Synthesis of Polyacids by Copolymerization of l-Lactide with MTC-COOH Using Zn[(acac)(L)H2O] Complex as an Initiator
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Functional Carbonate Monomers (Benzyl 5-Methyl-2-oxo-1,3-dioxane-5-carboxylate and 5-Methyl-2-oxo-1,3-dioxane-5-carboxylic Acid)
2.3. General Procedure for the Synthesis of the Zinc Initiator; Zn[(acac)(L)H2O] (Where: L-N-(2-Pyridin-4-ylethylidene) Phenylalaninate Ligand)
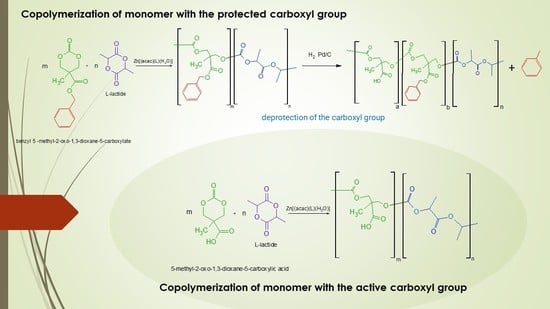
2.4. Copolymerization Procedures
2.5. Measurements
3. Results
3.1. Copolymerization of L-Lactide with 5-Methyl-2-oxo-1,3-dioxane-5-carboxylate (MTC-Bz)
3.2. Obtaining Polyacides, Poly (L-Lactide-co-MTC-COOH), via the Deprotection of Poly (L-Lactide-co-MTC-Bz)
3.3. Direct One Step Synthesis of Copolymer of MTC-COOH with LA
3.4. Comparison of the Copolymerization Course of Equimolar Amounts of L-Lactide with MTC-Bz and L-Lactide with MTC-COOH
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dubey, S.P.; Thakur, V.K.; Krishnaswamy, S.; Abhyankar, H.A.; Marchante, V.; Brighton, J.L. Progress in environmental-friendly polymer nanocomposite material from PLA: Synthesis, processing and applications. Vacuum 2017, 146, 655–663. [Google Scholar] [CrossRef] [Green Version]
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, Structures, and Advanced Applications of Nanocomposites from Biorenewable Resources. Chem. Rev. 2020, 17, 9304–9362. [Google Scholar] [CrossRef] [PubMed]
- Veld, P.J.A.; Dijkstra, P.J.; Feijen, J. Synthesis of biodegradable polyesteramides with pendant functional groups. Die Makromol. Chem. 1992, 11, 2713–2730. [Google Scholar] [CrossRef]
- Yokoe, M.; Aoi, K.; Okada, M. Biodegradable polymers based on renewable resources. IX. Synthesis and degradation behaviour of polycarbonates based on 1,4:3,6-dianhydrohexitols and tartaric acid derivatives with pendant functional groups. J. Polym. Sci. Part A 2005, 43, 3909–3919. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, J.; Liu, X.; Chang, J.; Cao, A. Synthesis and Characterization of Poly(butylene succinate-co-butylene malate): A New Biodegradable Copolyester Bearing Hydroxyl Pendant Groups. Biomacromolecules 2003, 4, 437–445. [Google Scholar]
- Yuen, A.Y.; Bossion, A.; Veloso, A.; Mecerreyes, D.; Hedrick, J.L.; Dove, A.P.; Sardon, H. Efficient polymerization and post-modification of N-substituted eight-membered cyclic carbonates containing allyl groups. Polym. Chem. 2018, 9, 2458–2467. [Google Scholar] [CrossRef]
- Becker, G.; Wurm, F.R. Functional biodegradable polymers via ring-opening polymerization of monomers without protective groups. Chem. Soc. Rev. 2018, 47, 7739–7782. [Google Scholar]
- Taguchi, K.; Yano, S.; Hiratani, K.; Minoura, N.; Okahata, Y. Ring-Opening Polymerization of 3(S)-[(Benzyloxycarbonyl) methyl]-l,4-dioxane-2,5-dione: A New Route to a Poly (a-hydroxy acid) with Pendant Carboxyl Groups. Macromolecules 1988, 21, 3338–3340. [Google Scholar]
- Moya-Lopez, C.; Bravo, I.; Castro-Osma, J.A.; Chapron, D.; Bourson, P.; Vagner, C.; Cochez, M.; Leoné, N.; Lara-Sánchez, A.; Alonso-Moreno, C.; et al. Synthesis of High Molecular Weight Stereo-Di-Block Copolymers Driven by a Co-Initiator Free Catalyst. Polymers 2022, 14, 232. [Google Scholar] [CrossRef]
- Jaworska, J.; Kawalec, M.; Pastusiak, M.; Reczynska, K.; Janeczek, H.; Lewicka, K.; Pamula, E.; Dobrzynski, P. Biodegradable Polycarbonates Containing Side Carboxyl Groups—Synthesis, Properties, and Degradation Study. J. Pol. Sci. Part A Polym. Chem. 2017, 55, 2756–2769. [Google Scholar]
- Al-Azemi, T.F.; Bisht, K.S. One-Step Synthesis of Polycarbonates Bearing Pendant Carboxyl Groups by Lipase-Catalyzed Ring-Opening Polymerization. J. Pol. Sci. Part A 2002, 40, 1267–1274. [Google Scholar] [CrossRef]
- Wang, H.F.; Jia, H.Z.; Zhu, J.Y.; Chu, Y.F.; Feng, J.; Zhang, X.Z.; Zhuo, R.X. One-Step Preparation and pH-Tunable Self-Aggregation of Amphoteric Aliphatic Polycarbonates Bearing Plenty of Amine and Carboxyl Groups. Macromol. Biosci. 2012, 12, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Dobrzynski, P.; Pastusiak, M.; Jaworska, J.; Kaczmarczyk, B.; Kwiecien, M.; Kawalec, M. Zirconium (IV) Acetylacetonate: Ring-Opening Initiator Mediating One-Step Synthesis of Biodegradable Polyacids. Adv. Polym. Technol. 2019, 2019, 3761430. [Google Scholar] [CrossRef] [Green Version]
- Barczyńska-Felusiak, R.; Pastusiak, M.; Rychter, P.; Kaczmarczyk, B.; Sobota, M.; Wanic, A.; Kaps, A.; Jaworska-Kik, M.; Orchel, A.; Dobrzyński, P. Synthesis of the Bacteriostatic Poly(l-Lactide) by Using Zinc (II)[(acac)(L)H2O] (L = Aminoacid-Based Chelate Ligands) as an Effective ROP Initiator. Int. J. Mol. Sci. 2021, 22, 6950. [Google Scholar] [CrossRef]
- Kalinin, K.T.; Sedush, N.G.; Dmitryakov, P.V.; Chvalun, S.N. Kinetics of D,L–Lactide Polymerization Initiated with Zirconium Acetylacetonate. ChemistryOpen 2020, 9, 1027–1032. [Google Scholar] [CrossRef]
- Dobrzynski, P. Initiation process of L-lactide polymerization carried out with zirconium (IV) acetylacetonate. J. Polym. Sci. A Polym. Chem. 2004, 42, 1886–1900. [Google Scholar] [CrossRef]
- Nederberg, F.; Trang, V.; Pratt, R.C.; Mason, A.F.; Frank, C.W.; Waymouth, R.M.; Hedrick, J.L. New ground for organic catalysis: A ring-opening polymerization approach to hydrogels. Biomacromolecules 2007, 8, 3294–3297. [Google Scholar] [CrossRef]
- Pratt, R.C.; Nederberg, F.; Waymouth, R.M.; Hedrick, J.L. Tagging alcohols with cyclic carbonate: A versatile equivalent of (meth)acrylate for ring-opening polymerization. Chem. Commun. 2008, 1, 114–116. [Google Scholar] [CrossRef]
- Hu, X.; Chen, X.; Liu, S.; Shi, Q.; Jing, X. Novel Aliphatic Poly(ester-carbonate) with Pendant Al-lyl Ester Groups and its Folic Acid Functionalization. J. Pol. Sci. Part A Pol. Chem. 2008, 46, 1852–1861. [Google Scholar] [CrossRef]
- Dobrzynski, P.; Kasperczyk, J. Synthesis of biodegradable copolymers with low-toxicity zirconium compounds. V. Multiblock and random copolymers of L-lactide with trimethylene carbonate obtained in copolymerizations initiated with zirconium (IV) acetylacetonate. J. Polym. Sci. A Polym. Chem. 2006, 44, 3184–3201. [Google Scholar] [CrossRef]
- Pastusiak, M.; Jaworska, J.; Kawalec, M.; Kasperczyk, J.; Dobrzynski, P. Obtaining aliphatic branched polycarbonates via simple copolymerization of trimethylene carbonate with cyclic carbonate containing pendant ester groups. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 808–819. [Google Scholar] [CrossRef]














| No. | (LA/MTC-Bz)0 (% mol.) | Time (h) | Conv. (%) | (LA: MTC-Bz)N (% mol.) | LLL | LM | Mw (kDa) | Đ | Tg (°C) | ΔHm (J/g) | Tm (°C) | R |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 100:0 | 72 | 97 | 100:0 | -- | -- | 88 | 2.1 | 41.4 | 9.7 | 100.3 | -- |
| 2 | 70:30 | 96 | 96 | 70:30 | 6.1 | 2.7 | 34 | 2.9 | 32.5 | 3.7 | 81.8 | 0.4 |
| 3 | 50:50 | 96 | 97 | 49:51 | 3.1 | 3.1 | 43 | 5.9 | 26.9 | 2.4 | 81.7 | 0.6 |
| 4 | 30:70 | 72 | 95 | 31:69 | 1.5 | 3.4 | 477 * | 76.0 * | 22.9 | 2.5 | 80.8 | 0.7 |
| 5 | 0:100 | 48 | ~65 | 0:100 | -- | -- | n/d * | n/d * | 1.3 | 5.1 | 77.6 | -- |
| No. | LA/MTCCOOH/MTC-Bz (% mol.) | LLL | LM | Mw (kDa) | Đ | Tg (°C) | ΔHm (J/g) | Tm (°C) | R |
|---|---|---|---|---|---|---|---|---|---|
| 2H | 69:23:8 | 5.8 | 2.8 | 24 | 2.2 | 32.7 | --- | --- | 0.4 |
| 3H | 50:43:7 | 3.1 | 3.1 | 32 | 2.9 | 33.3 | 29.2 | 113 | 0.6 |
| 4H | 30:30:40 | 1.4 | 3.3 | 63 | 4.4 | 30.1 | 13.5 | 111 | 0.7 |
| No. | (La/MTC-COOH)0 (% mol.) | (La/MTC-COOH)n (% mol.) | Conv. (%) | LLL | LM | Mw (kDa) | Đ | Tg (°C) | ΔHm (J/g) | Tm (°C) | R |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 70:30 | 62:38 | 95 | 3.1 | 1.9 | 32 | 2.1 | 41.4 | -- | -- | 0.7 |
| 2 | 50:50 | 48:52 | 97 | 2.0 | 2.1 | 21 | 2.5 | 45.8 | 8.7 | 114 | 0.7 |
| 3 | 30:70 | 31:69 | 98 | 1.5 | 3.3 | 49 | 3.8 | 49.6 | 16.7 | 101 | 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaworska, J.; Sobota, M.; Pastusiak, M.; Kawalec, M.; Janeczek, H.; Rychter, P.; Lewicka, K.; Dobrzyński, P. Synthesis of Polyacids by Copolymerization of l-Lactide with MTC-COOH Using Zn[(acac)(L)H2O] Complex as an Initiator. Polymers 2022, 14, 503. https://doi.org/10.3390/polym14030503
Jaworska J, Sobota M, Pastusiak M, Kawalec M, Janeczek H, Rychter P, Lewicka K, Dobrzyński P. Synthesis of Polyacids by Copolymerization of l-Lactide with MTC-COOH Using Zn[(acac)(L)H2O] Complex as an Initiator. Polymers. 2022; 14(3):503. https://doi.org/10.3390/polym14030503
Chicago/Turabian StyleJaworska, Joanna, Michał Sobota, Małgorzata Pastusiak, Michał Kawalec, Henryk Janeczek, Piotr Rychter, Kamila Lewicka, and Piotr Dobrzyński. 2022. "Synthesis of Polyacids by Copolymerization of l-Lactide with MTC-COOH Using Zn[(acac)(L)H2O] Complex as an Initiator" Polymers 14, no. 3: 503. https://doi.org/10.3390/polym14030503
APA StyleJaworska, J., Sobota, M., Pastusiak, M., Kawalec, M., Janeczek, H., Rychter, P., Lewicka, K., & Dobrzyński, P. (2022). Synthesis of Polyacids by Copolymerization of l-Lactide with MTC-COOH Using Zn[(acac)(L)H2O] Complex as an Initiator. Polymers, 14(3), 503. https://doi.org/10.3390/polym14030503