Magnetic Extraction of Weathered Tire Wear Particles and Polyethylene Microplastics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Accelerated Ageing
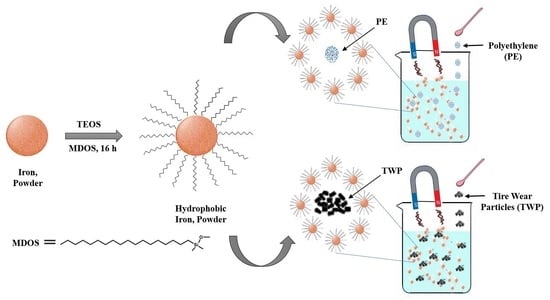
2.3. Synthesis of Fe@SiO2/MDOS Nanocomposite
2.4. Characterization of Fe@SiO2/MDOS, PE and TWP
2.5. Recovery of PE and TWP
3. Results and Discussion
3.1. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy
3.2. Thermogravimetric Analysis
3.3. Scanning Electron Microscopy
3.4. Magnetic Extraction Experiments
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a Consensus on the Definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, J.; Xing, B. Environmental Source, Fate, and Toxicity of Microplastics. J. Hazard. Mater. 2021, 407, 124357. [Google Scholar] [CrossRef]
- Wu, P.; Huang, J.; Zheng, Y.; Yang, Y.; Zhang, Y.; He, F.; Chen, H.; Quan, G.; Yan, J.; Li, T.; et al. Environmental Occurrences, Fate, and Impacts of Microplastics. Ecotoxicol. Environ. Saf. 2019, 184, 109612. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, J.; Sun, C.; Jiang, F.; Ju, P.; Qu, L.; Zheng, Y.; He, C. Detection of Microplastics in Local Marine Organisms Using a Multi-Technology System. Anal. Methods 2019, 11, 78–87. [Google Scholar] [CrossRef]
- Rocha-Santos, T.; Duarte, A.C. A Critical Overview of the Analytical Approaches to the Occurrence, the Fate and the Behavior of Microplastics in the Environment. TrAC Trends Anal. Chem. 2015, 65, 47–53. [Google Scholar] [CrossRef]
- Vinay Kumar, B.N.; Löschel, L.A.; Imhof, H.K.; Löder, M.G.J.; Laforsch, C. Analysis of Microplastics of a Broad Size Range in Commercially Important Mussels by Combining FTIR and Raman Spectroscopy Approaches. Environ. Pollut. 2021, 269, 116147. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Zhang, X.; Hu, J. Methods for Separating Microplastics from Complex Solid Matrices: Comparative Analysis. J. Hazard. Mater. 2021, 409, 124640. [Google Scholar] [CrossRef] [PubMed]
- Nabi, I.; Bacha, A.U.R.; Zhang, L. A Review on Microplastics Separation Techniques from Environmental Media. J. Clean. Prod. 2022, 337, 130458. [Google Scholar] [CrossRef]
- Nguyen, B.; Claveau-Mallet, D.; Hernandez, L.M.; Xu, E.G.; Farner, J.M.; Tufenkji, N. Separation and Analysis of Microplastics and Nanoplastics in Complex Environmental Samples. Acc. Chem. Res. 2019, 52, 858–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinn, B.; Murphy, F.; Ewins, C. Validation of Density Separation for the Rapid Recovery of Microplastics from Sediment. Anal. Methods 2017, 9, 1491–1498. [Google Scholar] [CrossRef]
- Cabernard, L.; Roscher, L.; Lorenz, C.; Gerdts, G.; Primpke, S. Comparison of Raman and Fourier Transform Infrared Spectroscopy for the Quantification of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2018, 52, 13279–13288. [Google Scholar] [CrossRef]
- Kovochich, M.; Liong, M.; Parker, J.A.; Oh, S.C.; Lee, J.P.; Xi, L.; Kreider, M.L.; Unice, K.M. Chemical Mapping of Tire and Road Wear Particles for Single Particle Analysis. Sci. Total Environ. 2021, 757, 144085. [Google Scholar] [CrossRef]
- Vindedahl, A.M.; Strehlau, J.H.; Arnold, W.A.; Penn, R.L. Organic Matter and Iron Oxide Nanoparticles: Aggregation, Interactions, and Reactivity. Environ. Sci. Nano 2016, 3, 494–505. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, W.; Cai, Z.; Han, B.; Qian, T.; Zhao, D. An Overview of Preparation and Applications of Stabilized Zero-Valent Iron Nanoparticles for Soil and Groundwater Remediation. Water Res. 2016, 100, 245–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grbic, J.; Nguyen, B.; Guo, E.; You, J.B.; Sinton, D.; Rochman, C.M. Magnetic Extraction of Microplastics from Environmental Samples. Environ. Sci. Technol. Lett. 2019, 6, 68–72. [Google Scholar] [CrossRef]
- Misra, A.; Zambrzycki, C.; Kloker, G.; Kotyrba, A.; Anjass, M.H.; Franco Castillo, I.; Mitchell, S.G.; Güttel, R.; Streb, C. Water Purification and Microplastics Removal Using Magnetic Polyoxometalate-Supported Ionic Liquid Phases (MagPOM-SILPs). Angew. Chem. Int. Ed. 2020, 59, 1601–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhao, J.; Liu, Z.; Tian, S.; Lu, J.; Mu, R.; Yuan, H. Coagulation Removal of Microplastics from Wastewater by Magnetic Magnesium Hydroxide and PAM. J. Water Process Eng. 2021, 43, 102250. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, S.; Su, Y.; Wu, D.; Zhao, Y.; Xie, B. Removal of Microplastics from Aqueous Solutions by Magnetic Carbon Nanotubes. Chem. Eng. J. 2021, 406, 126804. [Google Scholar] [CrossRef]
- Kang, J.; Zhou, L.; Duan, X.; Sun, H.; Ao, Z.; Wang, S. Degradation of Cosmetic Microplastics via Functionalized Carbon Nanosprings. Matter 2019, 1, 745–758. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Zhang, S.; Zhao, J.; Ma, J.; Wu, H.; Sun, H.; Cheng, S. Experimental Study on Removal of Microplastics from Aqueous Solution by Magnetic Force Effect on the Magnetic Sepiolite. Sep. Purif. Technol. 2022, 288, 120564. [Google Scholar] [CrossRef]
- Budhiraja, V.; Urh, A.; Horvat, P.; Krzan, A. Synergistic Adsorption of Organic Pollutants on Weathered Polyethylene Microplastics. Polymers 2022, 14, 2674. [Google Scholar] [CrossRef]
- Julienne, F.; Lagarde, F.; Delorme, N. Influence of the Crystalline Structure on the Fragmentation of Weathered Polyolefines. Polym. Degrad. Stab. 2019, 170, 109012. [Google Scholar] [CrossRef]
- Cai, J.; Guo, J.; Ji, M.; Yang, W.; Wang, C.; Fu, S. Preparation and Characterization of Multiresponsive Polymer Composite Microspheres with Core-Shell Structure. Colloid Polym. Sci. 2007, 285, 1607–1615. [Google Scholar] [CrossRef]
- Xie, W.; Wang, H. Immobilized Polymeric Sulfonated Ionic Liquid on Core-Shell Structured Fe3O4/SiO2 Composites: A Magnetically Recyclable Catalyst for Simultaneous Transesterification and Esterifications of Low-Cost Oils to Biodiesel. Renew. Energy 2020, 145, 1709–1719. [Google Scholar] [CrossRef]
- Widjonarko, D.M.; Jumina, J.; Kartini, I. Nuryono Phosphonate Modified Silica for Adsorption of Co(II), Ni(II), Cu(II), and Zn(II). Indones. J. Chem. 2014, 14, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Ahangaran, F.; Hassanzadeh, A.; Nouri, S. Surface Modification of Fe3O4@SiO2 Microsphere by Silane Coupling Agent. Int. Nano Lett. 2013, 3, 1–5. [Google Scholar] [CrossRef]
- Fan, C.; Huang, Y.Z.; Lin, J.N.; Li, J. Microplastic Constituent Identification from Admixtures by Fourier-Transform Infrared (FTIR) Spectroscopy: The Use of Polyethylene Terephthalate (PET), Polyethylene (PE), Polypropylene (PP), Polyvinyl Chloride (PVC) and Nylon (NY) as the Model Constituent. Environ. Technol. Innov. 2021, 23, 101798. [Google Scholar] [CrossRef]
- Ceccarini, A.; Corti, A.; Erba, F.; Modugno, F.; La Nasa, J.; Bianchi, S.; Castelvetro, V. The Hidden Microplastics: New Insights and Figures from the Thorough Separation and Characterization of Microplastics and of Their Degradation Byproducts in Coastal Sediments. Environ. Sci. Technol. 2018, 52, 5634–5643. [Google Scholar] [CrossRef] [PubMed]
- Almond, J.; Sugumaar, P.; Wenzel, M.N.; Hill, G.; Wallis, C. Determination of the Carbonyl Index of Polyethylene and Polypropylene Using Specified Area under Band Methodology with ATR-FTIR Spectroscopy. E-Polymers 2020, 20, 369–381. [Google Scholar] [CrossRef]
- Gharehdashli, A.; Mortazavi, S.; Rashidi, H. Photodegradation of Low-Density Polyethylene with Prooxidant and Photocatalyst. J. Appl. Polym. Sci. 2020, 137, 48979. [Google Scholar] [CrossRef]
- Baensch-Baltruschat, B.; Kocher, B.; Stock, F.; Reifferscheid, G. Tyre and Road Wear Particles (TRWP)—A Review of Generation, Properties, Emissions, Human Health Risk, Ecotoxicity, and Fate in the Environment. Sci. Total Environ. 2020, 733, 137823. [Google Scholar] [CrossRef] [PubMed]
- Analysis of Elastomers with Different Carbon Black Contents by TGA. Available online: https://www.mt.com/at/de/home/supportive_content/matchar_apps/MatChar_HB453.html (accessed on 11 November 2022).
- Shariati-Rad, M.; Irandoust, M.; Amri, S.; Feyzi, M.; Ja’fari, F. Magnetic Solid Phase Adsorption, Preconcentration and Determination of Methyl Orange in Water Samples Using Silica Coated Magnetic Nanoparticles and Central Composite Design. Int. Nano Lett. 2014, 4, 91–101. [Google Scholar] [CrossRef]
- Herath, A.; Salehi, M. Studying the Combined Influence of Microplastics’ Intrinsic and Extrinsic Characteristics on Their Weathering Behavior and Heavy Metal Transport in Storm Runoff. Environ. Pollut. 2022, 308, 119628. [Google Scholar] [CrossRef]
- Son, C.E.; Choi, S.S. Preparation and Characterization of Model Tire-Road Wear Particles. Polymers 2022, 14, 1512. [Google Scholar] [CrossRef] [PubMed]
- van Hoek, J.W.; Heideman, G.; Noordermeer, J.W.M.; Dierkes, W.K.; Blume, A. Implications of the Use of Silica as Active Filler in Passenger Car Tire Compounds on Their Recycling Options. Materials 2019, 12, 725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beghetto, V.; Sole, R.; Buranello, C.; Al-Abkal, M.; Facchin, M. Recent Advancements in Plastic Packaging Recycling: A Mini-Review. Materials 2021, 14, 4782. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, X.; Gao, W.; Zhang, Y.; He, D. Removal of Microplastics from Water by Magnetic Nano-Fe3O4. Sci. Total Environ. 2022, 802, 149838. [Google Scholar] [CrossRef]
- Liu, P.; Zhan, X.; Wu, X.; Li, J.; Wang, H.; Gao, S. Effect of Weathering on Environmental Behavior of Microplastics: Properties, Sorption and Potential Risks. Chemosphere 2020, 242, 125193. [Google Scholar] [CrossRef]
- Luo, H.; Liu, C.; He, D.; Xu, J.; Sun, J.; Li, J.; Pan, X. Environmental Behaviors of Microplastics in Aquatic Systems: A Systematic Review on Degradation, Adsorption, Toxicity and Biofilm under Aging Conditions. J. Hazard. Mater. 2022, 423, 126915. [Google Scholar] [CrossRef]
- Enfrin, M.; Hachemi, C.; Hodgson, P.D.; Jegatheesan, V.; Vrouwenvelder, J.; Callahan, D.L.; Lee, J.; Dumée, L.F. Nano/Micro Plastics—Challenges on Quantification and Remediation: A Review. J. Water Process Eng. 2021, 42, 102128. [Google Scholar] [CrossRef]
- Luo, H.; Zhao, Y.; Li, Y.; Xiang, Y.; He, D.; Pan, X. Aging of Microplastics Affects Their Surface Properties, Thermal Decomposition, Additives Leaching and Interactions in Simulated Fluids. Sci. Total Environ. 2020, 714, 136862. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.M.A.; Sheng, J.; Zimba, P.V.; Zhu, L.; Fadare, O.O.; Haley, C.; Wang, M.; Phillips, T.D.; Conkle, J.; Xu, W. Testing an Iron Oxide Nanoparticle-Based Method for Magnetic Separation of Nanoplastics and Microplastics from Water. Nanomaterials 2022, 12, 2348. [Google Scholar] [CrossRef]
- Gunawardana, C.; Goonetilleke, A.; Egodawatta, P.; Dawes, L.; Kokot, S. Source Characterisation of Road Dust Based on Chemical and Mineralogical Composition. Chemosphere 2012, 87, 163–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klöckner, P.; Seiwert, B.; Weyrauch, S.; Escher, B.I.; Reemtsma, T.; Wagner, S. Comprehensive Characterization of Tire and Road Wear Particles in Highway Tunnel Road Dust by Use of Size and Density Fractionation. Chemosphere 2021, 279, 130530. [Google Scholar] [CrossRef]
- Wang, J.; Sun, C.; Huang, Q.X.; Chi, Y.; Yan, J.H. Adsorption and Thermal Degradation of Microplastics from Aqueous Solutions by Mg/Zn Modified Magnetic Biochars. J. Hazard. Mater. 2021, 419, 126486. [Google Scholar] [CrossRef]
- Rhein, F.; Scholl, F.; Nirschl, H. Magnetic Seeded Filtration for the Separation of Fine Polymer Particles from Dilute Suspensions: Microplastics. Chem. Eng. Sci. 2019, 207, 1278–1287. [Google Scholar] [CrossRef]
- Ramage, S.J.F.F.; Pagaling, E.; Haghi, R.K.; Dawson, L.A.; Yates, K.; Prabhu, R.; Hillier, S.; Devalla, S. Rapid Extraction of High- and Low-Density Microplastics from Soil Using High-Gradient Magnetic Separation. Sci. Total Environ. 2022, 831, 154912. [Google Scholar] [CrossRef]
- Wang, C.; Huang, R.; Sun, R.; Yang, J.; Dionysiou, D.D. Microplastics Separation and Subsequent Carbonization: Synthesis, Characterization, and Catalytic Performance of Iron/Carbon Nanocomposite. J. Clean. Prod. 2022, 330, 129901. [Google Scholar] [CrossRef]
- Zandieh, M.; Liu, J. Removal and Degradation of Microplastics Using the Magnetic and Nanozyme Activities of Bare Iron Oxide Nanoaggregates. Angew. Chem. Int. Ed. 2022, 61, e202212013. [Google Scholar] [CrossRef]
- Rhein, F.; Nirschl, H.; Kaegi, R. Separation of Microplastic Particles from Sewage Sludge Extracts Using Magnetic Seeded Filtration. Water Res. X 2022, 17, 100155. [Google Scholar] [CrossRef]
- Bhore, R.K.; KAMBLE, S.B. Nano Adsorptive Extraction of Diverse Microplastics from the Potable and Seawater Using Organo-Polyoxometalate Magnetic Nanotricomposites. SSRN Electron. J. 2022, 10, 108720. [Google Scholar]
- Ye, H.; Wang, Y.; Liu, X.; Xu, D.; Yuan, H.; Sun, H.; Wang, S.; Ma, X. Magnetically Steerable Iron Oxides-Manganese Dioxide Core–Shell Micromotors for Organic and Microplastic Removals. J. Colloid Interface Sci. 2021, 588, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Sarcletti, M.; Park, H.; Wirth, J.; Englisch, S.; Eigen, A.; Drobek, D.; Vivod, D.; Friedrich, B.; Tietze, R.; Alexiou, C.; et al. The Remediation of Nano-/Microplastics from Water. Mater. Today 2021, 48, 38–46. [Google Scholar] [CrossRef]
- Rius-Ayra, O.; Bouhnouf-Riahi, O.; Llorca-Isern, N. Superhydrophobic and Sustainable Nanostructured Powdered Iron for the Efficient Separation of Oil-in-Water Emulsions and the Capture of Microplastics. ACS Appl. Mater. Interfaces 2020, 12, 45629–45640. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.J.; Park, J.W.; Jung, S.M. Repeatable Separation of Microplastics Integrating Mineral Oil Extraction and a PDMS-Ni Foam Adsorbent in Real Soil. Chem. Eng. J. 2022, 429, 132517. [Google Scholar] [CrossRef]
- Sun, M.; Chen, W.; Fan, X.; Tian, C.; Sun, L.; Xie, H. Cooperative Recyclable Magnetic Microsubmarines for Oil and Microplastics Removal from Water. Appl. Mater. Today 2020, 20, 100682. [Google Scholar] [CrossRef]





| Polymer | Tos (°C) | Tmid (°C) | Tmax (°C) | Tp (°C) |
|---|---|---|---|---|
| PE | 485.96 | 513.25 | 525.17 | 515.92 |
| Weathered PE1000h | 466.97 | 505.64 | 521.73 | 510.22 |
| TWP | 342.41 | 481.46 | 727.62 | 472.00 723.00 |
| Weathered TWP1000h | 333.09 | 488.40 | 740.51 | 472.00 731.00 |
| Material | MPs Type | MPs Size | Removal Efficiency | Reference |
|---|---|---|---|---|
| Fe-Hexadecyltrimethoxysilane | HDPE, PP, PS, PU 1, PVC, PET | 200–1000 µm | 49–105% | [15] |
| Magnetic polyoxometalate ionic-liquids | PS | 1 or 10 µm | 100% | [16] |
| Magnetic Carbon Nanotubes | PE, PA, PET | 48 µm | 100% | [18] |
| Magnetic Sepiolite | PE | 48 µm | 98.4% | [20] |
| Nano-Fe3O4 | PE, PP, PS, PET | 200–900 µm | 62.83–86.87% | [38] |
| Fe-polydimethylsiloxane | LDPE 2 PS | 2–5 mm 100–1000 nm | 100% 90% | [43] |
| Magnetic biochar | PS | 1 µm | 94.8% | [46] |
| Magnetic seeded filtration, Fe3O4 | PVC, PMMA | 2.06–5.98 µm | 95% | [47] |
| Magnetic steel collector wire | PE, PET, PTFE 3 | 63 µm–2 mm | 87–97% | [48] |
| Carbon/iron nanocomposite | PS | 147 µm | 100% | [49] |
| Fe3O4 | PE, PP, PVC, PS, PET | 20–800 µm | 100% | [50] |
| Magnetic seeded filtration, Fe3O4 | LDPE, PP, PVC, PS, PET | 100 µm | 80–100% | [51] |
| Fe3O4 polyoxometalate/n-octylamine | PS, PET, Polysulphone | 1–500 µm | 83–99% | [52] |
| Fe2O3-MnO2 micromotor | PE | 0.8 µm | 10% | [53] |
| Fe3O4 | PS, PMMA | 105–970 nm | - | [54] |
| Fe-Lauric Acid | HDPE | 273–1250 µm | 100% | [55] |
| Polydimethylsiloxane-Ni Foam | LDPE, PP, PS, PC, PET, PVC, PTFE | 70–1200 µm | 86.25–100% | [56] |
| Magnetic microsubmarines, sunflower pollon grains | PS | 40–100 µm | 70–75% | [57] |
| Fe@SiO2/MDOS | PE TWP | <100 µm 300–600 µm | - 59–77% | Present Work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budhiraja, V.; Mušič, B.; Krzan, A. Magnetic Extraction of Weathered Tire Wear Particles and Polyethylene Microplastics. Polymers 2022, 14, 5189. https://doi.org/10.3390/polym14235189
Budhiraja V, Mušič B, Krzan A. Magnetic Extraction of Weathered Tire Wear Particles and Polyethylene Microplastics. Polymers. 2022; 14(23):5189. https://doi.org/10.3390/polym14235189
Chicago/Turabian StyleBudhiraja, Vaibhav, Branka Mušič, and Andrej Krzan. 2022. "Magnetic Extraction of Weathered Tire Wear Particles and Polyethylene Microplastics" Polymers 14, no. 23: 5189. https://doi.org/10.3390/polym14235189
APA StyleBudhiraja, V., Mušič, B., & Krzan, A. (2022). Magnetic Extraction of Weathered Tire Wear Particles and Polyethylene Microplastics. Polymers, 14(23), 5189. https://doi.org/10.3390/polym14235189