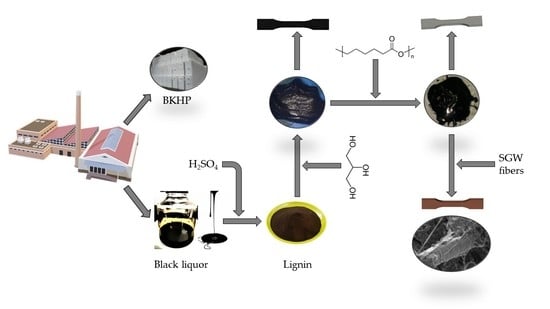
Valorization of Kraft Lignin from Black Liquor in the Production of Composite Materials with Poly(caprolactone) and Natural Stone Groundwood Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Precipitation and Thermoplasticization of Lignin
2.2.2. Development of Lignin and PCL Blends and Their Composites Reinforced with Natural Fibers
2.2.3. Materials Characterization
3. Results and Discussion
3.1. Black Liquor and Lignin Characterization
3.2. Thermoplastic Lignin Characterization
3.3. Blends
3.4. Composites
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosato, D. Plastics Engineered Product Design; Elsevier: Oxford, UK, 2003. [Google Scholar]
- Shogren, R.; Wood, D.; Orts, W.; Glenn, G. Plant-Based Materials and Transitioning to a Circular Economy. Sustain. Prod. Consum. 2019, 19, 194–215. [Google Scholar] [CrossRef]
- Soares, C.T.; de Mello Soares, C.T.; Ek, M.; Östmark, E.; Gällstedt, M.; Karlsson, S. Recycling of Multi-Material Multilayer Plastic Packaging: Current Trends and Future Scenarios. Resour. Conserv. Recycl. 2022, 176, 105905. [Google Scholar] [CrossRef]
- Philp, J.C.; Ritchie, R.J.; Guy, K. Biobased Plastics in a Bioeconomy. Trends Biotechnol. 2013, 31, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.A.; Domínguez-Robles, J.; Utomo, E.; Picco, C.J.; Corduas, F.; Mancuso, E.; Amir, M.N.; Bahar, M.A.; Sumarheni, S.; Donnelly, R.F.; et al. Poly(Caprolactone)-Based Subcutaneous Implant for Sustained Delivery of Levothyroxine. Int. J. Pharm. 2021, 607, 121011. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.A.; Domínguez-Robles, J.; McIlorum, V.J.; Gonzalez, Z.; Utomo, E.; Mancuso, E.; Lamprou, D.A.; Donnelly, R.F.; Larrañeta, E. Poly(Caprolactone)-Based Coatings on 3D-Printed Biodegradable Implants: A Novel Strategy to Prolong Delivery of Hydrophilic Drugs. Mol. Pharm. 2020, 17, 3487–3500. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Zuhri, M.Y.M.; Norrrahim, M.N.F.; Misenan, M.S.M.; Jenol, M.A.; Samsudin, S.A.; Nurazzi, N.M.; Asyraf, M.R.M.; Supian, A.B.M.; Bangar, S.P.; et al. Natural Fiber-Reinforced Polycaprolactone Green and Hybrid Biocomposites for Various Advanced Applications. Polymers 2022, 14, 182. [Google Scholar] [CrossRef]
- Utomo, E.; Domínguez-Robles, J.; Moreno-Castellanos, N.; Stewart, S.A.; Picco, C.J.; Anjani, Q.K.; Simón, J.A.; Peñuelas, I.; Donnelly, R.F.; Larrañeta, E. Development of Intranasal Implantable Devices for Schizophrenia Treatment. Int. J. Pharm. 2022, 624, 122061. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-Based Materials in Biomedical Applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Diaz-Gomez, L.; Utomo, E.; Shen, T.; Picco, C.J.; Alvarez-Lorenzo, C.; Concheiro, A.; Donnelly, R.F.; Larrañeta, E. Use of 3D Printing for the Development of Biodegradable Antiplatelet Materials for Cardiovascular Applications. Pharmaceuticals 2021, 14, 921. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Vyas, C.; Poologasundarampillai, G.; Pape, I.; Hinduja, S.; Mirihanage, W.; Bartolo, P. Structural Evolution of PCL during Melt Extrusion 3D Printing. Macromol. Mater. Eng. 2018, 303, 1700494. [Google Scholar] [CrossRef]
- Seyedsalehi, A.; Daneshmandi, L.; Barajaa, M.; Riordan, J.; Laurencin, C.T. Fabrication and Characterization of Mechanically Competent 3D Printed Polycaprolactone-Reduced Graphene Oxide Scaffolds. Sci. Rep. 2020, 10, 22210. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Robles, J.; Shen, T.; Cornelius, V.A.; Corduas, F.; Mancuso, E.; Donnelly, R.F.; Margariti, A.; Lamprou, D.A.; Larrañeta, E. Development of Drug Loaded Cardiovascular Prosthesis for Thrombosis Prevention Using 3D Printing. Mater. Sci. Eng. C 2021, 129, 112375. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Zhou, Y.; Chen, J.; Wan, Q. The Application of Polycaprolactone in Three-Dimensional Printing Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 2754. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Luo, B.; Xiang, S.; Ma, H.; Yu, Y.; Yang, W. 3D Printing of HA/PCL Composite Tissue Engineering Scaffolds. Adv. Ind. Eng. Polym. Res. 2019, 2, 196–202. [Google Scholar] [CrossRef]
- Melchor-Martínez, E.M.; Macías-Garbett, R.; Alvarado-Ramírez, L.; Araújo, R.G.; Sosa-Hernández, J.E.; Ramírez-Gamboa, D.; Parra-Arroyo, L.; Alvarez, A.G.; Monteverde, R.P.B.; Cazares, K.A.S.; et al. Towards a Circular Economy of Plastics: An Evaluation of the Systematic Transition to a New Generation of Bioplastics. Polymers 2022, 14, 1203. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Robles, J.; Cárcamo-Martínez, Á.; Stewart, S.A.; Donnelly, R.F.; Larrañeta, E.; Borrega, M. Lignin for Pharmaceutical and Biomedical Applications–Could This Become a Reality? Sustain. Chem. Pharm. 2020, 18, 100320. [Google Scholar] [CrossRef]
- Jääskeläinen, A.S.; Liitiä, T.; Mikkelson, A.; Tamminen, T. Aqueous Organic Solvent Fractionation as Means to Improve Lignin Homogeneity and Purity. Ind. Crops Prod. 2017, 103, 51–58. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Barros, J.; Serk, H.; Granlund, I.; Pesquet, E. The Cell Biology of Lignification in Higher Plants. Ann. Bot. 2015, 115, 1053–1074. [Google Scholar] [CrossRef] [Green Version]
- Chokshi, S.; Parmar, V.; Gohil, P.; Chaudhary, V. Chemical Composition and Mechanical Properties of Natural Fibers. J. Nat. Fibers 2020, 19, 3942–3953. [Google Scholar] [CrossRef]
- Sjöström, E.; Westermark, U. Chemical Composition of Wood and Pulps: Basic Constituents and Their Distribution. In Analytical Methods in Wood Chemistry, Pulping, and Papermaking; Springer: Berlin/Heidelberg, Germany, 1999; pp. 1–19. [Google Scholar]
- Marques, G.; Rencoret, J.; Gutiérrez, A.; del Río, J.C. Evaluation of the Chemical Composition of Different Non-Woody Plant Fibers Used for Pulp and Paper Manufacturing. Open Agric. J. 2014, 4, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Thakur, V.K.; Thakur, M.K. Recent Advances in Green Hydrogels from Lignin: A Review. Int. J. Biol. Macromol. 2015, 72, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Lora, J.H.; Glasser, W.G. Recent Industrial Applications of Lignin: A Sustainable Alternative to Nonrenewable Materials. J. Polym. Environ. 2002, 10, 39–48. [Google Scholar] [CrossRef]
- Bouajila, J.; Dole, P.; Joly, C.; Limare, A. Some Laws of a Lignin Plasticization. J. Appl. Polym. Sci. 2006, 102, 1445–1451. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Stewart, S.A.; Rendl, A.; González, Z.; Donnelly, R.F.; Larrañeta, E. Lignin and Cellulose Blends as Pharmaceutical Excipient for Tablet Manufacturing via Direct Compression. Biomolecules 2019, 9, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domínguez-robles, J.; Larrañeta, E.; Leon, M.; Martin, N.K.; Irwin, N.J.; Mutjé, P.; Tarrés, Q.; Delgado-aguilar, M. Lignin/Poly (Butylene Succinate) Composites with Antioxidant and Antibacterial Properties for Potential Biomedical Applications. Int. J. Biol. Macromol. 2020, 145, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Doherty, W.O.S.; Mousavioun, P.; Fellows, C.M. Value-Adding to Cellulosic Ethanol: Lignin Polymers. Ind. Crops Prod. 2011, 33, 259–276. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Dong, M.; Lu, Y.; Turley, A.; Jin, T.; Wu, C. Antimicrobial and Antioxidant Activities of Lignin from Residue of Corn Stover to Ethanol Production. Ind. Crops Prod. 2011, 34, 1629–1634. [Google Scholar] [CrossRef]
- Dai, P.; Liang, M.; Ma, X.; Luo, Y.; He, M.; Gu, X.; Gu, Q.; Hussain, I.; Luo, Z. Highly Efficient, Environmentally Friendly Lignin-Based Flame Retardant Used in Epoxy Resin. ACS Omega 2020, 5, 32084–32093. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, J.H.; Lee, S.Y.; Jang, S.K.; Kwak, H.W.; Kim, H.; Choi, I.G. Thermoplasticity Reinforcement of Ethanol Organosolv Lignin to Improve Compatibility in PLA-Based Ligno-Bioplastics: Focusing on the Structural Characteristics of Lignin. Int. J. Biol. Macromol. 2022, 209, 1638–1647. [Google Scholar] [CrossRef] [PubMed]
- Parit, M.; Jiang, Z. Towards Lignin Derived Thermoplastic Polymers. Int. J. Biol. Macromol. 2020, 165, 3180–3197. [Google Scholar] [CrossRef] [PubMed]
- Kammer, H.-W. Surface and Interfacial Tension of Polymer Melts—Thermodynamic Theory of the Interface between Immiscible Polymers. Z. Phys. Chem. 1977, 258, 1149–1161. [Google Scholar] [CrossRef]
- Baumberger, S. Starch-Lignin Films. In Chemical Modification, Properties, and Usage of Lignin; Springer: Boston, MA, USA, 2002; pp. 1–19. [Google Scholar]
- Li, J.; Chua, C.S. Transductive Inference for Color-Based Particle Filter Tracking. IEEE Int. Conf. Image Process. 2003, 3, 949–952. [Google Scholar] [CrossRef]
- Zhou, M.; Fakayode, O.A.; Ahmed Yagoub, A.E.G.; Ji, Q.; Zhou, C. Lignin Fractionation from Lignocellulosic Biomass Using Deep Eutectic Solvents and Its Valorization. Renew. Sustain. Energy Rev. 2022, 156, 111986. [Google Scholar] [CrossRef]
- Hong, S.; Shen, X.J.; Xue, Z.; Sun, Z.; Yuan, T.Q. Structure-Function Relationships of Deep Eutectic Solvents for Lignin Extraction and Chemical Transformation. Green Chem. 2020, 22, 7219–7232. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Sankaran, R.; Show, P.L.; Ibrahim, T.N.B.T.; Chew, K.W.; Culaba, A.; Chang, J.S. Pretreatment Methods for Lignocellulosic Biofuels Production: Current Advances, Challenges and Future Prospects. Biofuel Res. J. 2020, 7, 1115–1127. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Liu, X.; Yang, B.; Si, C.; Parvez, A.M.; Jang, J.; Ni, Y. Using Green γ-Valerolactone/Water Solvent to Decrease Lignin Heterogeneity by Gradient Precipitation. ACS Sustain. Chem. Eng. 2019, 7, 10112–10120. [Google Scholar] [CrossRef]
- Reixach, R.; Espinach, F.X.; Arbat, G.; Julián, F.; Delgado-Aguilar, M.; Puig, J.; Mutjé, P. Tensile Properties of Polypropylene Composites Reinforced with Mechanical, Thermomechanical, and Chemi-Thermomechanical Pulps from Orange Pruning. Bioresources 2015, 10, 4544–4556. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.; Fehrenbach, J.; Ulven, C.A. Hybridization of Hemp Fiber and Recycled-Carbon Fiber in Polypropylene Composites. Sustainability 2019, 11, 3163. [Google Scholar] [CrossRef] [Green Version]
- Serra, A.; Tarrés, Q.; Llop, M.; Reixach, R.; Mutjé, P.; Espinach, F.X. Recycling Dyed Cotton Textile Byproduct Fibers as Polypropylene Reinforcement. Text. Res. J. 2019, 89, 2113–2125. [Google Scholar] [CrossRef]
- Pickering, K.L.; Efendy, M.G.A.; Le, T.M. A Review of Recent Developments in Natural Fibre Composites and Their Mechanical Performance. Compos. Part A Appl. Sci. Manuf. 2016, 83, 98–112. [Google Scholar] [CrossRef] [Green Version]
- Graupner, N. Application of Lignin as Natural Adhesion Promoter in Cotton Fibre-Reinforced Poly(Lactic Acid) (PLA) Composites. J. Mater. Sci. 2008, 43, 5222–5229. [Google Scholar] [CrossRef]
- Wang, C.; Kelley, S.S.; Venditti, R.A. Lignin-Based Thermoplastic Materials. ChemSusChem 2016, 9, 770–783. [Google Scholar] [CrossRef]
- Park, C.-W.; Youe, J.; Namgung, H.-W.; Han, S.-Y.; Seo, P.-N.; Chae, H.-M.; Lee, S.-H. Effect of Lignocellulose Nanofibril and Polymeric Methylene Diphenyl Diisocyanate Addition on Plasticized Lignin/Polycaprolactone Composites. BioResources 2018, 13, 6802–6817. [Google Scholar] [CrossRef]
- Chihaoui, B.; Tarrés, Q.; Delgado-Aguilar, M.; Mutjé, P.; Boufi, S. Lignin-Containing Cellulose Fibrils as Reinforcement of Plasticized PLA Biocomposites Produced by Melt Processing Using PEG as a Carrier. Ind. Crops Prod. 2022, 175, 114287. [Google Scholar] [CrossRef]
- Alekhina, M.; Ershova, O.; Ebert, A.; Heikkinen, S.; Sixta, H. Softwood Kraft Lignin for Value-Added Applications: Fractionation and Structural Characterization. Ind. Crops Prod. 2015, 66, 220–228. [Google Scholar] [CrossRef]
- García, A.; Toledano, A.; Serrano, L.; Egüés, I.; González, M.; Marín, F.; Labidi, J. Characterization of Lignins Obtained by Selective Precipitation. Sep. Purif. Technol. 2009, 68, 193–198. [Google Scholar] [CrossRef]
- Sewring, T.; Theliander, H. Acid Precipitation of Kraft Lignin from Aqueous Solutions: The Influence of Anionic Specificity and Concentration Level of the Salt. Holzforschung 2019, 73, 937–945. [Google Scholar] [CrossRef]
- Faustino, H.; Gil, N.; Baptista, C.; Duarte, A.P. Antioxidant Activity of Lignin Phenolic Compounds Extracted from Kraft and Sulphite Black Liquors. Molecules 2010, 15, 9308–9322. [Google Scholar] [CrossRef] [Green Version]
- Stoklosa, R.J.; Velez, J.; Kelkar, S.; Saffron, C.M.; Thies, M.C.; Hodge, D.B. Correlating Lignin Structural Features to Phase Partitioning Behavior in a Novel Aqueous Fractionation of Softwood Kraft Black Liquor. Green Chem. 2013, 15, 2904–2912. [Google Scholar] [CrossRef]
- Cardoso, M.; de Oliveira, É.D.; Passos, M.L. Chemical Composition and Physical Properties of Black Liquors and Their Effects on Liquor Recovery Operation in Brazilian Pulp Mills. Fuel 2009, 88, 756–763. [Google Scholar] [CrossRef]
- Andreuccetti, M.T.; Santos Leite, B.; D’angelo, J.V.H. Eucalyptus Black Liquor-Density, Viscosity, Solids and Sodium Sulfate Contents Revisited. O Pap. 2011, 72, 52–57. [Google Scholar]
- Oliveira, R.C.P.; Mateus, M.; Santos, D.M.F. Chronoamperometric and Chronopotentiometric Investigation of Kraft Black Liquor. Int. J. Hydrogen Energy 2018, 43, 16817–16823. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Espinosa, E.; Savy, D.; Rosal, A.; Rodríguez, A. Biorefinery Process Combining Specel Process and Selective Lignin Precipitation Using Mineral Acids. BioResources 2016, 11, 7061–7077. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-robles, J.; Sánchez, R.; Díaz-carrasco, P.; Espinosa, E.; García-domínguez, M.T.; Rodríguez, A. Isolation and Characterization of Lignins from Wheat Straw: Application as Binder in Lithium Batteries. Int. J. Biol. Macromol. 2017, 104, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Robles, J.; Tamminen, T.; Liitiä, T.; Peresin, M.S.; Rodríguez, A.; Jääskeläinen, A.S. Aqueous Acetone Fractionation of Kraft, Organosolv and Soda Lignins. Int. J. Biol. Macromol. 2018, 106, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Huang, J.; He, Y.; Hu, C.; Zhang, Q.; Yin, X.; Wu, W.; Li, R.K.Y. Biodegradable and Renewable UV-Shielding Polylactide Composites Containing Hierarchical Structured POSS Functionalized Lignin. Int. J. Biol. Macromol. 2021, 188, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liu, T.; Deng, X.; Sun, Q.; Cao, X.; Feng, Y.; Wang, B.; Roy, V.A.L.; Li, R.K.Y. Ecofriendly UV-Protective Films Based on Poly(Propylene Carbonate) Biocomposites Filled with TiO2 Decorated Lignin. Int. J. Biol. Macromol. 2019, 126, 1030–1036. [Google Scholar] [CrossRef]
- Domínguez, J.C.; Oliet, M.; Alonso, M.V.; Gilarranz, M.A.; Rodríguez, F. Thermal Stability and Pyrolysis Kinetics of Organosolv Lignins Obtained from Eucalyptus Globulus. Ind. Crops Prod. 2008, 27, 150–156. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Sánchez, R.; Espinosa, E.; Savy, D.; Mazzei, P.; Piccolo, A.; Rodríguez, A. Isolation and Characterization of Gramineae and Fabaceae Soda Lignins. Int. J. Mol. Sci. 2017, 18, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, R.; Tomkinson, J.; Lloyd Jones, G. Fractional Characterization of Ash-AQ Lignin by Successive Extraction with Organic Solvents from Oil Palm EFB fibre. Polym. Degrad. Stab. 2000, 68, 111–119. [Google Scholar] [CrossRef]
- Mikus, P.Y.; Alix, S.; Soulestin, J.; Lacrampe, M.F.; Krawczak, P.; Coqueret, X.; Dole, P. Deformation Mechanisms of Plasticized Starch Materials. Carbohydr. Polym. 2014, 114, 450–457. [Google Scholar] [CrossRef]
- Alonso-González, M.; Felix, M.; Romero, A. Influence of the Plasticizer on Rice Bran-Based Eco-Friendly Bioplastics Obtained by Injection Moulding. Ind. Crops Prod. 2022, 180, 114767. [Google Scholar] [CrossRef]
- Upton, B.M.; Kasko, A.M. Strategies for the Conversion of Lignin to High-Value Polymeric Materials: Review and Perspective. Chem. Rev. 2016, 116, 2275–2306. [Google Scholar] [CrossRef] [PubMed]
- Yu, O.; Kim, K.H. Lignin to Materials: A Focused Review on Recent Novel Lignin Applications. Appl. Sci. 2020, 10, 4626. [Google Scholar] [CrossRef]
- Kaewtatip, K.; Thongmee, J. Effect of Kraft Lignin and Esterified Lignin on the Properties of Thermoplastic Starch. Mater. Des. 2013, 49, 701–704. [Google Scholar] [CrossRef]
- Hu, L.; Stevanovic, T.; Rodrigue, D. Compatibilization of Kraft Lignin-Polyethylene Composites Using Unreactive Compatibilizers. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Dammak, M.; Fourati, Y.; Tarrés, Q.; Delgado-Aguilar, M.; Mutjé, P.; Boufi, S. Blends of PBAT with Plasticized Starch for Packaging Applications: Mechanical Properties, Rheological Behaviour and Biodegradability. Ind. Crops Prod. 2020, 144, 112061. [Google Scholar] [CrossRef]
- Jumaidin, R.; Khiruddin, M.A.A.; Asyul Sutan Saidi, Z.; Salit, M.S.; Ilyas, R.A. Effect of Cogon Grass Fibre on the Thermal, Mechanical and Biodegradation Properties of Thermoplastic Cassava Starch Biocomposite. Int. J. Biol. Macromol. 2020, 146, 746–755. [Google Scholar] [CrossRef]
- Serra-Parareda, F.; Julián, F.; Espinosa, E.; Rodríguez, A.; Espinach, F.X.; Vilaseca, F. Feasibility of Barley Straw Fibers as Reinforcement in Fully Biobased Polyethylene Composites: Macro and Micro Mechanics of the Flexural Strength. Molecules 2020, 25, 2242. [Google Scholar] [CrossRef]
- Serra-Parareda, F.; Tarrés, Q.; Espinach, F.X.; Vilaseca, F.; Mutjé, P.; Delgado-Aguilar, M. Influence of Lignin Content on the Intrinsic Modulus of Natural Fibers and on the Stiffness of Composite Materials. Int. J. Biol. Macromol. 2020, 155, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Aguado, R.; Espinach, F.X.; Vilaseca, F.; Tarrés, Q.; Mutjé, P.; Delgado-Aguilar, M. Approaching a Zero-Waste Strategy in Rapeseed (Brassica napus) Exploitation: Sustainably Approaching Bio-Based Polyethylene Composites. Sustainability 2022, 14, 7942. [Google Scholar] [CrossRef]
- Kakou, C.A.; Arrakhiz, F.Z.; Trokourey, A.; Bouhfid, R.; Qaiss, A.; Rodrigue, D. Influence of Coupling Agent Content on the Properties of High Density Polyethylene Composites Reinforced with Oil Palm Fibers. Mater. Des. 2014, 63, 641–649. [Google Scholar] [CrossRef]
- Mulinari, D.R.; Voorwald, H.J.C.; Cioffi, M.O.H.; da Silva, M.L.C.P.; da Cruz, T.G.; Saron, C. Sugarcane Bagasse Cellulose/HDPE Composites Obtained by Extrusion. Compos. Sci. Technol. 2009, 69, 214–219. [Google Scholar] [CrossRef]
- López, J.P.; Méndez, J.A.; el Mansouri, N.-E.; Mutjé, P.; Vilaseca, F. Mean Intrinsic Tensile Properties of Stone Groundwood Fibers from Softwood. Bioresources 2011, 6, 5037–5049. [Google Scholar] [CrossRef]
- Serra-Parareda, F.; Tarrés, Q.; Delgado-Aguilar, M.; Espinach, F.X.; Mutjé, P.; Vilaseca, F. Biobased Composites from Biobased-Polyethylene and Barley Thermomechanical Fibers: Micromechanics of Composites. Materials 2019, 12, 4182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundholm, J. Mechanical Pulping; Fapet Oy: Dublin, OH, USA, 1999; Volume 5, ISBN 9525216055. [Google Scholar]
- Alzagameem, A.; Klein, S.E.; Bergs, M.; Do, X.T.; Korte, I.; Dohlen, S.; Hüwe, C.; Kreyenschmidt, J.; Kamm, B.; Larkins, M.; et al. Antimicrobial Activity of Lignin and Lignin-Derived Cellulose and Chitosan Composites against Selected Pathogenic and Spoilage Microorganisms. Polymers 2019, 11, 670. [Google Scholar] [CrossRef] [Green Version]
- Yun, J.; Wei, L.; Li, W.; Gong, D.; Qin, H.; Feng, X.; Li, G.; Ling, Z.; Wang, P.; Yin, B. Isolating High Antimicrobial Ability Lignin from Bamboo Kraft Lignin by Organosolv Fractionation. Front. Bioeng. Biotechnol. 2021, 9, 683796. [Google Scholar] [CrossRef]
- Lourençon, T.V.; de Lima, G.G.; Ribeiro, C.S.P.; Hansel, F.A.; Maciel, G.M.; da Silva, K.; Winnischofer, S.M.B.; de Muniz, G.I.B.; Magalhães, W.L.E. Antioxidant, Antibacterial and Antitumoural Activities of Kraft Lignin from Hardwood Fractionated by Acid Precipitation. Int. J. Biol. Macromol. 2021, 166, 1535–1542. [Google Scholar] [CrossRef]
- Luzi, F.; Yang, W.; Ma, P.; Torre, L.; Puglia, D. Lignin-Based Materials with Antioxidant and Antimicrobial Properties. In Lignin-Based Materials for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 291–326. [Google Scholar]





| Thermoplastic Lignin and Poly(Caprolactone) Blends | |||
|---|---|---|---|
| Sample | Thermoplastic Lignin Content (wt.%) | PCL Content (wt.%) | SGW Content (wt.%) |
| TPL | 100 | 0 | - |
| 90TPL/10PCL | 90 | 10 | - |
| 80TPL/20PCL | 80 | 20 | - |
| 70TPL/30PCL | 70 | 30 | - |
| 60TPL/40PCL | 60 | 40 | - |
| 50TPL/50PCL | 50 | 50 | - |
| 40TPL/60PCL | 40 | 60 | - |
| 30TPL/70PCL | 30 | 70 | - |
| 20TPL/80PCL | 20 | 80 | - |
| 10TPL/90PCL | 10 | 90 | - |
| PCL | 0 | 100 | - |
| Composites with SGW | |||
| Sample | TPL/PCL Blend Content (wt.%) | SGW Content (wt.%) | |
| 80TPL/20PCL + 10SGW | 90 | 10 | |
| 80TPL/20PCL + 20SGW | 80 | 20 | |
| 80TPL/20PCL + 30SGW | 70 | 30 | |
| 80TPL/20PCL + 40SGW | 60 | 40 | |
| Black Liquor | |||
|---|---|---|---|
| Density (g/mL) | Total Dry Solids (g/L) | Acid spent (mL/L BL) | Lignin Extraction (g/L) |
| 1.176 | 28.8 | 290 | 15.3 |
| Lignin | |||
| Density (g/mL) | Ash (%) | Klason Lignin (%) | Acid-Soluble Lignin (%) |
| 1.384 | 13.12 | 44.72 | 6.93 |
| Glycerin (wt.%) | VG | Density (g/mL) |
|---|---|---|
| 10 | 0.109 | 1.37 ± 0.01 |
| 20 | 0.215 | 1.36 ± 0.01 |
| 30 | 0.320 | 1.34 ± 0.02 |
| 40 | 0.423 | 1.33 ± 0.01 |
| Glycerin (wt.%) | Tensile Properties | Flexural Properties | ||||
|---|---|---|---|---|---|---|
| Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation at Break (%) | Flexural Strength (MPa) | Flexural Modulus (MPa) | Elongation (%) | |
| 10 | 0.112 ± 0.536 | 215 ± 36 | 3.945 ± 1.126 | 1.874 ± 0.784 | 39 ± 12 | 1.322 ± 0.742 |
| 20 | 0.084 ± 0.697 | 119 ± 21 | 0.719 ± 0.542 | 0.682 ± 0.587 | 14 ± 16 | 0.466 ± 0.312 |
| 30 | 0.035 ± 0.342 | 21 ± 19 | 0.077 ± 0.345 | 0.123 ± 0.421 | 2 ± 7 | 0.081 ± 0.154 |
| 40 | - | - | - | - | - | - |
| Thermoplastic Lignin | PCL (wt.%) | VPCL | Density (g/mL) |
|---|---|---|---|
| 90% lignin + 10% glycerin | 10 | 0.108 | 1.36 ± 0.01 |
| 20 | 0.214 | 1.35 ± 0.01 | |
| 30 | 0.318 | 1.33 ± 0.01 | |
| 40 | 0.420 | 1.32 ± 0.01 | |
| 50 | 0.521 | 1.31 ± 0.02 | |
| 60 | 0.620 | 1.30 ± 0.01 | |
| 70 | 0.717 | 1.29 ± 0.01 | |
| 80 | 0.813 | 1.28 ± 0.01 | |
| 90 | 0.907 | 1.27 ± 0.02 | |
| 100 | 1.000 | 1.26 ± 0.01 | |
| 80% lignin + 20% glycerin | 10 | 0.107 | 1.36 ± 0.01 |
| 20 | 0.212 | 1.34 ± 0.01 | |
| 30 | 0.316 | 1.33 ± 0.02 | |
| 40 | 0.418 | 1.32 ± 0.01 | |
| 50 | 0.518 | 1.31 ± 0.01 | |
| 60 | 0.617 | 1.30 ± 0.01 | |
| 70 | 0.715 | 1.29 ± 0.01 | |
| 80 | 0.811 | 1.27 ± 0.01 | |
| 90 | 0.906 | 1.27 ± 0.01 | |
| 100 | 1.000 | 1.26 ± 0.01 | |
| 70% lignin + 30% glycerin | 10 | 0.106 | 1.36 ± 0.01 |
| 20 | 0.210 | 1.34 ± 0.01 | |
| 30 | 0.313 | 1.33 ± 0.01 | |
| 40 | 0.415 | 1.32 ± 0.01 | |
| 50 | 0.516 | 1.31 ± 0.01 | |
| 60 | 0.615 | 1.30 ± 0.01 | |
| 70 | 0.713 | 1.29 ± 0.01 | |
| 80 | 0.810 | 1.27 ± 0.01 | |
| 90 | 0.906 | 1.27 ± 0.01 | |
| 100 | 1.000 | 1.26 ± 0.01 |
| SGW Fibers (wt.%) | VF | Density (g/mL) | Width (µm) | Aspect Ratio 1 | ||
|---|---|---|---|---|---|---|
| 0 | 0.100 | 1.345 ± 0.06 | - | - | - | - |
| 10 | 0.100 | 1.345 ± 0.04 | 205 | 953 | 33.6 | 6.1 |
| 20 | 0.199 | 1.346 ± 0.07 | 195 | 859 | 33.4 | 5.8 |
| 30 | 0.299 | 1.346 ± 0.05 | 182 | 727 | 32.2 | 5.7 |
| 40 | 0.399 | 1.347 ± 0.05 | 172 | 627 | 30.3 | 5.7 |
| SGW Fibers (wt.%) | Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation at Break (%) | Flexural Strength (MPa) | Flexural Modulus (MPa) | Elongation (%) |
|---|---|---|---|---|---|---|
| 0 | 1.679 ± 0.543 | 282 ± 36 | 4.79 ± 1.05 | 2.669 ± 0.743 | 61 ± 12 | 2.048 ± 0.912 |
| 10 | 3.762 ± 0.875 | 642 ± 79 | 3.59 ± 0.87 | 8.008 ± 1.103 | 325 ± 34 | 1.568 ± 0.423 |
| 20 | 6.245 ± 0.674 | 1174 ± 46 | 2.32 ± 0.92 | 15.34 ± 1.546 | 529 ± 29 | 1.175 ± 0.687 |
| 30 | 8.269 ± 1.279 | 1567 ± 87 | 1.65 ± 0.63 | 22.47 ± 1.429 | 734 ± 41 | 1.010 ± 0.443 |
| 40 | 11.217 ± 1.875 | 1872 ± 64 | 1.24 ± 0.57 | 26.84 ± 2.061 | 1083 ± 63 | 0.880 ± 0.162 |
| SGW Fibers (wt.%) | First Assumption | Second Assumption | |||
|---|---|---|---|---|---|
| 10 | 1.55 | 250 | 0.09 | 132 | 0.18 |
| 20 | 1.20 | 250 | 0.11 | 147 | 0.18 |
| 30 | 0.92 | 250 | 0.10 | 142 | 0.18 |
| 40 | 0.73 | 250 | 0.11 | 150 | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarrés, Q.; Aguado, R.; Domínguez-Robles, J.; Larrañeta, E.; Delgado-Aguilar, M. Valorization of Kraft Lignin from Black Liquor in the Production of Composite Materials with Poly(caprolactone) and Natural Stone Groundwood Fibers. Polymers 2022, 14, 5178. https://doi.org/10.3390/polym14235178
Tarrés Q, Aguado R, Domínguez-Robles J, Larrañeta E, Delgado-Aguilar M. Valorization of Kraft Lignin from Black Liquor in the Production of Composite Materials with Poly(caprolactone) and Natural Stone Groundwood Fibers. Polymers. 2022; 14(23):5178. https://doi.org/10.3390/polym14235178
Chicago/Turabian StyleTarrés, Quim, Roberto Aguado, Juan Domínguez-Robles, Eneko Larrañeta, and Marc Delgado-Aguilar. 2022. "Valorization of Kraft Lignin from Black Liquor in the Production of Composite Materials with Poly(caprolactone) and Natural Stone Groundwood Fibers" Polymers 14, no. 23: 5178. https://doi.org/10.3390/polym14235178
APA StyleTarrés, Q., Aguado, R., Domínguez-Robles, J., Larrañeta, E., & Delgado-Aguilar, M. (2022). Valorization of Kraft Lignin from Black Liquor in the Production of Composite Materials with Poly(caprolactone) and Natural Stone Groundwood Fibers. Polymers, 14(23), 5178. https://doi.org/10.3390/polym14235178