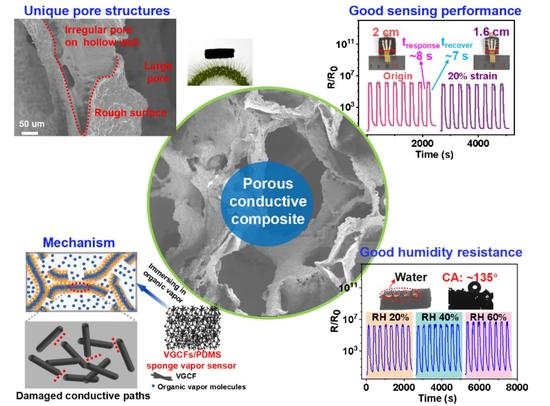
Sensitive Organic Vapor Sensors Based on Flexible Porous Conductive Composites with Multilevel Pores and Thin, Rough, Hollow-Wall Structure
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Film-Shaped Composites
2.3. Preparation of Porous Composites
2.4. Characterization
2.4.1. General Techniques
2.4.2. Characterization of Vapor-Sensing Behaviors
3. Results and Discussion
3.1. Fabrication and Structural Characterization of Porous CPS Composites
3.2. Mechanical Properties and Structural Stability
3.3. Static Organic Vapor-Sensing Behaviors
3.4. Flowing Organic Vapor-Sensing Behaviors
3.5. Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esteves, C.H.A.; Iglesias, B.-A.; Li, R.W.C.; Ogawa, T.; Araki, K.; Gruber, J. New composite porphyrin-conductive polymer gas sensors for application in electronic noses. Sens. Actuators B Chem. 2014, 193, 136–141. [Google Scholar] [CrossRef]
- Farea, M.-A.; Mohammed, H.-Y.; Shirsat, S.-M.; Sayyad, P.-W.; Ingle, N.-N.; Al-Gahouari, T.; Mahadik, M.M.; Bodkhe, G.A.; Shirsat, M.D. Hazardous gases sensors based on conducting polymer composites: Review. Chem. Phys. Lett. 2021, 776, 138703. [Google Scholar] [CrossRef]
- Jain, R.; Lei, Y.; Maric, R. Ultra-low NO2 detection by gamma WO3 synthesized by Reactive Spray Deposition Technology. Sens. Actuators B Chem. 2016, 236, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, T.; Hamedsoltani, L. Graphene/graphite/molecularly imprinted polymer nanocomposite as the highly selective gas sensor for nitrobenzene vapor recognition. J. Environ. Chem. Eng. 2014, 2, 1514–1526. [Google Scholar] [CrossRef]
- Park, H.-J.; Kim, W.-J.; Lee, H.-K.; Lee, D.-S.; Shin, J.-H.; Jun, Y.; Yun, Y.-J. Highly flexible, mechanically stable, and sensitive NO2 gas sensors based on reduced graphene oxide nanofibrous mesh fabric for flexible electronics. Sens. Actuators B Chem. 2018, 257, 846–852. [Google Scholar] [CrossRef]
- Chiou, J.-C.; Wu, C.-C.; Lin, T.-M. Sensitivity Enhancement of Acetone Gas Sensor using Polyethylene Glycol/Multi-Walled Carbon Nanotubes Composite Sensing Film with Thermal Treatment. Polymers 2019, 11, 423. [Google Scholar] [CrossRef] [Green Version]
- Chiou, J.-C.; Wu, C.-C. A Wearable and Wireless Gas-Sensing System Using Flexible Polymer/Multi-Walled Carbon Nanotube Composite Films. Polymers 2017, 9, 457. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zheng, W.; Kumar, R.; Kumar, M.; Zhang, J. Conducting polymer-based nanostructures for gas sensors. Coord. Chem. Rev. 2022, 462, 214517. [Google Scholar] [CrossRef]
- Pötschke, P.; Andres, T.; Villmow, T.; Pegel, S.; Brünig, H.; Kobashi, K.; Fischer, D.; Häussler, L. Liquid sensing properties of fibres prepared by melt spinning from poly(lactic acid) containing multi-walled carbon nanotubes. Compos. Sci. Technol. 2010, 70, 343–349. [Google Scholar] [CrossRef]
- Zhang, B.; Dong, X.; Fu, R.; Zhao, B.; Zhang, M. The sensibility of the composites fabricated from polystyrene filling multi-walled carbon nanotubes for mixed vapors. Compos. Sci. Technol. 2008, 68, 1357–1362. [Google Scholar] [CrossRef]
- Tabačiarová, J.; Krajči, J.; Pionteck, J.; Reuter, U.; Omastová, M.; Mičušík, M. Styrene Butadiene Rubber/Carbon Filler-Based Vapor Sensors. Macromol. Chem. Phys. 2016, 217, 1149–1160. [Google Scholar] [CrossRef]
- Shang, S.; Zeng, W.; Tao, X.-m. Investigation on the electrical response behaviors of multiwalled carbon nanotube/polyurethane composite in organic solvent vapors. Sens. Actuators B Chem. 2012, 166–167, 330–337. [Google Scholar] [CrossRef]
- Chen, S.-G.; Hu, J.-W.; Zhang, M.-Q.; Li, M.-W.; Rong, M.-Z. Gas sensitivity of carbon black/waterborne polyurethane composites. Carbon 2004, 42, 645–651. [Google Scholar] [CrossRef]
- Zhang, B.; Fu, R.; Zhang, M.; Dong, X.; Wang, L.; Pittman, C.U. Gas sensitive vapor grown carbon nanofiber/polystyrene sensors. Mater. Res. Bull. 2006, 41, 553–562. [Google Scholar] [CrossRef]
- Wang, N.; Xu, Z.; Qu, Y.; Zheng, G.; Dai, K.; Liu, C.; Shen, C. Liquid-sensing behaviors of carbon black/polyamide 6/high-density polyethylene composite containing ultrafine conductive electrospun fibrous network. Colloid Polym. Sci. 2016, 294, 1343–1350. [Google Scholar] [CrossRef]
- Zhang, R.-Q.; Wang, L.-B.; Bai, R.-X.; Luo, Y.-L.; Xu, F.; Chen, Y.-S. Sensitive conductive polymer nanocomposites from multiwalled carbon nanotube coated with polypyrrole and hydroxyl-terminated poly(butadiene-co-acrylonitile) polyurethane for detection of chloroform vapor. Compos. Part B Eng. 2019, 173, 106894. [Google Scholar] [CrossRef]
- Arshak, K.; Moore, E.; Cavanagh, L.; Harris, J.; McConigly, B.; Cunniffe, C.; Lyons, G.; Clifford, S. Determination of the electrical behaviour of surfactant treated polymer/carbon black composite gas sensors. Compos. Part A Appl. Sci. Manuf. 2005, 36, 487–491. [Google Scholar] [CrossRef]
- Castro, M.; Lu, J.; Bruzaud, S.; Kumar, B.; Feller, J.-F. Carbon nanotubes/poly(ε-caprolactone) composite vapour sensors. Carbon 2009, 47, 1930–1942. [Google Scholar] [CrossRef]
- Philip, B.; Abraham, J.-K.; Chandrasekhar, A.; Varadan, V.K. Carbon nanotube/PMMA composite thin films for gas-sensing applications. Smart Mater. Struct. 2003, 12, 935–939. [Google Scholar] [CrossRef]
- Zhang, B.; Fu, R.-W.; Zhang, M.-Q.; Dong, X.-M.; Lan, P.-L.; Qiu, J.-S. Preparation and characterization of gas-sensitive composites from multi-walled carbon nanotubes/polystyrene. Sens. Actuators B Chem. 2005, 109, 323–328. [Google Scholar] [CrossRef]
- Chen, S.-G.; Hu, J.-W.; Zhang, M.-Q.; Rong, M.-Z.; Zheng, Q. Improvement of gas sensing performance of carbon black/waterborne polyurethane composites: Effect of crosslinking treatment. Sens. Actuators B Chem. 2006, 113, 361–369. [Google Scholar] [CrossRef]
- Zhang, B.; Fu, R.; Zhang, M.; Dong, X.; Zhao, B.; Wang, L.; Pittman, C.-U. Studies of the vapor-induced sensitivity of hybrid composites fabricated by filling polystyrene with carbon black and carbon nanofibers. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1884–1889. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Dai, K.; Zheng, G.; Liu, C.; Chen, J.; Shen, C. Tuning of vapor sensing behaviors of eco-friendly conductive polymer composites utilizing ramie fiber. Sens. Actuators B Chem. 2015, 221, 1279–1289. [Google Scholar] [CrossRef]
- Gao, L.; Liu, C.; Peng, Y.; Deng, J.; Hou, S.; Cheng, Y.; Huang, W.; Yu, J. Ultrasensitive flexible NO2 gas sensors via multilayer porous polymer film. Sens. Actuators B Chem. 2022, 368, 132113. [Google Scholar] [CrossRef]
- Fan, Q.; Qin, Z.; Villmow, T.; Pionteck, J.; Pötschke, P.; Wu, Y.; Voit, B.; Zhu, M. Vapor sensing properties of thermoplastic polyurethane multifilament covered with carbon nanotube networks. Sens. Actuators B Chem. 2011, 156, 63–70. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Pionteck, J.; Zhou, Z.; Weng, W.; Luo, X.; Qin, Z.; Voit, B.; Zhu, M. Flexible poly(styrene-butadiene-styrene)/carbon nanotube fiber based vapor sensors with high sensitivity, wide detection range, and fast response. Sens. Actuators B Chem. 2018, 256, 896–904. [Google Scholar] [CrossRef]
- Xu, F.; Guo, S.; Luo, Y.-L. Novel THTBN/MWNTs-OH polyurethane conducting composite thin films for applications in detection of volatile organic compounds. Mater. Chem. Phys. 2014, 145, 222–231. [Google Scholar] [CrossRef]
- Wisser, F.M.; Grothe, J.; Kaskel, S. Nanoporous polymers as highly sensitive functional material in chemiresistive gas sensors. Sens. Actuators B Chem. 2016, 223, 166–171. [Google Scholar] [CrossRef]
- Qiang, F.; Dai, S.-W.; Zhao, L.; Gong, L.-X.; Zhang, G.-D.; Jiang, J.-X.; Tang, L.-C. An insulating second filler tuning porous conductive composites for highly sensitive and fast responsive organic vapor sensor. Sens. Actuators B Chem. 2019, 285, 254–263. [Google Scholar] [CrossRef]
- He, H.; Xu, X.-B.; Zhang, D.-F. An aligned macro-porous carbon nanotube/waterborne polyurethane sensor for the detection of flowing organic vapors. Sens. Actuators B Chem. 2013, 176, 940–944. [Google Scholar] [CrossRef]
- Wang, C.; Yu, H.-Y.; Miao, Z.; Ge, D.; Abdalkarim, S.Y.H.; Yao, J. Interface Growth of PANI-ZnO Nanohybrids on a Self-Formed Grapefruit Peel Aerogel to Construct a Quick Self-Restored Gas Sensor. ACS Sustain. Chem. Eng. 2022, 10, 6573–6583. [Google Scholar] [CrossRef]
- Cao, C.-F.; Wang, P.-H.; Zhang, J.-W.; Guo, K.-Y.; Li, Y.; Xia, Q.-Q.; Zhang, G.-D.; Zhao, L.; Chen, H.; Wang, L.; et al. One-step and green synthesis of lightweight, mechanically flexible and flame-retardant polydimethylsiloxane foam nanocomposites via surface-assembling ultralow content of graphene derivative. Chem. Eng. J. 2020, 393, 124724. [Google Scholar] [CrossRef]
- Li, Y.; Cao, C.-F.; Li, S.-N.; Huang, N.-J.; Mao, M.; Zhang, J.-W.; Wang, P.-H.; Guo, K.-Y.; Gong, L.-X.; Zhang, G.-D.; et al. In situ reactive self-assembly of a graphene oxide nano-coating in polymer foam materials with synergistic fire shielding properties. J. Mater. Chem. A 2019, 7, 27032–27040. [Google Scholar] [CrossRef]
- Li, K.; Dai, K.; Xu, X.; Zheng, G.; Liu, C.; Chen, J.; Shen, C. Organic vapor sensing behaviors of carbon black/poly (lactic acid) conductive biopolymer composite. Colloid Polym. Sci. 2013, 291, 2871–2878. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Ha, S.-C.; Yang, H.; Kim, Y.-T. Gas sensor measurement system capable of sampling volatile organic compounds (VOCs) in wide concentration range. Sens. Actuators B Chem. 2007, 122, 211–218. [Google Scholar] [CrossRef]
- Wu, Q.; Gong, L.-X.; Li, Y.; Cao, C.-F.; Tang, L.-C.; Wu, L.; Zhao, L.; Zhang, G.-D.; Li, S.-N.; Gao, J.; et al. Efficient Flame Detection and Early Warning Sensors on Combustible Materials Using Hierarchical Graphene Oxide/Silicone Coatings. ACS Nano 2018, 12, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.-W.; Gu, Y.-L.; Zhao, L.; Zhang, W.; Gao, C.-H.; Wu, Y.-X.; Shen, S.-C.; Zhang, C.; Kong, T.-T.; Li, Y.-T.; et al. Bamboo-inspired mechanically flexible and electrically conductive polydimethylsiloxane foam materials with designed hierarchical pore structures for ultra-sensitive and reliable piezoresistive pressure sensor. Compos. Part B Eng. 2021, 225, 109243. [Google Scholar] [CrossRef]
- Zhao, L.; Qiang, F.; Dai, S.-W.; Shen, S.-C.; Huang, Y.-Z.; Huang, N.-J.; Zhang, G.-D.; Guan, L.-Z.; Gao, J.-F.; Song, Y.-H.; et al. Construction of sandwich-like porous structure of graphene-coated foam composites for ultrasensitive and flexible pressure sensors. Nanoscale 2019, 11, 10229–10238. [Google Scholar] [CrossRef]
- Daneshkhah, A.; Shrestha, S.; Agarwal, M.; Varahramyan, K. Poly(vinylidene fluoride-hexafluoropropylene) composite sensors for volatile organic compounds detection in breath. Sens. Actuators B Chem. 2015, 221, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Park, E.-J.; Choi, J.; Hong, J.; Shim, S.-E. Polyurethane/PEG-modified MWCNT composite film for the chemical vapor sensor application. Synth. Met. 2010, 160, 566–574. [Google Scholar] [CrossRef]
- Liu, H.; Huang, W.; Yang, X.; Dai, K.; Zheng, G.; Liu, C.; Shen, C.; Yan, X.; Guo, J.; Guo, Z. Organic vapor sensing behaviors of conductive thermoplastic polyurethane–graphene nanocomposites. J. Mater. Chem. C 2016, 4, 4459–4469. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A.; Vollebregt, S. Effect of Humidity on Gas Sensing Performance of Carbon Nanotube Gas Sensors Operated at Room Temperature. IEEE Sens. J. 2021, 21, 5763–5770. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, T.-T.; Zhou, J.-H.; Nie, F.; Zhang, C.; Shen, F.-X.; Dai, S.-W.; Pan, H.-T.; Gong, L.-X.; Zhao, L. Sensitive Organic Vapor Sensors Based on Flexible Porous Conductive Composites with Multilevel Pores and Thin, Rough, Hollow-Wall Structure. Polymers 2022, 14, 4809. https://doi.org/10.3390/polym14224809
Kong T-T, Zhou J-H, Nie F, Zhang C, Shen F-X, Dai S-W, Pan H-T, Gong L-X, Zhao L. Sensitive Organic Vapor Sensors Based on Flexible Porous Conductive Composites with Multilevel Pores and Thin, Rough, Hollow-Wall Structure. Polymers. 2022; 14(22):4809. https://doi.org/10.3390/polym14224809
Chicago/Turabian StyleKong, Ting-Ting, Jia-Hai Zhou, Feng Nie, Chao Zhang, Fei-Xiang Shen, Shou-Wei Dai, Hong-Tao Pan, Li-Xiu Gong, and Li Zhao. 2022. "Sensitive Organic Vapor Sensors Based on Flexible Porous Conductive Composites with Multilevel Pores and Thin, Rough, Hollow-Wall Structure" Polymers 14, no. 22: 4809. https://doi.org/10.3390/polym14224809
APA StyleKong, T. -T., Zhou, J. -H., Nie, F., Zhang, C., Shen, F. -X., Dai, S. -W., Pan, H. -T., Gong, L. -X., & Zhao, L. (2022). Sensitive Organic Vapor Sensors Based on Flexible Porous Conductive Composites with Multilevel Pores and Thin, Rough, Hollow-Wall Structure. Polymers, 14(22), 4809. https://doi.org/10.3390/polym14224809