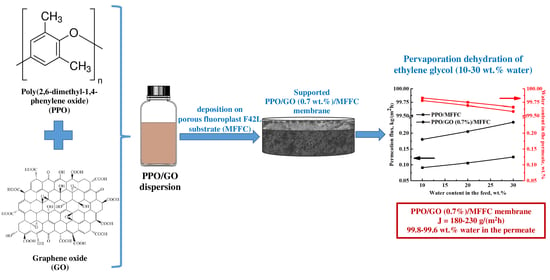
Novel Mixed Matrix Membranes Based on Polyphenylene Oxide Modified with Graphene Oxide for Enhanced Pervaporation Dehydration of Ethylene Glycol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Pervaporation
2.4. Fourier-Transform Infrared Spectroscopy (FTIR)
2.5. Nuclear Magnetic Resonance (NMR)
2.6. Scanning Electron Microscopy (SEM)
2.7. Atomic Force Microscopy (AFM)
2.8. Thermogravimetric Analysis (TGA)
2.9. Contact Angle Measurements
2.10. Swelling Experiments
3. Results
3.1. Transport Properties of the PPO-Based Membranes
3.2. The Characterization of PPO-Based Membranes
3.2.1. The Study of the Dense Membranes
3.2.2. The Study of the Supported Membranes
3.3. Membrane Performance Comparison in Pervaporation Separation of EG/Water Mixture
| Membrane | Temperature, °C | Permeation Flux, g/(m2·h) | Separation Factor (β) | Reference |
|---|---|---|---|---|
| Dense membranes | ||||
| PPO/GO (0.7%) | 22 | 78 | 4491 | This study |
| PIM-1 (polymer of intrinsic microporosity) | 30 | 51 | 39 | [7] |
| PPO/heteroarm stars with C60 center (5%) | 50 | 21 | 11,240 | [23] |
| Polybenzimidazole/polyetherimide dual-layer hollow fiber membrane | 60 | 115 | 1763 | [13] |
| Supported membranes | ||||
| PPO/GO (0.7%)/MFFC | 22 | 180 | 4082 | This study |
| Polyethylenimine-poly(acrylic acid) (PEI/PAA) complex/PA | 22 | 12 | 415 | [12] |
| PVA/buckypaper | 30 | 26 | 802 | [4] |
| 1-butyl-3-methylimidazolium tetrafluoroborate-PVA (70/30)/buckypaper | 30 | 102 | 1014 | |
| Chitosan/PS | 35 | 300 | 104 | [10] |
| PVA/PS | 60 | 360 | 987 | [60] |
| Polyelectrolyte complex/GO (3 wt.%)/PS | 60 | 961 | 1191 | [11] |
| GFT1001 (PVA/PAN) | 75 | 244 | 1116 | [61] |
| GFT1000 (PVA/PAN) | 75 | 56 | 141 | |
| GFT1510 (PVA/PAN) | 75 | 1700 | 591 | |
| DEG167 (PVA/PAN) | 75 | 500 | 991 | |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Guo, R.; Hu, C.; Li, B.; Jiang, Z. Pervaporation separation of ethylene glycol/water mixtures through surface crosslinked PVA membranes: Coupling effect and separation performance analysis. J. Memb. Sci. 2007, 289, 191–198. [Google Scholar] [CrossRef]
- Huang, R.Y.M.; Shao, P.; Feng, X.; Anderson, W.A. Separation of Ethylene Glycol−Water Mixtures Using Sulfonated Poly(ether ether ketone) Pervaporation Membranes: Membrane Relaxation and Separation Performance Analysis. Ind. Eng. Chem. Res. 2002, 41, 2957–2965. [Google Scholar] [CrossRef]
- Halakoo, E.; Feng, X. Self-assembled membranes from polyethylenimine and graphene oxide for pervaporation dehydration of ethylene glycol. J. Memb. Sci. 2020, 616, 118583. [Google Scholar] [CrossRef]
- Ong, Y.T.; Tan, S.H. Synthesis of the novel symmetric buckypaper supported ionic liquid membrane for the dehydration of ethylene glycol by pervaporation. Sep. Purif. Technol. 2015, 143, 135–145. [Google Scholar] [CrossRef]
- Wu, J.-K.; Yin, M.-J.; Han, W.; Wang, N.; An, Q.-F. Development of high-performance polyelectrolyte-complex-nanoparticle-based pervaporation membranes via convenient tailoring of charged groups. J. Mater. Sci. 2020, 55, 12607–12620. [Google Scholar] [CrossRef]
- Penkova, A.V.; Acquah, S.F.; Piotrovskiy, L.B.; Markelov, D.A.; Semisalova, A.S.; Kroto, H.W. Fullerene derivatives as nano-additives in polymer composites. Russ. Chem. Rev. 2017, 86, 530–566. [Google Scholar] [CrossRef]
- Wu, X.M.; Guo, H.; Soyekwo, F.; Zhang, Q.G.; Lin, C.X.; Liu, Q.L.; Zhu, A.M. Pervaporation Purification of Ethylene Glycol Using the Highly Permeable PIM-1 Membrane. J. Chem. Eng. Data 2016, 61, 579–586. [Google Scholar] [CrossRef]
- Shahverdi, M.; Baheri, B.; Rezakazemi, M.; Motaee, E.; Mohammadi, T. Pervaporation study of ethylene glycol dehydration through synthesized (PVA-4A)/polypropylene mixed matrix composite membranes. Polym. Eng. Sci. 2013, 53, 1487–1493. [Google Scholar] [CrossRef]
- Hu, S.Y.; Zhang, Y.; Lawless, D.; Feng, X. Composite membranes comprising of polyvinylamine-poly(vinyl alcohol) incorporated with carbon nanotubes for dehydration of ethylene glycol by pervaporation. J. Memb. Sci. 2012, 417–418, 34–44. [Google Scholar] [CrossRef]
- Feng, X. Pervaporation with chitosan membranes. I. Separation of water from ethylene glycol by a chitosan/polysulfone composite membrane. J. Memb. Sci. 1996, 116, 67–76. [Google Scholar] [CrossRef]
- Wu, J.-K.; Ye, C.-C.; Zhang, W.-H.; Wang, N.-X.; Lee, K.-R.; An, Q.-F. Construction of well-arranged graphene oxide/polyelectrolyte complex nanoparticles membranes for pervaporation ethylene glycol dehydration. J. Memb. Sci. 2019, 577, 104–112. [Google Scholar] [CrossRef]
- Zhang, Y.; Rhim, J.W.; Feng, X. Improving the stability of layer-by-layer self-assembled membranes for dehydration of alcohol and diol. J. Memb. Sci. 2013, 444, 22–31. [Google Scholar] [CrossRef]
- Wang, Y.; Chung, T.S.; Neo, B.W.; Gruender, M. Processing and engineering of pervaporation dehydration of ethylene glycol via dual-layer polybenzimidazole (PBI)/polyetherimide (PEI) membranes. J. Memb. Sci. 2011, 378, 339–350. [Google Scholar] [CrossRef]
- Villaluenga, J.P.G.; Godino, P.; Khayet, M.; Seoane, B.; Mengual, J.I. Pervaporation of Alcohols and Methyl t ert -Butyl Ether through a Dense Poly(2,6-dimethyl-1,4-phenylene oxide) Membrane. Ind. Eng. Chem. Res. 2004, 43, 2548–2555. [Google Scholar] [CrossRef]
- Polotskaya, G.; Biryulin, Y.; Pientka, Z.; Brozova, L.; Bleha, M. Transport Properties of Fullerene–Polyphenylene Oxide Homogeneous Membranes. Fuller. Nanotub. Carbon Nanostruct. 2005, 12, 365–369. [Google Scholar] [CrossRef]
- Tyan, N.S.; Polotskaya, G.A.; Meleshko, T.K.; Yakimansky, A.V.; Pientka, Z. Influence of the Molecular Polyimide Brush on the Gas Separation Properties of Polyphenylene Oxide. Russ. J. Appl. Chem. 2019, 92, 360–366. [Google Scholar] [CrossRef]
- Pulyalina, A.; Rostovtseva, V.; Polotskaya, G.; Vinogradova, L.; Zoolshoev, Z.; Simonova, M.; Hairullin, A.; Toikka, A.; Pientka, Z. Hybrid macromolecular stars incorporated poly(phenylene oxide) membranes: Organization, physical, and gas separation properties. Polymer 2019, 172, 355–364. [Google Scholar] [CrossRef]
- Jung, B.; Yoon, J.K.; Kim, B.; Rhee, H.-W. Effect of molecular weight of polymeric additives on formation, permeation properties and hypochlorite treatment of asymmetric polyacrylonitrile membranes. J. Memb. Sci. 2004, 243, 45–57. [Google Scholar] [CrossRef]
- Polotskaya, G.A.; Penkova, A.V.; Toikka, A.M. Fullerene-containing polyphenylene oxide membranes for pervaporation. Desalination 2006, 200, 400–402. [Google Scholar] [CrossRef]
- Penkova, A.; Polotskaya, G.; Toikka, A. Pervaporation composite membranes for ethyl acetate production. Chem. Eng. Process. Process Intensif. 2015, 87, 81–87. [Google Scholar] [CrossRef]
- Polotskaya, G.; Pulyalina, A.; Lebedev, V.; Török, G.; Rudakova, D.; Vinogradova, L. Novel view at hybrid membranes containing star macromolecules using neutron scattering and pervaporation dehydration of acetic acid. Mater. Des. 2020, 186, 108352. [Google Scholar] [CrossRef]
- Moulik, S.; Kumar, K.P.; Bohra, S.; Sridhar, S. Pervaporation performance of PPO membranes in dehydration of highly hazardous mmh and udmh liquid propellants. J. Hazard. Mater. 2015, 288, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Rostovtseva, V.; Pulyalina, A.; Rudakova, D.; Vinogradova, L.; Polotskaya, G. Strongly Selective Polymer Membranes Modified with Heteroarm Stars for the Ethylene Glycol Dehydration by Pervaporation. Membranes 2020, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Polotskaya, G.A.; Krasnopeeva, E.L.; Kalyuzhnaya, L.M.; Saprykina, N.N.; Vinogradova, L.V. Mixed matrix membranes with hybrid star-shaped macromolecules for mono- and dihydric alcohols pervaporation. Sep. Purif. Technol. 2015, 143, 192–200. [Google Scholar] [CrossRef]
- Polotskaya, G.A.; Lebedev, V.T.; Pulyalina, A.Y.; Vinogradova, L.V. Structure and transport properties of pervaporation membranes based on polyphenylene oxide and heteroarm star polymers. Pet. Chem. 2016, 56, 920–930. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Ashtiani, S.; Khoshnamvand, M.; Shaliutina-Kolešová, A.; Bouša, D.; Sofer, Z.; Friess, K. Co0·5Ni0·5FeCrO4 spinel nanoparticles decorated with UiO-66-based metal-organic frameworks grafted onto GO and O-SWCNT for gas adsorption and water purification. Chemosphere 2020, 255, 126966. [Google Scholar] [CrossRef]
- Mahmoud, K.A.; Mansoor, B.; Mansour, A.; Khraisheh, M. Functional graphene nanosheets: The next generation membranes for water desalination. Desalination 2015, 356, 208–225. [Google Scholar] [CrossRef]
- Chong, J.Y.; Wang, B.; Mattevi, C.; Li, K. Dynamic microstructure of graphene oxide membranes and the permeation flux. J. Memb. Sci. 2018, 549, 385–392. [Google Scholar] [CrossRef]
- Sun, P.; Wang, K.; Zhu, H. Recent Developments in Graphene-Based Membranes: Structure, Mass-Transport Mechanism and Potential Applications. Adv. Mater. 2016, 28, 2287–2310. [Google Scholar] [CrossRef]
- Ma, J.; Ping, D.; Dong, X. Recent Developments of Graphene Oxide-Based Membranes: A Review. Membranes 2017, 7, 52. [Google Scholar] [CrossRef]
- Guan, K.; Liu, G.; Matsuyama, H.; Jin, W. Graphene-based membranes for pervaporation processes. Chin. J. Chem. Eng. 2020, 28, 1755–1766. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Liamin, V.; Lahderanta, E.; Ermakov, S.; Penkova, A. Mixed matrix membranes based on sodium alginate modified by fullerene derivatives with L-amino acids for pervaporation isopropanol dehydration. J. Mater. Sci. 2021. [Google Scholar] [CrossRef]
- Kuzminova, A.; Dmitrenko, M.; Zolotarev, A.; Korniak, A.; Poloneeva, D.; Selyutin, A.; Emeline, A.; Yushkin, A.; Foster, A.; Budd, P.; et al. Novel Mixed Matrix Membranes Based on Polymer of Intrinsic Microporosity PIM-1 Modified with Metal-Organic Frameworks for Removal of Heavy Metal Ions and Food Dyes by Nanofiltration. Membranes 2021, 12, 14. [Google Scholar] [CrossRef]
- Mural, P.K.S.; Madras, G.; Bose, S. Polymeric membranes derived from immiscible blends with hierarchical porous structures, tailored bio-interfaces and enhanced flux: Potential and key challenges. Nano-Struct. Nano-Objects 2018, 14, 149–165. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Liamin, V.; Kuzminova, A.; Mazur, A.; Lahderanta, E.; Ermakov, S.; Penkova, A. Novel Mixed Matrix Sodium Alginate–Fullerenol Membranes: Development, Characterization, and Study in Pervaporation Dehydration of Isopropanol. Polymers 2020, 12, 864. [Google Scholar] [CrossRef] [PubMed]
- Penkova, A.V.; Dmitrenko, M.E.; Ermakov, S.S.; Toikka, A.M.; Roizard, D. Novel green PVA-fullerenol mixed matrix supported membranes for separating water-THF mixtures by pervaporation. Environ. Sci. Pollut. Res. 2017, 25, 20354–20362. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.W. Membrane Technology and Applications; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Baker, R.W.; Wijmans, J.G.; Huang, Y. Permeability, permeance and selectivity: A preferred way of reporting pervaporation performance data. J. Memb. Sci. 2010, 348, 346–352. [Google Scholar] [CrossRef]
- Polotskaya, G.A.; Penkova, A.V.; Toikka, A.M.; Pientka, Z.; Brozova, L.; Bleha, M. Transport of small molecules through polyphenylene oxide membranes modified by fullerene. Sep. Sci. Technol. 2007, 42, 333–347. [Google Scholar] [CrossRef]
- Dmitrenko, M.E.; Penkova, A.V.; Atta, R.R.; Zolotarev, A.A.; Plisko, T.V.; Mazur, A.S.; Solovyev, N.D.; Ermakov, S.S. The development and study of novel membrane materials based on polyphenylene isophthalamide—Pluronic F127 composite. Mater. Des. 2019, 165, 107596. [Google Scholar] [CrossRef]
- Khayet, M.; Villaluenga, J.P.G.; Valentin, J.L.; López-Manchado, M.A.; Mengual, J.I.; Seoane, B. Filled poly(2,6-dimethyl-1,4-phenylene oxide) dense membranes by silica and silane modified silica nanoparticles: Characterization and application in pervaporation. Polymer 2005, 46, 9881–9891. [Google Scholar] [CrossRef]
- Faykov, I.I.; Rostovtseva, V.A.; Tyan, N.S.; Pulyalina, A.Y. A Deep Eutectic Solvent as a Modifier of Polyphenylene Oxide Membranes for Acetic Acid Dehydration. Membr. Membr. Technol. 2021, 3, 124–130. [Google Scholar] [CrossRef]
- Samanta, H.S.; Ray, S.K.; Das, P.; Singha, N.R. Separation of acid-water mixtures by pervaporation using nanoparticle filled mixed matrix copolymer membranes. J. Chem. Technol. Biotechnol. 2012, 87, 608–622. [Google Scholar] [CrossRef]
- Jehle, W.; Staneff, T.; Wagner, B.; Steinwandel, J. Separation of glycol and water from coolant liquids by evaporation, reverse osmosis and pervaporation. J. Memb. Sci. 1995, 102, 9–19. [Google Scholar] [CrossRef]
- Rostovtseva, V.; Pulyalina, A.; Dubovenko, R.; Faykov, I.; Subbotina, K.; Saprykina, N.; Novikov, A.; Vinogradova, L.; Polotskaya, G. Enhancing Pervaporation Membrane Selectivity by Incorporating Star Macromolecules Modified with Ionic Liquid for Intensification of Lactic Acid Dehydration. Polymers 2021, 13, 1811. [Google Scholar] [CrossRef] [PubMed]
- Abdelhalim, A.O.E.; Sharoyko, V.V.; Meshcheriakov, A.A.; Luttsev, M.D.; Potanin, A.A.; Iamalova, N.R.; Zakharov, E.E.; Ageev, S.V.; Petrov, A.V.; Vasina, L.V.; et al. Synthesis, characterisation and biocompatibility of graphene–L-methionine nanomaterial. J. Mol. Liq. 2020, 314, 113605. [Google Scholar] [CrossRef]
- Abdelhalim, A.O.E.; Sharoyko, V.V.; Meshcheriakov, A.A.; Martynova, S.D.; Ageev, S.V.; Iurev, G.O.; Al Mulla, H.; Petrov, A.V.; Solovtsova, I.L.; Vasina, L.V.; et al. Reduction and functionalization of graphene oxide with L-cysteine: Synthesis, characterization and biocompatibility. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102284. [Google Scholar] [CrossRef]
- Nagendra, B.; Cozzolino, A.; Daniel, C.; Rizzo, P.; Guerra, G.; Auriemma, F.; De Rosa, C.; D’Alterio, M.C.; Tarallo, O.; Nuzzo, A. Two Nanoporous Crystalline Forms of Poly(2,6-dimethyl-1,4-phenylene)oxide and Related Co-Crystalline Forms. Macromolecules 2019, 52, 9646–9656. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Alhoshan, M.S.; Ali, F.A.A.; Mishra, U.; Hamid, A.A. Thin-Film Nanocomposite Membrane Incorporated with Porous Zn-Based Metal–Organic Frameworks: Toward Enhancement of Desalination Performance and Chlorine Resistance. ACS Appl. Mater. Interfaces 2021, 13, 28818–28831. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.K.; Alam, J.; Ansari, M.A.; Alhoshan, M.; Alam, M.; Kaushik, A. Selective ion removal and antibacterial activity of silver-doped multi-walled carbon nanotube / polyphenylsulfone nanocomposite membranes. Mater. Chem. Phys. 2019, 233, 102–112. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Liamin, V.; Kuzminova, A.; Lahderanta, E.; Solovyev, N.; Penkova, A. Modification Approaches to Enhance Dehydration Properties of Sodium Alginate-Based Pervaporation Membranes. Membranes 2021, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Polotskaya, G.A.; Penkova, A.V.; Pientka, Z.; Toikka, A.M. Polymer membranes modified by fullerene C60 for pervaporation of organic mixtures. Desalin. Water Treat. 2010, 14, 83–88. [Google Scholar] [CrossRef]
- Manshad, S.; Sazegar, M.R.; Mohd Nawawi, M.G.; bin Hassan, H. Fabrication of nanohybrid polyetherimide/graphene oxide membranes: Biofuel dehydration by pervaporation process. RSC Adv. 2016, 6, 103888–103894. [Google Scholar] [CrossRef]
- Abdul Wahab, M.S.; Rahman, S.A.; Samah, R.A. Hydrophilic enhancement of Polysulfone membrane via Graphene Oxide embedded thin film nanocomposite for Isopropanol dehydration. Vacuum 2020, 180, 109569. [Google Scholar] [CrossRef]
- Huang, A.; Feng, B. Synthesis of novel graphene oxide-polyimide hollow fiber membranes for seawater desalination. J. Memb. Sci. 2018, 548, 59–65. [Google Scholar] [CrossRef]
- Mokhtarzadeh, S.; Agbolaghi, S.; Mansourpanah, Y. Novel Branched Polyamide/Poly(acrylonitrile)/Graphene Oxide Membranes for Separation of Chlorinated Volatile Organic Compounds from Water via Pervaporation. Macromol. Res. 2020, 28, 797–804. [Google Scholar] [CrossRef]
- Farivar, F.; Lay Yap, P.; Karunagaran, R.U.; Losic, D. Thermogravimetric Analysis (TGA) of Graphene Materials: Effect of Particle Size of Graphene, Graphene Oxide and Graphite on Thermal Parameters. C 2021, 7, 41. [Google Scholar] [CrossRef]
- Favre, E.; Bounaceur, R.; Roizard, D. Biogas, membranes and carbon dioxide capture. J. Memb. Sci. 2009, 328, 11–14. [Google Scholar] [CrossRef]
- Reid, R.C.; Prausnitz, J.M.; Polimg, B.E. The Properties of Gases & Liquids, 4th ed.; McGraw-Hill Book Company: New York, NY, USA, 1987. [Google Scholar]













| Membrane | Surface Parameters | |
|---|---|---|
| Ra, nm | Rq, nm | |
| PPO | 3.8 ± 0.2 | 6.6 ± 0.2 |
| PPO/GO (0.1%) | 3.3 ± 0.5 | 5.9 ± 0.5 |
| PPO/GO (0.3%) | 3.5 ± 0.5 | 5.8 ± 0.5 |
| PPO/GO (0.5%) | 5.2 ± 0.5 | 7.2 ± 0.5 |
| PPO/GO (0.7%) | 5.5 ± 0.5 | 7.5 ± 0.5 |
| PPO/GO (0.9%) | 5.7 ± 0.5 | 7.9 ± 0.5 |
| Membrane | Contact Angle of Water, ° | Swelling Degree, % | |
|---|---|---|---|
| Water | Ethylene Glycol | ||
| PPO | 89 ± 2 | 1.4 | 3.4 |
| PPO/GO (0.1%) | 89 ± 2 | 1.8 | 3.5 |
| PPO/GO (0.3%) | 87 ± 2 | 2.7 | 3.5 |
| PPO/GO (0.5%) | 86 ± 2 | 3.5 | 4.4 |
| PPO/GO (0.7%) | 85 ± 2 | 6.4 | 7.1 |
| PPO/GO (0.9%) | 83 ± 2 | 6.6 | 7.7 |
| Membrane | Surface Parameters | |
|---|---|---|
| Ra, nm | Rq, nm | |
| PPO/MFFC | 33.5 | 68.1 |
| PPO/GO (0.7%)/MFFC | 54.6 | 82.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dmitrenko, M.; Chepeleva, A.; Liamin, V.; Mazur, A.; Semenov, K.; Solovyev, N.; Penkova, A. Novel Mixed Matrix Membranes Based on Polyphenylene Oxide Modified with Graphene Oxide for Enhanced Pervaporation Dehydration of Ethylene Glycol. Polymers 2022, 14, 691. https://doi.org/10.3390/polym14040691
Dmitrenko M, Chepeleva A, Liamin V, Mazur A, Semenov K, Solovyev N, Penkova A. Novel Mixed Matrix Membranes Based on Polyphenylene Oxide Modified with Graphene Oxide for Enhanced Pervaporation Dehydration of Ethylene Glycol. Polymers. 2022; 14(4):691. https://doi.org/10.3390/polym14040691
Chicago/Turabian StyleDmitrenko, Mariia, Anastasia Chepeleva, Vladislav Liamin, Anton Mazur, Konstantin Semenov, Nikolay Solovyev, and Anastasia Penkova. 2022. "Novel Mixed Matrix Membranes Based on Polyphenylene Oxide Modified with Graphene Oxide for Enhanced Pervaporation Dehydration of Ethylene Glycol" Polymers 14, no. 4: 691. https://doi.org/10.3390/polym14040691
APA StyleDmitrenko, M., Chepeleva, A., Liamin, V., Mazur, A., Semenov, K., Solovyev, N., & Penkova, A. (2022). Novel Mixed Matrix Membranes Based on Polyphenylene Oxide Modified with Graphene Oxide for Enhanced Pervaporation Dehydration of Ethylene Glycol. Polymers, 14(4), 691. https://doi.org/10.3390/polym14040691