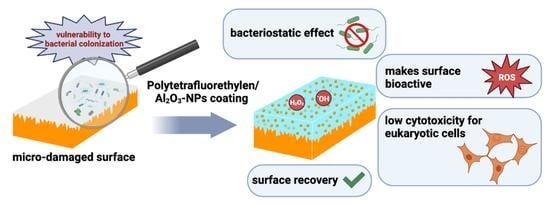
A Polytetrafluoroethylene (PTFE) and Nano-Al2O3 Based Composite Coating with a Bacteriostatic Effect against E. coli and Low Cytotoxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Al2O3 Nanoparticles
2.2. Hydrodynamic Diameter and ζ-Potential of Nanoparticles Measurements
2.3. Composite Fabrication & Preparation of Coatings from Composite Material
2.4. Hydrogen Peroxide Concentration Measurement
2.5. Hydroxyl Radicals Concentration Measurement
2.6. Long-Lived Reactive Protein Species Concentration Measurement
2.7. Quantitative Determination of 8-Oxoguanine Using the ELISA Method
2.8. Antibacterial Activity Assay
2.9. Protocol of Manipulations with Animals
2.10. Preparation of Primary Cultures of Mouse Lung Fibroblasts
2.11. Cytotoxicity Assay
2.12. Statistic
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brooks, J.D.; Flint, S.H. Biofilms in the food industry: Problems and potential solutions. Int. J. Food Sci. Technol. 2008, 43, 2163–2176. [Google Scholar] [CrossRef]
- Simpson Beauchamp, C.; Dourou, D.; Geornaras, I.; Yoon, Y.; Scanga, J.A.; Belk, K.E.; Smith, G.C.; Nychas, G.J.E.; Sofos, J.N. Transfer, Attachment, and Formation of Biofilms by Escherichia coli O157: H7 on Meat-Contact Surface Materials. J. Food Sci. 2012, 77, M343–M347. [Google Scholar] [CrossRef] [PubMed]
- Sofos, J.N.; Geornaras, I. Overview of current meat hygiene and safety risks and summary of recent studies on biofilms, and control of Escherichia coli O157: H7 in nonintact, and Listeria monocytogenes in ready-to-eat, meat products. Meat Sci. 2010, 86, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Simakin, A.V.; Sarimov, R.M.; Kurilov, A.D.; Chausov, D.N. Novel Biocompatible with Animal Cells Composite Material Based on Organosilicon Polymers and Fullerenes with Light-Induced Bacteriostatic Properties. Nanomaterials 2021, 11, 2804. [Google Scholar] [CrossRef] [PubMed]
- Barkhudarov, E.M.; Kossyi, I.A.; Anpilov, A.M.; Ivashkin, P.I.; Artem’ev, K.V.; Moryakov, I.V.; Misakyan, M.A.; Christofi, N.; Burmistrov, D.E.; Smirnova, V.V.; et al. New Nanostructured Carbon Coating Inhibits Bacterial Growth, but Does Not Influence on Animal Cells. Nanomaterials 2020, 10, 2130. [Google Scholar] [CrossRef]
- Hemeg, H.A. Nanomaterials for alternative antibacterial therapy. Int. J. Nanomed. 2017, 12, 8211–8225. [Google Scholar] [CrossRef] [Green Version]
- Beyth, N.; Houri-Haddad, Y.; Domb, A. Alternative antimicrobial approach: Nanoantimicrobial materials. Evid-Based Complement. Altern. 2015, 2015, 246012. [Google Scholar]
- Naseem, T.; Durrani, T. The role of some important metal oxide nanoparticles for wastewater and antibacterial applications: A review. Environ. Chem. Ecotoxicol. 2021, 3, 59–75. [Google Scholar] [CrossRef]
- Jagadeeshan, S.; Parsanathan, R. Nano-metal Oxides for Antibacterial Activity. In Advanced Nanostructured Materials for Environmental Remediation; Naushad, M., Rajendran, S., Gracia, F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 59–90. [Google Scholar]
- Kadiyala, U.; Turali-Emre, E.S.; Bahng, J.H.; Kotov, N.A.; VanEpps, J.S. Unexpected insights into antibacterial activity of zinc oxide nanoparticles against methicillin resistant Staphylococcus aureus (MRSA). Nanoscale 2018, 10, 4927–4939. [Google Scholar] [CrossRef] [PubMed]
- Aldeen, T.S.; Mohamed, H.E.A.; Maaza, M. ZnO nanoparticles prepared via a green synthesis approach: Physical properties, photocatalytic and antibacterial activity. J. Phys. Chem. Solids 2022, 160, 110313. [Google Scholar] [CrossRef]
- Burmistrov, D.E.; Yanykin, D.V.; Paskhin, M.O.; Nagaev, E.V.; Efimov, A.D.; Kaziev, A.V.; Ageychenkov, D.G.; Gudkov, S.V. Additive Production of a Material Based on an Acrylic Polymer with a Nanoscale Layer of Zno Nanorods Deposited Using a Direct Current Magnetron Discharge: Morphology, Photoconversion Properties, and Biosafety. Materials 2021, 14, 6586. [Google Scholar] [CrossRef] [PubMed]
- Chausov, D.N.; Burmistrov, D.E.; Kurilov, A.D.; Bunkin, N.F.; Astashev, M.E.; Simakin, A.V.; Vedunova, M.V.; Gudkov, S.V. New Organosilicon Composite Based on Borosiloxane and Zinc Oxide Nanoparticles Inhibits Bacterial Growth, but Does Not Have a Toxic Effect on the Development of Animal Eukaryotic Cells. Materials 2021, 14, 6281. [Google Scholar] [CrossRef] [PubMed]
- Buarki, F.; AbuHassan, H.; Al Hannan, F.; Henari, F. Green Synthesis of Iron Oxide Nanoparticles Using Hibiscus rosa sinensis Flowers and Their Antibacterial Activity. J. Nanotechnol. 2022, 2022, 5474645. [Google Scholar] [CrossRef]
- Zakariya, N.A.; Majeed, S.; Jusof, W.H.W. Investigation of antioxidant and antibacterial activity of iron oxide nanoparticles (IONPS) synthesized from the aqueous extract of Penicillium spp. Sens. Int. 2022, 3, 100164. [Google Scholar] [CrossRef]
- Ouerghi, O.; Geesi, M.H.; Riadi, Y.; Ibnouf, E.O. Limon-citrus extract as a capping/reducing agent for the synthesis of titanium dioxide nanoparticles: Characterization and antibacterial activity. Green Chem. Lett. Rev. 2022, 15, 483–490. [Google Scholar] [CrossRef]
- Gudata, L.; Saka, A.; Tesfaye, J.L.; Shanmugam, R.; Dwarampudi, L.P.; Nagaprasad, N.; Stalin, B.; Krishnaraj, R. Investigation of TiO2 Nanoparticles Using Leaf Extracts of Lippia adoensis (Kusaayee) for Antibacterial Activity. J. Nanomater. 2022, 2022, 3881763. [Google Scholar] [CrossRef]
- Alavi, M. Bacteria and fungi as major bio-sources to fabricate silver nanoparticles with antibacterial activities. Expert Rev. Anti-Infect. Ther. 2022, 20, 897–906. [Google Scholar] [CrossRef]
- Balachandar, R.; Navaneethan, R.; Biruntha, M.; Kumar, K.K.A.; Govarthanan, M.; Karmegam, N. Antibacterial activity of silver nanoparticles phytosynthesized from Glochidion candolleanum leaves. Mater. Lett. 2022, 311, 131572. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Serov, D.A.; Astashev, M.E.; Semenova, A.A.; Lisitsyn, A.B. Ag2O Nanoparticles as a Candidate for Antimicrobial Compounds of the New Generation. Pharmaceuticals 2022, 15, 968. [Google Scholar] [CrossRef]
- Naguib, G.H.; Nassar, H.M.; Hamed, M.T. Antimicrobial properties of dental cements modified with zein-coated magnesium oxide nanoparticles. Bioact. Mater. 2022, 8, 49–56. [Google Scholar] [CrossRef]
- Rangrazi, A.; Daneshmand, M.S.; Ghazvini, K.; Shafaee, H. Effects of Magnesium Oxide Nanoparticles Incorporation on Shear Bond Strength and Antibacterial Activity of an Orthodontic Composite: An In Vitro Study. Biomimetics 2022, 7, 133. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Maldonado, A.; Bustos-Guadarrama, S.; Espinoza-Gomez, H.; Flores-López, L.Z.; Ramirez-Acosta, K.; Alonso-Nuñez, G.; Cadena-Nava, R.D. Green synthesis of copper nanoparticles using different plant extracts and their antibacterial activity. J. Environ. Chem. Eng. 2022, 10, 107130. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Menon, S.; Kumar, S.V.; Tambuwala, M.M.; Bakshi, H.A.; Mehta, M.; Satija, S.; Gupta, G.; Chellappan, D.K.; Thangavelu, L. Antibacterial and antioxidant potential of biosynthesized copper nanoparticles mediated through Cissus arnotiana plant extract. J. Photochem. Photobiol. B Biol. 2019, 197, 111531. [Google Scholar] [CrossRef]
- Nabila, M.I.; Kannabiran, K. Biosynthesis, characterization and antibacterial activity of copper oxide nanoparticles (CuO NPs) from actinomycetes. Biocatal. Agric. Biotechnol. 2018, 15, 56–62. [Google Scholar] [CrossRef]
- Chen, J.; Mao, S.; Xu, Z.; Ding, W. Various antibacterial mechanisms of biosynthesized copper oxide nanoparticles against soilborne Ralstonia solanacearum. RSC Adv. 2019, 9, 3788–3799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gudkov, S.V.; Burmistrov, D.E.; Smirnova, V.V.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of Al2O3 Nanoparticles. Nanomaterials 2022, 12, 2635. [Google Scholar] [CrossRef]
- Radziun, E.; Wilczyńska, J.D.; Książek, I.; Nowak, K.; Anuszewska, E.; Kunicki, A.; Olszyna, A.; Ząbkowski, T. Assessment of the cytotoxicity of aluminium oxide nanoparticles on selected mammalian cells. Toxicol. Vitr. 2011, 25, 1694–1700. [Google Scholar] [CrossRef]
- Ng, A.M.C.; Guo, M.Y.; Leung, Y.H.; Chan, C.M.; Wong, S.W.; Yung, M.M.; Ma, A.P.; Djurišić, A.B.; Leung, F.C.; Leung, K.M. Metal oxide nanoparticles with low toxicity. J. Photochem. Photobiol. B Biol. 2015, 151, 17–24. [Google Scholar] [CrossRef]
- Balakrishnan, S.B.; Kuppu, S.; Thambusamy, S. Biologically important alumina nanoparticles modified polyvinylpyrrolidone scaffolds in vitro characterizations and it is in vivo wound healing efficacy. J. Mol. Struct. 2021, 1246, 131195. [Google Scholar] [CrossRef]
- Tavakolian, S.; Ahari, H.; Givianrad, M.H.; Hosseini, H. Improving the Barrier Properties of Food Packaging by Al2O3@ TiO2 & Al2O3@ SiO2 Nanoparticles. Food Bioprocess Technol. 2021, 14, 1287–1300. [Google Scholar]
- Dhanumalayan, E.; Joshi, G.M. Performance properties and applications of polytetrafluoroethylene (PTFE)—A review. Adv. Compos. Hybrid Mater. 2018, 1, 247–268. [Google Scholar] [CrossRef]
- Demling, A.; Elter, C.; Heidenblut, T.; Bach, F.-W.; Hahn, A.; Schwestka-Polly, R.; Stiesch, M.; Heuer, W. Reduction of biofilm on orthodontic brackets with the use of a polytetrafluoroethylene coating. Eur. J. Orthod. 2010, 32, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Rungraeng, N.; Cho, Y.-C.; Yoon, S.H.; Jun, S. Carbon nanotube-polytetrafluoroethylene nanocomposite coating for milk fouling reduction in plate heat exchanger. J. Food Eng. 2012, 111, 218–224. [Google Scholar] [CrossRef]
- Zaporojtchenko, V.; Podschun, R.; Schürmann, U.; Kulkarni, A.; Faupel, F. Physico-chemical and antimicrobial properties of co-sputtered Ag–Au/PTFE nanocomposite coatings. Nanotechnology 2006, 17, 4904. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Liang, X.; Vorstius, J.; Keatch, R.; Corner, G.; Nabi, G.; Davidson, F.; Gadd, G.M.; Zhao, Q. Enhanced antibacterial and antiadhesive activities of silver-PTFE nanocomposite coating for urinary catheters. ACS Biomater. Sci. Eng. 2019, 5, 2804–2814. [Google Scholar] [CrossRef] [PubMed]
- Simakin, A.V.; Baimler, I.V.; Smirnova, V.V.; Uvarov, O.V.; Kozlov, V.A.; Gudkov, S.V. Evolution of the Size Distribution of Gold Nanoparticles under Laser Irradiation. Phys. Wave Phenom. 2021, 29, 102–107. [Google Scholar] [CrossRef]
- Sevostyanov, M.A.; Baikin, A.S.; Kaplan, M.A.; Kolmakov, A.G.; Gudkov, S.V.; Rebezov, M.B.; Garnov, S.V. A β Ti–20Nb–10Ta–5Zr Alloy with the Surface Structured on the Micro- and Nanoscale. Dokl. Phys. 2021, 66, 14–16. [Google Scholar] [CrossRef]
- Shtarkman, I.; Gudkov, S.; Chernikov, A.; Bruskov, V. Effect of amino acids on X-ray-induced hydrogen peroxide and hydroxyl radical formation in water and 8-oxoguanine in DNA. Biochem. Mosc. 2008, 73, 470–478. [Google Scholar] [CrossRef]
- Chernikov, A.; Gudkov, S.; Shtarkman, I.; Bruskov, V. Oxygen effect in heat-mediated damage to DNA. Biofizika 2007, 52, 244–251. [Google Scholar]
- Gudkov, S.V.; Guryev, E.L.; Gapeyev, A.B.; Sharapov, M.G.; Bunkin, N.F.; Shkirin, A.V.; Zabelina, T.S.; Glinushkin, A.P.; Sevost’yanov, M.A.; Belosludtsev, K.N. Unmodified hydrated C60 fullerene molecules exhibit antioxidant properties, prevent damage to DNA and proteins induced by reactive oxygen species and protect mice against injuries caused by radiation-induced oxidative stress. Nanomed. Nanotechnol. Biol. Med. 2019, 15, 37–46. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Astashev, M.E.; Baimler, I.V.; Uvarov, O.V.; Voronov, V.V.; Simakin, A.V. Laser-Induced Optical Breakdown of an Aqueous Colloidal Solution Containing Terbium Nanoparticles: The Effect of Oxidation of Nanoparticles. J. Phys. Chem. B 2022, 126, 5678–5688. [Google Scholar] [CrossRef]
- Baimler, I.; Simakin, A.; Uvarov, O.; Volkov, M.Y.; Gudkov, S. Generation of hydroxyl radicals during laser breakdown of aqueous solutions in the presence of Fe and Cu nanoparticles of different sizes. Phys. Wave Phenom. 2020, 28, 107–110. [Google Scholar] [CrossRef]
- Gudkov, S.; Garmash, S.; Shtarkman, I.; Chernikov, A.; Karp, O.; Bruskov, V. Long-lived protein radicals induced by X-ray irradiation are the source of reactive oxygen species in aqueous medium. In Doklady. Biochemistry and Biophysics; Springer: Berlin/Heidelberg, Germany, 2010; p. 1. [Google Scholar]
- Sharapov, M.; Novoselov, V.; Penkov, N.; Fesenko, E.; Vedunova, M.; Bruskov, V.; Gudkov, S. Protective and adaptogenic role of peroxiredoxin 2 (Prx2) in neutralization of oxidative stress induced by ionizing radiation. Free Radic. Biol. Med. 2019, 134, 76–86. [Google Scholar] [CrossRef]
- Ivanov, V.E.; Usacheva, A.M.; Chernikov, A.V.; Bruskov, V.I.; Gudkov, S.V. Formation of long-lived reactive species of blood serum proteins induced by low-intensity irradiation of helium-neon laser and their involvement in the generation of reactive oxygen species. J. Photochem. Photobiol. B Biol. 2017, 176, 36–43. [Google Scholar] [CrossRef]
- Barkhudarov, E. New nanostructured coating of nanosized amorphous carbon inhibits bacterial growth, but does not have a toxic effect on the development of animal eukaryotic cells. Nanomaterials 2020, 10, 2130. [Google Scholar] [CrossRef]
- Lema, C.; Varela-Ramirez, A.; Aguilera, R.J. Differential nuclear staining assay for high-throughput screening to identify cytotoxic compounds. Curr. Cell. Biochem. 2011, 1, 1. [Google Scholar]
- Dutta, R.K.; Nenavathu, B.P.; Gangishetty, M.K.; Reddy, A. Studies on antibacterial activity of ZnO nanoparticles by ROS induced lipid peroxidation. Colloids Surf. B Biointerfaces 2012, 94, 143–150. [Google Scholar] [CrossRef]
- Zuo, L.; Zhou, T.; Pannell, B.; Ziegler, A.; Best, T.M. Biological and physiological role of reactive oxygen species–the good, the bad and the ugly. Acta Physiol. 2015, 214, 329–348. [Google Scholar] [CrossRef]
- Manohar, A.; Park, J.; Geleta, D.D.; Krishnamoorthi, C.; Thangam, R.; Kang, H.; Lee, J. Synthesis and characterization of ZnO nanoparticles for photocatalysis, antibacterial and cytotoxicity in kidney cancer (A498) cell lines. J. Alloy Compd. 2021, 874, 159868. [Google Scholar] [CrossRef]
- Mohamad Sukri, S.N.A.; Shameli, K.; Mei-Theng Wong, M.; Teow, S.-Y.; Chew, J.; Ismail, N.A. Cytotoxicity and antibacterial activities of plant-mediated synthesized zinc oxide (ZnO) nanoparticles using Punica granatum (pomegranate) fruit peels extract. J. Mol. Struct. 2019, 1189, 57–65. [Google Scholar] [CrossRef]
- Zhao, X.; Drlica, K. Reactive oxygen species and the bacterial response to lethal stress. Curr. Opin. Microbiol. 2014, 21, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Fortini, P.; Pascucci, B.; Parlanti, E.; D’errico, M.; Simonelli, V.; Dogliotti, E. 8-Oxoguanine DNA damage: At the crossroad of alternative repair pathways. Mutat. Res. /Fundam. Mol. Mech. Mutagen. 2003, 531, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Slone, J.; Liu, W.; Barnes, R.; Opresko, P.L.; Wark, L.; Mai, S.; Horvath, S.; Huang, T. Premature aging is associated with higher levels of 8-oxoguanine and increased DNA damage in the Polg mutator mouse. Aging Cell 2022, 21, e13669. [Google Scholar] [CrossRef]
- Abdel-Naby, A.S.; Nabil, S.; Aldulaijan, S.; Ababutain, I.M.; Alghamdi, A.I.; Almubayedh, S.; Khalil, K.D. Synthesis, Characterization of Chitosan-Aluminum Oxide Nanocomposite for Green Synthesis of Annulated Imidazopyrazol Thione Derivatives. Polymers 2021, 13, 1160. [Google Scholar] [CrossRef]
- El Nahrawy, A.M.; Abou Hammad, A.B.; Abdel-Aziz, M.S.; Wassel, A. Spectroscopic and antimicrobial activity of hybrid chitosan/silica membranes doped with Al2O3 nanoparticles. Silicon 2019, 11, 1677–1685. [Google Scholar] [CrossRef]
- Yakdoumi, F.Z.; Hadj-Hamou, A.S. Effectiveness assessment of TiO2-Al2O3 nano-mixture as a filler material for improvement of packaging performance of PLA nanocomposite films. J. Polym. Eng. 2020, 40, 848–858. [Google Scholar] [CrossRef]
- Astashev, M.; Sarimov, R.; Serov, D.; Matveeva, T.; Simakin, A.; Ignatenko, D.; Burmistrov, D.; Smirnova, V.; Kurilov, A.; Mashchenko, V. Antibacterial behavior of organosilicon composite with nano aluminum oxide without influencing animal cells. React. Funct. Polym. 2022, 170, 105143. [Google Scholar] [CrossRef]
- Bala, T.; Armstrong, G.; Laffir, F.; Thornton, R. Titania–silver and alumina–silver composite nanoparticles: Novel, versatile synthesis, reaction mechanism and potential antimicrobial application. J. Colloid Interface Sci. 2011, 356, 395–403. [Google Scholar] [CrossRef] [Green Version]
- Geoprincy, G.; Gandhi, N.; Renganathan, S. Novel antibacterial effects of alumina nanoparticles on Bacillus cereus and Bacillus subtilis in comparison with antibiotics. Int. J. Pharm Pharm Sci 2012, 4, 544–548. [Google Scholar]
- Jwad, K.H.; Saleh, T.H.; Abd-Alhamza, B. Preparation of Aluminum oxide nanoparticles by laser ablation and a study of their applications as antibacterial and wounds healing agent. Nano Biomed. Eng 2019, 11, 313–319. [Google Scholar] [CrossRef]
- Vyas, S.; Shukla, A.; Shivhare, S.; Upadhyay, N. Facile synthesis and characterization of polyaniline (PANI)–Aluminium oxide (Al2O3) nanocomposites by using chemical oxidative polymerization. In Proceedings of the AIP Conference Proceedings, Arau, Malaysia, 23–24 July 2020; p. 020192. [Google Scholar]
- Khajeh Mehrizi, M.; Mashroteh, H.; Nabizadeh Moghadam Noghabi, N. Effect of Chitosan, Aluminum Oxide and Silver Nanoparticles on Antibacterial, Deodorizing and Moisture Absorption Properties of Nonwoven Polyester Fabrics for Use in Medical Textiles. Med. Lab. J. 2016, 10, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Fajardo, C.; Saccà, M.; Costa, G.; Nande, M.; Martin, M. Impact of Ag and Al2O3 nanoparticles on soil organisms: In vitro and soil experiments. Sci. Total Environ. 2014, 473, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Yin, L.H.; Meng, T.A.; Pu, Y.P. ZnO, TiO2, SiO2, and Al2O3 Nanoparticles-induced Toxic Effects on Human Fetal Lung Fibroblasts. Biomed Env. Sci 2011, 24, 661–669. [Google Scholar]
- Muzammil, S.; Khurshid, M.; Nawaz, I.; Siddique, M.H.; Zubair, M.; Nisar, M.A.; Imran, M.; Hayat, S. Aluminium oxide nanoparticles inhibit EPS production, adhesion and biofilm formation by multidrug resistant Acinetobacter baumannii. Biofouling 2020, 36, 492–504. [Google Scholar] [CrossRef]
- Chen, M.; Zamora, P.O.; Som, P.; Peña, L.A.; Osaki, S. Cell attachment and biocompatibility of polytetrafluoroethylene (PTFE) treated with glow-discharge plasma of mixed ammonia and oxygen. J. Biomater. Sci. Polym. Ed. 2003, 14, 917–935. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, M.; Niederer, K.; Becker, M.; Raynaud, C.M.; Vahl, C.-F.; Frey, H. Tailoring novel PTFE surface properties: Promoting cell adhesion and antifouling properties via a wet chemical approach. Bioconj. Chem. 2016, 27, 1216–1221. [Google Scholar] [CrossRef]
- Yao, C.; Storey, D.; Webster, T.J. Nanostructured metal coatings on polymers increase osteoblast attachment. Int. J. Nanomed. 2007, 2, 487–492. [Google Scholar]
- Webster, M.; Witkin, K.L.; Cohen-Fix, O. Sizing up the nucleus: Nuclear shape, size and nuclear-envelope assembly. J. Cell Sci. 2009, 122, 1477–1486. [Google Scholar] [CrossRef] [Green Version]
- Rose, R.; Peschke, N.; Nigi, E.; Gelléri, M.; Ritz, S.; Cremer, C.; Luhmann, H.J.; Sinning, A. Chromatin compaction precedes apoptosis in developing neurons. Commun. Biol. 2022, 5, 797. [Google Scholar] [CrossRef]












| № | Composition | Size, nm | Concentration | Type of Microorganism | Biological Effect | Ref. |
|---|---|---|---|---|---|---|
| 1 | Chitosan-coated Al2O3-NPs films | <50 | 0.05, 0.1 g/mL | S. aureus, P. aeruginosa, S. epidermidis | bacteriostatic | [56] |
| 2 | Chitosan/SiO2 nanocomposite with Al2O3 | - | - | S. aureus, P. aeruginosa, C. albicans, A. niger | bacteriostatic | [57] |
| 3 | PLA/Al2O3 | 30 | - | P. aeruginosa & E. coli | bacteriostatic | [58] |
| 4 | Al2O3/borosiloxane composite | 45 | 0.001–0.1 wt% | E. coli | bacteriostatic | [59] |
| 5 | Al2O3–Ag composite nanoparticles | 100–200 | 1–50 wt% | E. coli & S. epidermidis | bacteriostatic | [60] |
| 6 | Bulk Al2O3 | 100–200 | MIC: 100 µg | B. cereus, B. subtilis, K. pneumoniae, V. cholerae | bacteriostatic | [61] |
| 7 | Bulk Al2O3 | 10–60 | 25–100 µg/mL | E. coli, P. aeruginosa, S. aureus | bacteriostatic | [62] |
| 8 | PANI–Al2O3 NPs composite | - | 5, 10 mg/mL | B. subtilis & E. coli | bacteriostatic | [63] |
| 9 | Al2O3 coated by chitosan | 80 | 0.025 mg/mL | S. aureus | bacteriostatic | [64] |
| 10 | Bulk Al2O3 | <50 | 1–10 g/L | B. cereus & P. stutzeri | bacteriostatic | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burmistrov, D.E.; Serov, D.A.; Simakin, A.V.; Baimler, I.V.; Uvarov, O.V.; Gudkov, S.V. A Polytetrafluoroethylene (PTFE) and Nano-Al2O3 Based Composite Coating with a Bacteriostatic Effect against E. coli and Low Cytotoxicity. Polymers 2022, 14, 4764. https://doi.org/10.3390/polym14214764
Burmistrov DE, Serov DA, Simakin AV, Baimler IV, Uvarov OV, Gudkov SV. A Polytetrafluoroethylene (PTFE) and Nano-Al2O3 Based Composite Coating with a Bacteriostatic Effect against E. coli and Low Cytotoxicity. Polymers. 2022; 14(21):4764. https://doi.org/10.3390/polym14214764
Chicago/Turabian StyleBurmistrov, Dmitriy E., Dmitriy A. Serov, Aleksander V. Simakin, Ilya V. Baimler, Oleg V. Uvarov, and Sergey V. Gudkov. 2022. "A Polytetrafluoroethylene (PTFE) and Nano-Al2O3 Based Composite Coating with a Bacteriostatic Effect against E. coli and Low Cytotoxicity" Polymers 14, no. 21: 4764. https://doi.org/10.3390/polym14214764
APA StyleBurmistrov, D. E., Serov, D. A., Simakin, A. V., Baimler, I. V., Uvarov, O. V., & Gudkov, S. V. (2022). A Polytetrafluoroethylene (PTFE) and Nano-Al2O3 Based Composite Coating with a Bacteriostatic Effect against E. coli and Low Cytotoxicity. Polymers, 14(21), 4764. https://doi.org/10.3390/polym14214764