modified with C-MOF/MWCNTs and TBPBP as Building Insulation Materials: Synergistic Effect and Corresponding Mechanism for Enhancing Fire and Smoke Safety. Polymers. 2022; 14(17):3630. https://doi.org/10.3390/polym14173630
Chicago/Turabian StyleBo, Guangxu, Xiaoling Xu, Xiaoke Tian, Jinyong Yan, Xingjian Su, and Yunjun Yan. 2022. "Bio-Based Rigid Polyurethane Foams Modified with C-MOF/MWCNTs and TBPBP as Building Insulation Materials: Synergistic Effect and Corresponding Mechanism for Enhancing Fire and Smoke Safety" Polymers 14, no. 17: 3630. https://doi.org/10.3390/polym14173630
APA StyleBo, G., Xu, X., Tian, X., Yan, J., Su, X., & Yan, Y. (2022). Bio-Based Rigid Polyurethane Foams Modified with C-MOF/MWCNTs and TBPBP as Building Insulation Materials: Synergistic Effect and Corresponding Mechanism for Enhancing Fire and Smoke Safety. Polymers, 14(17), 3630. https://doi.org/10.3390/polym14173630