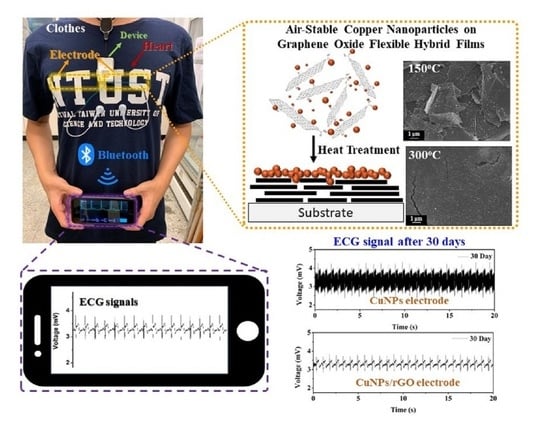
Immobilization of Air-Stable Copper Nanoparticles on Graphene Oxide Flexible Hybrid Films for Smart Clothes
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Branched Polyester Polymeric Dispersant
2.3. Preparation of the CuNPs/PTT/GO Nanohybrids
2.4. Preparation of the CuNPs/PTT/GO Film Electrode
2.5. Characterization and Instruments
3. Results and Discussion
3.1. Dispersion Characteristic of CuNPs/PTT/GO Nanohybrids
3.2. Preparation and Surface Morphology of CuNPs/PTT/GO Composite Films
3.3. Antioxidation and High Conductivity Stability of CuNPs/PTT/GO Composite Films
3.4. Signal Measurement with the ECG Smart Clothes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- An, B.W.; Shin, J.H.; Kim, S.Y.; Kim, J.; Ji, S.; Park, J.; Lee, Y.; Jang, J.; Park, Y.G.; Cho, E.; et al. Smart Sensor Systems for Wearable Electronic Devices. Polymers 2017, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Yun, K.S. ECG Monitoring Garment Using Conductive Carbon Paste for Reduced Motion Artifacts. Polymers 2017, 9, 439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celik, N.; Manivannan, N.; Strudwick, A.; Balanchendran, W. Graphene-Enabled Electrodes for Electrocardiogram Monitoring. Nanomaterials 2016, 6, 156. [Google Scholar] [CrossRef] [Green Version]
- Yun, I.; Jeung, J.; Lim, H.; Kang, J.; Lee, S.; Park, S.; Seong, S.; Park, S.; Cho, K.; Chung, Y. Stable Bioelectric Signal Acquisition Using an Enlarged Surface-Area Flexible Skin Electrode. ACS Appl. Electron. Mater. 2021, 3, 1842–1851. [Google Scholar] [CrossRef]
- Bu, C.; Li, F.; Yin, K.; Pang, J.; Wang, L.; Wang, K. Research Progress and Prospect of Triboelectric Nanogenerators as Self-Powered Human Body Sensors. ACS Appl. Electron. Mater. 2020, 2, 863–878. [Google Scholar] [CrossRef]
- Chiu, C.W.; Li, J.W.; Huang, C.Y.; Yang, S.S.; Soong, Y.C.; Lin, C.L.; Lee, J.C.; Lee Sanchez, W.A.; Cheng, C.C.; Suen, M.C. Controlling the Structures, Flexibility, Conductivity Stability of Three-Dimensional Conductive Networks of Silver Nanoparticles/Carbon-Based Nanomaterials with Nanodispersion and their Application in Wearable Electronic Sensors. Nanomaterials 2020, 10, 1009. [Google Scholar] [CrossRef]
- Dong, S.; Han, B.; Ou, J.; Li, Z.; Han, L.; Yu, X. Electrically Conductive Behaviors and Mechanisms of Short-Cut Super-Fine Stainless Wire Reinforced Reactive Powder Concrete. Cem. Concr. Compos. 2016, 720, 48–65. [Google Scholar] [CrossRef] [Green Version]
- Han, W.B.; Ko, G.J.; Jang, T.M.; Hwang, S.W. Materials, Devices, and Applications for Wearable and Implantable Electronics. ACS Appl. Electron. Mater. 2021, 3, 485–503. [Google Scholar] [CrossRef]
- Li, S.; Xiao, X.; Hu, J.; Dong, M.; Zhang, Y.; Xu, R.; Wang, X.; Islam, J. Recent Advances of Carbon-Based Flexible Strain Sensors in Physiological Signal Monitoring. ACS Appl. Electron. Mater. 2020, 2, 2282–2300. [Google Scholar]
- Su, M.; Li, F.; Chen, S.; Huang, Z.; Qin, M.; Li, W.; Zhang, X.; Song, Y. Nanoparticle Based Curve Arrays for Multirecognition Flexible Electronics. Adv. Mater. 2015, 28, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Chiu, C.W. Facile Fabrication of Stretchable and Flexible Nanofiber Carbon Film Sensing Electrode by Electrospinning and its Application in Smart Clothing for ECG and EMG Monitoring. ACS Appl. Electron. Mater. 2020, 3, 676–686. [Google Scholar] [CrossRef]
- Song, Y.; Fan, R.Q.; Xing, K.; Du, X.; Su, T.; Wang, P.; Yang, Y.L. Insight into the Controllable Synthesis of Cu (I)/Cu (II) Metal–Organic Complexes: Size-Exclusive Selective Dye Adsorption and Semiconductor Properties. Cryst. Growth Des. 2017, 17, 2549–2559. [Google Scholar] [CrossRef]
- Guan, Z.; Chen, F.; Liu, Z.; Lv, P.; Chen, M.; Guo, M.; Li, X.; Teng, F.; Chen, S.; Tang, A. Compositional Engineering of Multinary Cu–In–Zn-Based Semiconductor Nanocrystals for Efficient and Solution-Processed Red-emitting Quantum-Dot Light-Emitting Diodes. Org. Electron. 2019, 74, 46–51. [Google Scholar] [CrossRef]
- Avancini, E.; Carron, R.; Weiss, T.P.; Andres, C.; Bürki, M.; Schreiner, C.; Figi, R.; Romanyuk, Y.E.; Buecheler, S.; Tiwari, A.N. Effects of Rubidium Fluoride and Potassium Fluoride Postdeposition Treatments on Cu (In, Ga) Se2 Thin Films and Solar Cell Performance. Chem. Mater. 2017, 29, 9695–9704. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Wang, Y.; Chen, W.; Chen, Q.; Wu, J.; Kuznetsov, A.; Du, X. 18.87%-Efficient Inverted Pyramid Structured Silicon Solar Cell by One-Step Cu-Assisted Texturization Technique. Sol. Energy Mater Sol. Cells 2017, 166, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Li, Y.; Luo, G.; Shi, K.; Liu, E.; Zhou, J. Using a Dynamic Inhibition Concept to Achieve Content-Controllable Synthesis of N-Coordinated Cu Atoms as Reversible Active Site toward Super Li-Ion Capacitors. Adv. Energy Mater. 2020, 10, 2002644. [Google Scholar] [CrossRef]
- Niu, H.; Liu, Y.; Mao, B.; Xin, N.; Jia, H.; Shi, W. In-situ Embedding MOFs-Derived Copper Sulfide Polyhedrons in Carbon Nanotube Networks for Hybrid Supercapacitor with Superior Energy Density. Electrochim. Acta 2020, 329, 135130. [Google Scholar] [CrossRef]
- Cu, Y.T.; Tran, M.V.; Ho, C.H.; Nguyen, P.H. Relationship between Workability and Rheological Parameters of Self-Compacting Concrete Used for Vertical Pump up to Supertall Buildings. J. Build. Eng. 2020, 32, 101786. [Google Scholar] [CrossRef]
- Shalaby, R.M.; Kamal, M.; Ali, E.A.M.; Gumaan, M.S. Design and Properties of New Lead-Free Solder Joints Using Sn-3.5 Ag-Cu Solder. Silicon 2018, 10, 1861–1871. [Google Scholar] [CrossRef]
- Kubo, Y.; Tanaka, H.; Saito, Y.; Mizoguchi, A. Fabrication of a Bilayer Structure of Cu and Polyimide to Realize Circuit Microminiaturization and High Interfacial Adhesion in Flexible Electronic Devices. ACS Appl. Mater. Interfaces 2018, 10, 44589–44602. [Google Scholar] [CrossRef]
- Yang, S.; Wang, D.; Liu, H.; Liu, C.; Xie, X.; Xu, Z.; Liu, Z. Highly Stable Activated Carbon Composite Material to Selectively Capture Gas-Phase Elemental Mercury from Smelting Flue Gas: Copper Polysulfide Modification. Chem. Eng. J. 2019, 358, 1235–1242. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A.; Durán-Martín, D.; Moreno-Tost, R.; Santamaría-González, J.; Mérida-Robles, J.; Mariscal, R.; Maireles-Torres, P. Gas-Phase Hydrogenation of Furfural to Furfuryl Alcohol Over Cu/ZnO Catalysts. J. Catal. 2016, 336, 107–115. [Google Scholar] [CrossRef]
- Dastkhoon, M.; Ghaedi, M.; Asfaram, A.; Arabi, M.; Ostovan, A.; Goudarzi, A. Cu@SnS/SnO2 Nanoparticles as Novel Sorbent for Dispersive Micro Solid Phase Extraction of Atorvastatin in Human Plasma and Urine Samples by High-Performance Liquid Chromatography with UV Detection: Application of Central Composite Design (CCD). Ultrason. Sonochem. 2017, 36, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Wang, Y.; Xu, K.; Li, N.; Zhang, H.; Yang, Q. A Novel Polymeric Ionic Liquid-Coated Magnetic Multiwalled Carbon Nanotubes for The Solid-Phase Extraction of Cu, Zn-Superoxide Dismutase. Anal. Chim. Acta 2016, 939, 54–63. [Google Scholar] [CrossRef]
- Villaverde, M.M.; Bertero, N.M.; Garetto, T.F.; Marchi, A.J. Selective Liquid-Phase Hydrogenation of Furfural to Furfuryl Alcohol Over Cu-based Catalysts. Catal. Today 2013, 213, 87–92. [Google Scholar] [CrossRef]
- Sun, S.H.; Jung, S.C. Facile Synthesis of Bimetallic Ni-Cu Nanoparticles Using Liquid Phase Plasma Method. Korean J. Chem. Eng. 2016, 33, 1075–1079. [Google Scholar] [CrossRef]
- Vegas, V.G.; Villar-Alonso, M.; Gómez-García, C.J.; Zamora, F.; Amo-ochoa, P. Direct Formation of Sub-Micron and Nanoparticles of a Bioinspired Coordination Polymer Based on Copper with Adenine. Polymers 2017, 9, 565. [Google Scholar] [CrossRef] [Green Version]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal Nanoparticles Synthesis: An Overview on Methods of Preparation, Advantages and Disadvantages, and Applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Pozdnyakov, A.S.; Emel’Yanov, A.I.; Korzhova, S.A.; Kuznetsova, N.P.; Bolgova, Y.I.; Trofimova, O.M.; Semenova, T.A.; Prozorova, G.F. Green Synthesis of Stable Nanocomposites Containing Copper Nanoparticles Incorporated in Poly-N-vinylimidazole. Polymers 2021, 13, 3212. [Google Scholar] [CrossRef]
- Li, J.W.; Chen, Y.S.; Chen, Y.F.; Chen, J.X.; Kuo, C.F.J.; Chen, L.Y.; Chiu, C.W. Enhanced Efficiency of Dye-Sensitized Solar Cells Based on Polymer-Assisted Dispersion of Platinum Nanoparticles/Carbon Nanotubes Nanohybrid Films as FTO-Free Counter Electrodes. Polymers 2021, 13, 3103. [Google Scholar] [CrossRef]
- Jardón-Maximino, N.; Pérez-Alvarez, M.; Cadenas-Pliego, G.; Lugo-Uribe, L.E.; Cabello-Alvarado, C.; Mata-Padilla, J.M.; Barriga-Castro, E.D. Synthesis of Copper Nanoparticles Stabilized with Organic Ligands and Their Antimicrobial Properties. Polymers 2021, 13, 2846. [Google Scholar] [CrossRef]
- Chiu, C.W.; Ou, G.B. Facile Preparation of Highly Electrically Conductive Films of Silver Nanoparticles Finely Dispersed in Polyisobutylene-b-Poly(oxyethylene)-b-Polyisobutylene Triblock Copolymers and Graphene Oxide Hybrid Surfactants. RSC Adv. 2015, 5, 102462–102468. [Google Scholar] [CrossRef]
- Soong, Y.C.; Li, J.W.; Chen, Y.F.; Chen, J.X.; Sanchez, W.A.L.; Tsai, W.Y.; Chou, T.Y.; Cheng, C.C.; Chiu, C.W. Polymer-Assisted Dispersion of Boron Nitride/Graphene in a Thermoplastic Polyurethane Hybrid for Cooled Smart Clothes. ACS Omega 2021, 6, 28779–28787. [Google Scholar] [CrossRef]
- Soong, Y.C.; Chiu, C.W. Multilayered Graphene/Boron Nitride/Thermoplastic Polyurethane Composite Films with High Thermal Conductivity, Stretchability, and Washability for Adjustable-Cooling Smart Clothes. J. Colloid Interface Sci. 2021, 599, 611–619. [Google Scholar] [CrossRef]
- Chen, L.; Yu, G.; Chu, Y.; Zhang, J.; Hu, B.; Zhang, X. Effect of Three Types of Surfactants on Fabrication of Cu-Coated Graphite Powders. Adv. Powder Technol. 2014, 24, 281–287. [Google Scholar] [CrossRef]
- Hosseini, S.R.; Ghasemi, S.; Ghasemi, S.A. Effect of Surfactants on Electrocatalytic Performance of Copper Nanoparticles for Hydrogen Evolution Reaction. J. Mol. Liq. 2016, 222, 1068–1075. [Google Scholar] [CrossRef]
- Chiu, C.W.; Wu, M.T.; Lin, C.L.; Li, J.W.; Huang, C.Y.; Soong, Y.C.; Lee, J.C.M.; Lee Sanchez, W.A.; Lin, H.Y. Adsorption Performance for Reactive Blue 221 Dye of β-Chitosan/Polyamine Functionalized Graphene Oxide Hybrid Adsorbent with High Acid–Alkali Resistance Stability in Different Acid–Alkaline Environments. Nanomaterials 2020, 10, 748. [Google Scholar] [CrossRef]
- Mevold, A.H.; Hsu, W.W.; Hardiansyah, A.; Huang, L.Y.; Yang, M.C.; Liu, T.Y.; Liu, T.Y.; Chan, T.Y.; Wang, K.S.; Su, Y.A.; et al. Fabrication of Gold Nanoparticles/Graphene-PDDA Nanohybrids for Bio-Detection by SERS Nanotechnology. Nanoscale Res. Lett. 2015, 10, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.; Xu, J.; Lu, L.; Zhu, X.; Gao, Y.; Xing, H.; Yu, Y.F.; Ding, W.C.; Liu, Z. Copper Nanoparticle/Graphene Oxide/Single Wall Carbon Nanotube Hybrid Materials as Electrochemical Sensing Platform for Nonenzymatic Glucose Detection. J. Electroanal. Chem. 2016, 761, 118–124. [Google Scholar] [CrossRef]
- Xie, J.; Chen, Q.; Shen, H.; Li, G. Wearable Graphene Devices for Sensing. J. Electrochem. Soc. 2020, 167, 037541. [Google Scholar] [CrossRef]
- Chiu, C.W.; Ou, G.B.; Tsai, Y.H.; Lin, J.J. Immobilization of Silver Nanoparticles on Exfoliated Mica Nanosheets to Form Highly Conductive Nanohybrid Films. Nanotechnology 2015, 26, 465702. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.W.; Lee, Y.C.; Ou, G.B.; Cheng, C.C. Controllable 3D Hot-Junctions of Silver Nanoparticles Stabilized by Amphiphilic Tri-block Copolymer/Graphene Oxide Hybrid Surfactants for Use as Surface-Enhanced Raman Scattering Substrates. Ind. Eng. Chem. Res. 2017, 56, 2935–2942. [Google Scholar] [CrossRef]
- Huang, P.Y.; Chiu, C.W.; Huang, C.Y.; Shen, S.Y.; Lee, Y.C.; Cheng, C.C.; Jeng, R.J.; Lin, J.J. Facile Fabrication of Flexible Electrodes and Immobilization of Silver Nanoparticles on Nanoscale Silicate Platelets to Form Highly Conductive Nanohybrid Films for Wearable Electronic Devices. Nanomaterials 2020, 10, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, D.; Soroka, Y.; Ma’or, Z.; Oron, M.; Portugal-Cohen, M.; Brégégère, F.M.; Berhanu, D.; Valsami-Jones, E.; Hai, N.; Milner, Y. Evaluation of Topically Applied Copper (II) Oxide Nanoparticle Cytotoxicity in Human Skin Organ Culture. Toxicol. In Vitro 2013, 27, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, I.; Crosera, M.; Ortelli, S.; Blosi, M.; Adami, G.; Filon, F.L.; Costa, A.L. CuO Nanoparticle Penetration through Intact and Damaged Human Skin. New J. Chem. 2019, 43, 17033–17039. [Google Scholar] [CrossRef]
- Kwon, Y.T.; Kim, Y.S.; Lee, Y.; Kwon, S.; Lim, M.; Song, Y.; Choa, Y.H.; Yeo, W.H. Ultrahigh Conductivity and Superior Interfacial Adhesion of a Nanostructured, Photonic-Sintered Copper Membrane for Printed Flexible Hybrid Electronics. ACS Appl. Mater. Interfaces 2018, 10, 44071–44079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leu, Y.A.; Lu, Y.A.; Yeh, M.H.; Shih, P.T.; Shen, S.Y.; Ho, K.C.; Lin, J.J. Designing Novel Poly (Oxyalkylene)-Segmented Ester-Based Polymeric Dispersants for Efficient TiO2 Photoanodes of Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2018, 2018 10, 38394–38403. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chiu, C.W. Immobilization and 3D Hot-Junction Formation of Gold Nanoparticles on Two-Dimensional Silicate Nanoplatelets as Substrates for High-Efficiency Surface-Enhanced Raman Scattering Detection. Nanomaterials 2019, 9, 324. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Dai, S.; Wang, X.; He, X.; Wang, M.; Xi, Y.; Hu, C. Nanorod-Aggregated Flower-Like CuO Grown on a Carbon Fiber Fabric for a Super High Sensitive Non-Enzymatic Glucose Sensor. J. Mater. Chem. B 2015, 3, 5777–5785. [Google Scholar] [CrossRef]
- Shathi, M.A.; Chen, M.; Khoso, N.A.; Rahman, M.T.; Bhattacharjee, B. Graphene Coated Textile Based Highly Flexible and Washable Sports Bra for Human Health Monitoring. Mater. Des. 2020, 193, 108792. [Google Scholar] [CrossRef]
- Zahed, M.A.; Barman, S.C.; Sharifuzzaman, M.; Zhang, S.; Yoon, H.; Park, C.; Yoon, S.H.; Park, J.Y. Polyaziridine-Encapsulated Phosphorene-Incorporated Flexible 3D Porous Graphene for Multimodal Sensing and Energy Storage Applications. Adv. Funct. Mater. 2021, 31, 2009018. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Wang, Y.; Su, X.; Wang, C.; Zhang, M.; Jian, M.; Xia, K.; Liang, X.; Lu, H.; et al. Laser Writing of Janus Graphene/Kevlar Textile for Intelligent Protective Clothing. ACS Nano 2020, 14, 3219–3226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zhang, F.; Lu, K.; Abdulaziz, M.; Li, C.; Zhang, C.; Li, Y. Nano-Copper Enhanced Flexible Device for Simultaneous Measurement of Human Respiratory and Electro-Cardiac Activities. J. Nanobiotechnol. 2020, 18, 82. [Google Scholar] [CrossRef] [PubMed]







| CuNPs/PTT/GO | UV-Vis Absorption (nm) | Average CuNPs Sizeby TEM (nm) a | Sheet Resistance (Ω/sq) b |
|---|---|---|---|
| 1:1:0 | 571 | 63.3 | 6.92 × 10−2 |
| 20:20:1 | 577 | 35.9 | 3.45 × 100 |
| 10:10:1 | 579 | 36.7 | 8.12 × 10−2 |
| 5:5:1 | 585 | 14.2 | 9.41 × 10−2 |
| 1:1:1 | 598 | 44.0 | 4.27 × 101 |
| Materials | Mathod | Ohmic Resistance (Ohm) | Application | Reference |
|---|---|---|---|---|
| CuO/carbon fiber fabric | hydrothermal method | 0.67 | non-enzymatic glucose sensor | [49] |
| PEDOT:PSS/rGO | pad-dry-cure method | 125 | electrocardiogram | [50] |
| PEP/3DPG a | drop-cast | 105.3 | immunosensing, electrocardiogram recording, and microsupercapacitors (MSCs) | [51] |
| LIG b | laser-induced | 10.6 | electrocardiogram | [52] |
| Carbon fiber/CuNPs | electrochemical synthesized | 7.96 | electrocardiogram | [53] |
| AgNPs/PIB-POE-PIB/GO | heated | 0.012 | electrocardiogram | [6] |
| CuNPs/PTT/GO | heated | 0.0812 | electrocardiogram | Current study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, P.-Y.; Huang, C.-Y.; Li, J.-W.; Shen, S.-Y.; Cheng, C.-C.; Chiu, C.-W.; Jeng, R.-J.; Lin, J.-J. Immobilization of Air-Stable Copper Nanoparticles on Graphene Oxide Flexible Hybrid Films for Smart Clothes. Polymers 2022, 14, 237. https://doi.org/10.3390/polym14020237
Huang P-Y, Huang C-Y, Li J-W, Shen S-Y, Cheng C-C, Chiu C-W, Jeng R-J, Lin J-J. Immobilization of Air-Stable Copper Nanoparticles on Graphene Oxide Flexible Hybrid Films for Smart Clothes. Polymers. 2022; 14(2):237. https://doi.org/10.3390/polym14020237
Chicago/Turabian StyleHuang, Peng-Yang, Chen-Yang Huang, Jia-Wun Li, Sheng-Yen Shen, Chih-Chia Cheng, Chih-Wei Chiu, Ru-Jong Jeng, and Jiang-Jen Lin. 2022. "Immobilization of Air-Stable Copper Nanoparticles on Graphene Oxide Flexible Hybrid Films for Smart Clothes" Polymers 14, no. 2: 237. https://doi.org/10.3390/polym14020237
APA StyleHuang, P. -Y., Huang, C. -Y., Li, J. -W., Shen, S. -Y., Cheng, C. -C., Chiu, C. -W., Jeng, R. -J., & Lin, J. -J. (2022). Immobilization of Air-Stable Copper Nanoparticles on Graphene Oxide Flexible Hybrid Films for Smart Clothes. Polymers, 14(2), 237. https://doi.org/10.3390/polym14020237