Comparison of Protein Content, Availability, and Different Properties of Plant Protein Sources with Their Application in Packaging
Abstract
:1. Introduction
1.1. Opportunities of Protein Sources from Underutilized Biomass for Non-Food Application
1.2. Opportunities of Protein Sources for Edible Bioplastic
1.3. Challenges in the Utilization of Protein Sources
2. Method for the Formation of Protein-Based Packaging
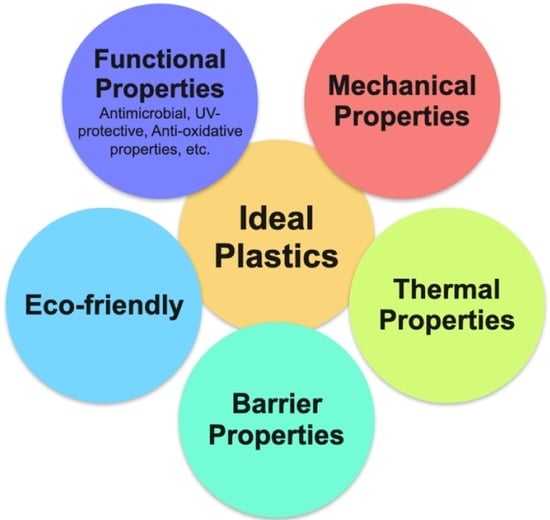
3. Comparison of Different Properties of Bioplastics
4. Synthesis Methods and Properties of Plant Protein-Based Bioplastics
4.1. Comparison of Different Formation Methods in Molding and Extrusion
4.2. Comparison of Film and Raw Material Treatment Methods
4.3. Comparison of Mechanical Properties
4.4. Comparison of Different Barrier Properties
4.5. Film Solubility and Water Uptake Capacity
4.6. Thermal Properties
4.7. Other Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, M.; Chen, C.; Song, B.; Shen, M.; Cao, W.; Yang, H.; Zeng, G.; Gong, J. A review of biodegradable plastics to biodegradable microplastics: Another ecological threat to soil environments? J. Clean. Prod. 2021, 312, 127816. [Google Scholar] [CrossRef]
- Iwata, T. Biodegradable and bio-based polymers: Future prospects of eco-friendly plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef] [PubMed]
- Shafqat, A.; Tahir, A.; Mahmood, A.; Tabinda, A.B.; Yasar, A.; Pugazhendhi, A. A review on environmental significance carbon foot prints of starch based bio-plastic: A substitute of conventional plastics. Biocatal. Agric. Biotechnol. 2020, 27, 101540. [Google Scholar] [CrossRef]
- Brodin, M.; Vallejos, M.; Opedal, M.T.; Area, M.C.; Chinga-Carrasco, G. Lignocellulosics as sustainable resources for production of bioplastics–A review. J. Clean. Prod. 2017, 162, 646–664. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Rempel, C. Processing and characteristics of canola protein-based biodegradable packaging: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 475–485. [Google Scholar] [CrossRef]
- Coppola, G.; Gaudio, M.T.; Lopresto, C.G.; Calabro, V.; Curcio, S.; Chakraborty, S. Bioplastic from Renewable Biomass: A Facile Solution for a Greener Environment. Earth Syst. Environ. 2021, 5, 231–251. [Google Scholar] [CrossRef]
- Nelson, D.L.; Lehninger, A.L.; Cox, M.M. Lehninger Principles of Biochemistry; Macmillan: Basingstoke, UK, 2008; ISBN 071677108X. [Google Scholar]
- Brändén, C.-I.; Tooze, J. Introduction to Protein Structure; Taylor & Francis: New York, NY, USA, 1999; ISBN 0815323050. [Google Scholar]
- Li-Chan, E.C.Y.; Lacroix, I.M.E. Properties of Proteins in Food Systems: An Introduction, 2nd ed.; Elsevier Ltd.: Vancouver, BC, Canada, 2018; ISBN 9780081007297. [Google Scholar]
- Carus, M.; Dammer, L. Food or non-food: Which agricultural feedstocks are best for industrial uses? Ind. Biotechnol. 2013, 9, 171–176. [Google Scholar] [CrossRef]
- De Corato, U.; De Bari, I.; Viola, E.; Pugliese, M. Assessing the main opportunities of integrated biorefining from agro-bioenergy co/by-products and agroindustrial residues into high-value added products associated to some emerging markets: A review. Renew. Sustain. Energy Rev. 2018, 88, 326–346. [Google Scholar] [CrossRef]
- Obruca, S.; Benesova, P.; Marsalek, L.; Marova, I. Use of lignocellulosic materials for PHA production. Chem. Biochem. Eng. Q. 2015, 29, 135–144. [Google Scholar] [CrossRef]
- Israni, N.; Shivakumar, S. Polyhydroxyalkanoate (PHA) biosynthesis from directly valorized ragi husk and sesame oil cake by Bacillus megaterium strain Ti3: Statistical optimization and characterization. Int. J. Biol. Macromol. 2020, 148, 20–30. [Google Scholar] [CrossRef]
- Anex, R.P.; Ogletree, A.L. Life-Cycle Assessment of Energy-Based Impacts of a Biobased Process for Producing 1,3-Propanediol. In Feedstocks for the Future; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2006; Volume 921, pp. 17–222. ISBN 9780841239340. [Google Scholar]
- Belletti, N.; Gatti, M.; Bottari, B.; Neviani, E.; Tabanelli, G.; Gardini, F. The size of native milk fat globules affects physico-chemical and sensory properties. J. Food Prot. 2009, 72, 2162–2169. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.; Arqués, J.L.; Rodríguez, R.; Nuñez, M.; Medina, M.; Talarico, T.L.; Casas, I.A.; Chung, T.C.; Dobrogosz, W.J.; Axelsson, L.; et al. We are IntechOpen, the world’s leading publisher of Open Access books Built by scientists, for scientists TOP 1%. Intech 1989, 32, 137–144. [Google Scholar]
- Cui, L.; Fan, X.; Wang, P.; Wang, Q.; Fu, G. Casein and transglutaminase-mediated modification of wool surface. Eng. Life Sci. 2011, 11, 201–206. [Google Scholar] [CrossRef]
- Costa, C.; Azoia, N.G.; Coelho, L.; Freixo, R.; Batista, P.; Pintado, M. Proteins derived from the dairy losses and by-products as raw materials for non-food applications. Foods 2021, 10, 135. [Google Scholar] [CrossRef]
- Jones, A.; Mandal, A.; Sharma, S. Protein-based bioplastics and their antibacterial potential. J. Appl. Polym. Sci. 2015, 132, 41931. [Google Scholar] [CrossRef]
- Iles, A.; Martin, A.N. Expanding bioplastics production: Sustainable business innovation in the chemical industry. J. Clean. Prod. 2013, 45, 38–49. [Google Scholar] [CrossRef]
- Bhunia, K.; Sablani, S.S.; Tang, J.; Rasco, B. Migration of chemical compounds from packaging polymers during microwave, conventional heat treatment, and storage. Compr. Rev. Food Sci. Food Saf. 2013, 12, 523–545. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made—Supplementary Information. Sci. Adv. 2017, 3, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Queiroz, A.U.B.; Collares-Queiroz, F.P. Innovation and industrial trends in bioplastics. Polym. Rev. 2009, 49, 65–78. [Google Scholar] [CrossRef]
- Pauling, L.; Corey, R.B.; Branson, H.R. The structure of proteins: Two hydrogen-bonded helical configurations of the polypeptide chain. Proc. Natl. Acad. Sci. USA 1951, 37, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Wittaya, T. Protein-based edible films: Characteristics and improvement of properties. In Structure and Function of Food Engineering; InTech Open: London, UK, 2012; pp. 43–70. [Google Scholar]
- Calva-Estrada, S.J.; Jiménez-Fernández, M.; Lugo-Cervantes, E. Protein-based films: Advances in the development of biomaterials applicable to food packaging. Food Eng. Rev. 2019, 11, 78–92. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Cheng, Y.; Wang, C.; Liu, H.; Bian, H.; Pan, Y.; Sun, J.; Han, W. Application of protein-based films and coatings for food packaging: A review. Polymers 2019, 11, 2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurley, B.R.A.; Ouzts, A.; Fischer, J.; Gomes, T. Paper presented at iapri world conference 2012 Effects of Private and Public Label Packaging on Consumer Purchase Patterns. Packag. Technol. Sci. 2013, 29, 399–412. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Schmid, M.; Müller, K. Whey protein-based packaging films and coatings. In Whey Proteins; Elsevier: Berlin, Germany, 2019; pp. 407–437. [Google Scholar]
- Bourtoom, T. Edible protein films: Properties enhancement. Int. Food Res. J. 2009, 16, 1–9. [Google Scholar]
- Cuq, B.; Gontard, N.; Cuq, J.-L.; Guilbert, S. Selected Functional Properties of Fish Myofibrillar Protein-Based Films as Affected by Hydrophilic Plasticizers. J. Agric. Food Chem. 1997, 45, 622–626. [Google Scholar] [CrossRef]
- Kolster, P.; Vereijken, J.M.; de Graaf, L.A. Protein modification and technical applications. In Plant Proteins from European Crops; Springer: Berlin/Heidelberg, Germany, 1998; ISBN 9783662037201. [Google Scholar]
- Yildirim, M.; Hettiarachchy, N.S.; Kalapathy, U. Properties of biopolymers from cross-linking whey protein isolate and soybean 11S globulin. J. Food Sci. 1996, 61, 1129–1132. [Google Scholar] [CrossRef]
- Tzoumaki, M.V.; Biliaderis, C.G.; Vasilakakis, M. Impact of edible coatings and packaging on quality of white asparagus (Asparagus officinalis L.) during cold storage. Food Chem. 2009, 117, 55–63. [Google Scholar] [CrossRef]
- Ghidelli, C.; Mateos, M.; Rojas-Argudo, C.; Pérez-Gago, M.B. Extending the shelf life of fresh-cut eggplant with a soy protein-cysteine based edible coating and modified atmosphere packaging. Postharvest Biol. Technol. 2014, 95, 81–87. [Google Scholar] [CrossRef]
- Marelli, B.; Brenckle, M.A.; Kaplan, D.L.; Omenetto, F.G. Silk Fibroin as Edible Coating for Perishable Food Preservation. Sci. Rep. 2016, 6, 25263. [Google Scholar] [CrossRef] [Green Version]
- Katiyar, V.; Ghosh, T. Nanotechnology in Edible Food Packaging: Food Preservation Practices for a Sustainable Future; Springer Nature: Berlin, Germany, 2021; ISBN 978-981-33-6168-3. [Google Scholar]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Murrieta-Martínez, C.L.; Soto-Valdez, H.; Pacheco-Aguilar, R.; Torres-Arreola, W.; Rodríguez-Felix, F.; Márquez Ríos, E. Edible protein films: Sources and behavior. Packag. Technol. Sci. 2018, 31, 113–122. [Google Scholar] [CrossRef]
- Babaei-Ghazvini, A.; Acharya, B.; Korber, D.R. Antimicrobial Biodegradable Food Packaging Based on Chitosan and Metal/Metal-Oxide Bio-Nanocomposites: A Review. Polymers 2021, 13, 2790. [Google Scholar] [CrossRef]
- Park, H.J.; Chinnan, M.S.; Shewfelt, R.L. Edible Coating Effects on Storage Life and Quality of Tomatoes. J. Food Sci. 1994, 59, 568–570. [Google Scholar] [CrossRef]
- Tulamandi, S.; Rangarajan, V.; Rizvi, S.S.H.; Singhal, R.S.; Chattopadhyay, S.K.; Saha, N.C. A biodegradable and edible packaging film based on papaya puree, gelatin, and defatted soy protein. Food Packag. Shelf Life 2016, 10, 60–71. [Google Scholar] [CrossRef]
- Thiruchelvi, R.; Das, A.; Sikdar, E. Bioplastics as better alternative to petro plastic. Mater. Today Proc. 2020, 37, 1634–1639. [Google Scholar] [CrossRef]
- Zimmermann, L.; Dombrowski, A.; Völker, C.; Wagner, M. Are bioplastics and plant-based materials safer than conventional plastics? In vitro toxicity and chemical composition. Environ. Int. 2020, 145, 106066. [Google Scholar] [CrossRef]
- Hernandez-Izquierdo, V.M.; Krochta, J.M. Thermoplastic processing of proteins for film formation—A review. J. Food Sci. 2008, 73, 30–39. [Google Scholar] [CrossRef]
- Montes-de-Oca-Ávalos, J.M.; Altamura, D.; Herrera, M.L.; Huck-Iriart, C.; Scattarella, F.; Siliqi, D.; Giannini, C.; Candal, R.J. Physical and structural properties of whey protein concentrate—Corn oil—TiO2 nanocomposite films for edible food-packaging. Food Packag. Shelf Life 2020, 26, 100590. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Bouroudian, E.; Perez-Puyana, V.; Guerrero, A.; Romero, A. Evaluation of different strengthening methods in the mechanical and functional properties of soy protein-based bioplastics. J. Clean. Prod. 2020, 262. [Google Scholar] [CrossRef]
- Cheng, H.N.; Ford, C.V.; He, Z. Evaluation of polyblends of cottonseed protein and polycaprolactone plasticized by cottonseed oil. Int. J. Polym. Anal. Charact. 2019, 24, 389–398. [Google Scholar] [CrossRef]
- Babaei-Ghazvini, A.; Acharya, B.; Korber, D.R. Multilayer photonic films based on interlocked chiral-nematic cellulose nanocrystals in starch/chitosan. Carbohydr. Polym. 2022, 275, 118709. [Google Scholar] [CrossRef] [PubMed]
- Babaei-Ghazvini, A.; Cudmore, B.; Dunlop, M.J.; Acharya, B.; Bissessur, R.; Ahmed, M.; Whelan, W.M. Effect of magnetic field alignment of cellulose nanocrystals in starch nanocomposites: Physicochemical and mechanical properties. Carbohydr. Polym. 2020, 247, 116688. [Google Scholar] [CrossRef] [PubMed]
- Shahabi-Ghahfarrokhi, I.; Babaei-Ghazvini, A. Using photo-modification to compatibilize nano-ZnO in development of starch-kefiran-ZnO green nanocomposite as food packaging material. Int. J. Biol. Macromol. 2019, 124, 922–930. [Google Scholar] [CrossRef]
- Sautter, C. Preface. In Green Gene Technology: Research in an Area of Social Conflict; Fiechter, A., Sautter, C., Eds.; Advances in Biochemical Engineering/Biotechnology Series 107; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar] [CrossRef]
- Lammens, T.M.; Franssen, M.C.R.; Scott, E.L.; Sanders, J.P.M. Availability of protein-derived amino acids as feedstock for the production of bio-based chemicals. Biomass Bioenergy 2012, 44, 168–181. [Google Scholar] [CrossRef]
- Kamal, H.; Le, C.F.; Salter, A.M.; Ali, A. Extraction of protein from food waste: An overview of current status and opportunities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2455–2475. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Z. Aqueous enzymatic extraction technology of oil and protein hydrolysates from rapeseed. Nongye Gongcheng Xuebao/Trans. Chin. Soc. Agric. Eng. 2007, 23, 213–219. [Google Scholar] [CrossRef]
- Parker, S. Principles and Practice. IFLA J. 2006, 32, 179–180. [Google Scholar] [CrossRef]
- Johansson, T. Development of Bioplastics from Oil Plant By-Products. Master’s Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, October 2010. [Google Scholar]
- Young, R.; Pellett, P.L. Plant proteins in relation to human and amino acid nutrition1’ 2 whereas. Am. J. Clin. Nutr. 2018, 59, 1203–1212. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Felix, M.; Bengoechea, C.; Guerrero, A. Proteins from agri-food industrial biowastes or co-products and their applications as green materials. Foods 2021, 10, 981. [Google Scholar] [CrossRef]
- Ravindran, V.; Blair, R. Feed resources for poultry production in Asia and the Pacific. III. Animal protein sources. Worlds. Poult. Sci. J. 1993, 49, 219–235. [Google Scholar] [CrossRef]
- Thammahiwes, S.; Riyajan, S.A.; Kaewtatip, K. Effect of Shrimp Shell Waste on the Properties of Wheat Gluten Based-Bioplastics. J. Polym. Environ. 2018, 26, 1775–1781. [Google Scholar] [CrossRef]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012, 49, 173–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahabi-Ghahfarrokhi, I.; Almasi, H.; Babaei-Ghazvini, A. Chapter 3—Characteristics of biopolymers from natural resources. In Processing and Development of Polysaccharide-Based Biopolymers for Packaging Applications; Zhang, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 49–95. ISBN 978-0-12-818795-1. [Google Scholar]
- Babaei-Ghazvini, A.; Shahabi-Ghahfarrokhi, I.; Goudarzi, V. Preparation of UV-protective starch/kefiran/ZnO nanocomposite as a packaging film: Characterization. Food Packag. Shelf Life 2018, 16, 103–111. [Google Scholar] [CrossRef]
- Babaei-Ghazvini, A.; Acharya, B. Humidity-Responsive Photonic Films and Coatings Based on Tuned Cellulose Nanocrystals/Glycerol/Polyethylene Glycol. Polymers 2021, 13, 3695. [Google Scholar] [CrossRef] [PubMed]
- Rad, V.F.; Babaei-Ghazvini, A.; Jamali, R.; Shahabi-Ghahfarrokhi, I.; Moradi, A.-R. Digital holographic microscopy for real-time investigation of 3D microstructural dynamics of starch-kefiran based nanocomposite. Appl. Opt. 2021, 60, 4706–4715. [Google Scholar] [CrossRef]
- Goudarzi, V.; Shahabi-Ghahfarrokhi, I.; Babaei-Ghazvini, A. Preparation of ecofriendly UV-protective food packaging material by starch/TiO2 bio-nanocomposite: Characterization. Int. J. Biol. Macromol. 2017, 95, 306–313. [Google Scholar] [CrossRef]
- Shahabi-Ghahfarrokhi, I.; Goudarzi, V.; Babaei-Ghazvini, A. Production of starch based biopolymer by green photochemical reaction at different UV region as a food packaging material: Physicochemical characterization. Int. J. Biol. Macromol. 2019, 122, 201–209. [Google Scholar] [CrossRef]
- Shahabi-Ghahfarrokhi, I.; Babaei-Ghazvini, A. Production of Biodegradable Packaging Material Based on Starch-kefiran-ZnO: Physical and Mechanical Characterization. Iran. J. Biosyst. Eng. 2019, 49, 557–565. [Google Scholar]
- Dordevic, D.; Necasova, L.; Antonic, B.; Jancikova, S.; Tremlová, B. Plastic cutlery alternative: Case study with biodegradable spoons. Foods 2021, 10, 1612. [Google Scholar] [CrossRef]
- Perez, V.; Felix, M.; Romero, A.; Guerrero, A. Characterization of pea protein-based bioplastics processed by injection moulding. Food Bioprod. Process. 2016, 97, 100–108. [Google Scholar] [CrossRef]
- Yue, H.B.; Cui, Y.D.; Shuttleworth, P.S.; Clark, J.H. Preparation and characterisation of bioplastics made from cottonseed protein. Green Chem. 2012, 14, 2009–2016. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.H.; Karim, A.A.; Seow, C.C. Effects of Water-Glycerol and Water-Sorbitol Interactions on the Physical Properties of Konjac Glucomannan Films. J. Food Sci. 2006, 71, E62–E67. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, J.H. Mechanical and thermal characteristics of pea starch films plasticized with monosaccharides and polyols. J. Food Sci. 2006, 71. [Google Scholar] [CrossRef]
- Wihodo, M.; Moraru, C.I. Physical and chemical methods used to enhance the structure and mechanical properties of protein films: A review. J. Food Eng. 2013, 114, 292–302. [Google Scholar] [CrossRef]
- Lackner, M. Bioplastics. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Peelman, N.; Ragaert, P.; De Meulenaer, B.; Adons, D.; Peeters, R.; Cardon, L.; Van Impe, F.; Devlieghere, F. Application of bioplastics for food packaging. Trends Food Sci. Technol. 2013, 32, 128–141. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.S.; Han, J.H. Physical and mechanical properties of pea-protein-based edible films. J. Food Sci. 2001, 66, 319–322. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Peponi, L.; López, D.; López, J.; Kenny, J.M. 12—An Overview of Nanoparticles Role in the Improvement of Barrier Properties of Bioplastics for Food Packaging Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128043028. [Google Scholar]
- Carvajal-Piñero, J.M.; Ramos, M.; Jiménez-Rosado, M.; Perez-Puyana, V.; Romero, A. Development of Pea Protein Bioplastics by a Thermomoulding Process: Effect of the Mixing Stage. J. Polym. Environ. 2019, 27, 968–978. [Google Scholar] [CrossRef]
- Gamero, S.; Jiménez-Rosado, M.; Romero, A.; Bengoechea, C.; Guerrero, A. Reinforcement of Soy Protein-Based Bioplastics Through Addition of Lignocellulose and Injection Molding Processing Conditions. J. Polym. Environ. 2019. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Zarate-Ramírez, L.S.; Romero, A.; Bengoechea, C.; Partal, P.; Guerrero, A. Bioplastics based on wheat gluten processed by extrusion. J. Clean. Prod. 2019, 239. [Google Scholar] [CrossRef]
- Sun, S.; Song, Y.; Zheng, Q. Thermo-molded wheat gluten plastics plasticized with glycerol: Effect of molding temperature. Food Hydrocoll. 2008, 22, 1006–1013. [Google Scholar] [CrossRef]
- Diani, J.; Gall, K. Finite strain 3D thermoviscoelastic constitutive model for shape memory polymers. Polymer Eng. Sci. 2006, 46, 486–492. [Google Scholar] [CrossRef]
- Alonso-González, M.; Felix, M.; Guerrero, A.; Romero, A. Effects of mould temperature on rice bran-based bioplastics obtained by injection moulding. Polymers 2021, 13, 398. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Tellez-Garay, A.M.; Castell-Perez, M.E. Physical and mechanical properties of peanut protein films. LWT—Food Sci. Technol. 2004, 37, 731–738. [Google Scholar] [CrossRef]
- Delgado, M.; Felix, M.; Bengoechea, C. Development of bioplastic materials: From rapeseed oil industry by products to added-value biodegradable biocomposite materials. Ind. Crops Prod. 2018, 125, 401–407. [Google Scholar] [CrossRef]
- Orliac, O.; Rouilly, A.; Silvestre, F.; Rigal, L. Effects of additives on the mechanical properties, hydrophobicity and water uptake of thermo-moulded films produced from sunflower protein isolate. Polymer 2002, 43, 5417–5425. [Google Scholar] [CrossRef]
- Félix, M.; Lucio-Villegas, A.; Romero, A.; Guerrero, A. Development of rice protein bio-based plastic materials processed by injection molding. Ind. Crops Prod. 2016, 79, 152–159. [Google Scholar] [CrossRef]
- Gómez-Heincke, D.; Martínez, I.; Stading, M.; Gallegos, C.; Partal, P. Improvement of mechanical and water absorption properties of plant protein based bioplastics. Food Hydrocoll. 2017, 73, 21–29. [Google Scholar] [CrossRef]
- Buffo, R.A.; Weller, C.L.; Gennadios, A. DigitalCommons @ University of Nebraska—Lincoln Films from Laboratory-Extracted Sorghum Kafirin Films from Laboratory-Extracted Sorghum Kafirin 1. Biol. Syst. Eng. Papers Publ. 1997, 105, 473–475. [Google Scholar]
- Yue, H.B.; Fernandez-Blazquez, J.P.; Shuttleworth, P.S.; Cui, Y.D.; Ellis, G. Thermomechanical relaxation and different water states in cottonseed protein derived bioplastics. RSC Adv. 2014, 4, 32320–32326. [Google Scholar] [CrossRef]
- Sharma, L.; Singh, C. Sesame protein based edible films: Development and characterization. Food Hydrocoll. 2016, 61, 139–147. [Google Scholar] [CrossRef]
- Bourtoom, T. Factors Affecting the Properties of Edible Film Prepared from Mung Bean Proteins. Int. Food Res. J. 2008, 15, 167–180. [Google Scholar]
- Bamdad, F.; Goli, A.H.; Kadivar, M. Preparation and characterization of proteinous film from lentil (Lens culinaris): Edible film from lentil (Lens culinaris). Food Res. Int. 2006, 39, 106–111. [Google Scholar] [CrossRef]
- Han, J.; Shin, S.H.; Park, K.M.; Kim, K.M. Characterization of physical, mechanical, and antioxidant properties of soy protein-based bioplastic films containing carboxymethylcellulose and catechin. Food Sci. Biotechnol. 2015, 24, 939–945. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Perez-Puyana, V.; Sánchez-Cid, P.; Guerrero, A.; Romero, A. Incorporation of zno nanoparticles into soy protein-based bioplastics to improve their functional properties. Polymers 2021, 13, 486. [Google Scholar] [CrossRef]
- Aguilar, J.M.; Bengoechea, C.; Pérez, E.; Guerrero, A. Effect of different polyols as plasticizers in soy based bioplastics. Ind. Crops Prod. 2020, 153, 112522. [Google Scholar] [CrossRef]
- Fernández-Espada, L.; Bengoechea, C.; Sandía, J.A.; Cordobés, F.; Guerrero, A. Development of novel soy-protein-based superabsorbent matrixes through the addition of salts. J. Appl. Polym. Sci. 2019, 136, 1–10. [Google Scholar] [CrossRef]
- Patel, A.; Panchal, T.; Thomas, M.; Gupte, A.; Patel, J. Preparation and characterization of biodegradable packaging film using groundnut protein isolate. Eur. Biomass Conf. Exhib. Proc. 2016, 2016, 1048–1055. [Google Scholar] [CrossRef]
- Sun, Q.; Sun, C.; Xiong, L. Mechanical, barrier and morphological properties of pea starch and peanut protein isolate blend films. Carbohydr. Polym. 2013, 98, 630–637. [Google Scholar] [CrossRef]
- Jang, S.A.; Shin, Y.J.; Song, K. Bin Effect of rapeseed protein-gelatin film containing grapefruit seed extract on “Maehyang” strawberry quality. Int. J. Food Sci. Technol. 2011, 46, 620–625. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Moisture sensitivity, optical, mechanical and structural properties of whey protein-based edible films incorporated with rapeseed oil. Food Technol. Biotechnol. 2016, 54, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Z.; Li, Y.; Yang, Y.; Ju, X.; He, R. The preparation and physiochemical characterization of rapeseed protein hydrolysate-chitosan composite films. Food Chem. 2019, 272, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Gennadios, A.; Weller, C.L.; Testin, R.F. Biological Systems Engineering: Papers and Publications Modification of Physical and Barrier Properties of Edible Wheat Gluten-Based Films Modification of Physical and Barrier Properties of Edible Wheat Gluten-Based films. Cereal Chem. 1993, 70, 426–429. [Google Scholar]
- Cho, S.Y.; Lee, S.Y.; Rhee, C. Edible oxygen barrier bilayer film pouches from corn zein and soy protein isolate for olive oil packaging. LWT—Food Sci. Technol. 2010, 43, 1234–1239. [Google Scholar] [CrossRef]
- Ikeguchi, A.; Moriyama, H. Measurement method of ventilation rate with tracer gas method in open type livestock houses. In Proceedings of the XVIIth World Congress of the International Commission of Agricultural and Biosystems Engineering, Quebec City, QC, Canada, 13–17 June 2010; pp. 1–10. [Google Scholar]
- Weller, C.L.; Gennadios, A.; Saraiva, R.A. Edible bilayer films from zein and grain sorghum wax or carnauba wax. LWT—Food Sci. Technol. 1998, 31, 279–285. [Google Scholar] [CrossRef]
- Tapia-Blácido, D.; Sobral, P.J.; Menegalli, F.C. Development and characterization of biofilms based on Amaranth flour (Amaranthus caudatus). J. Food Eng. 2005, 67, 215–223. [Google Scholar] [CrossRef]
- Salgado, P.R.; Molina Ortiz, S.E.; Petruccelli, S.; Mauri, A.N. Biodegradable sunflower protein films naturally activated with antioxidant compounds. Food Hydrocoll. 2010, 24, 525–533. [Google Scholar] [CrossRef]
- Ayhllon-Meixueiro, F.; Vaca-Garcia, C.; Silvestre, F. Biodegradable films from isolate of sunflower (Helianthus annuus) proteins. J. Agric. Food Chem. 2000, 48, 3032–3036. [Google Scholar] [CrossRef] [Green Version]
- Acquah, C.; Zhang, Y.; Dubé, M.A.; Udenigwe, C.C. Formation and characterization of protein-based films from yellow pea (Pisum sativum) protein isolate and concentrate for edible applications. Curr. Res. Food Sci. 2020, 2, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, C.; Pinte, J.; Gontard, N.; Gastaldi, E. Wheat gluten-coated papers for bio-based food packaging: Structure, surface and transfer properties. Food Res. Int. 2010, 43, 1395–1401. [Google Scholar] [CrossRef]
- Ciapponi, R.; Turri, S.; Levi, M. Mechanical reinforcement by microalgal biofiller in novel thermoplastic biocompounds from plasticized gluten. Materials 2019, 12, 1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso-González, M.; Ramos, M.; Bengoechea, C.; Romero, A.; Guerrero, A. Evaluation of Composition on Processability and Water Absorption of Wheat Gluten-Based Bioplastics. J. Polym. Environ. 2021, 29, 1434–1443. [Google Scholar] [CrossRef]
- Thammahiwes, S.; Riyajan, S.A.; Kaewtatip, K. Preparation and properties of wheat gluten based bioplastics with fish scale. J. Cereal Sci. 2017, 75, 186–191. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Felix, M.; Romero, A.; Guerrero, A. Development of pea protein-based bioplastics with antimicrobial properties. J. Sci. Food Agric. 2017, 97, 2671–2674. [Google Scholar] [CrossRef]
- Jang, S.A.; Lim, G.O.; Song, K. Bin Preparation and Mechanical Properties of Edible Rapeseed Protein Films. J. Food Sci. 2011, 76, 218–223. [Google Scholar] [CrossRef]
- Rocca-Smith, J.R.; Marcuzzo, E.; Karbowiak, T.; Centa, J.; Giacometti, M.; Scapin, F.; Venir, E.; Sensidoni, A.; Debeaufort, F. Effect of lipid incorporation on functional properties of wheat gluten based edible films. J. Cereal Sci. 2016, 69, 275–282. [Google Scholar] [CrossRef]



| Protein | Source | Protein Percent (%/DM) | Top Global Producers | References |
|---|---|---|---|---|
| Oilseed cakes/meals | Canola/Rapeseed | 33.9 | Canada, China, Europe, Russia, Asia (India, China, Indonesia) | [59,60,61,62] |
| Coconut | 25.2 | Malaysia, Indonesia | ||
| Cottonseed | 40.3 | Southern US, Brazil, Asia | ||
| Groundnut | 49.5 | Asia (India, China) | ||
| Mustard | 39.5 | India | ||
| Olive | 6.3 | Mediterranean countries of Spain, Italy, Greece, Tunisia | ||
| Palm kernel | 18.6 | Malaysia, Indonesia, China | ||
| Sesame | 35.6 | Asia (Burma, India, China), Africa, South America | ||
| Soybean | 47.5 | North and South America (USA, Brazil and Argentina), Asia | ||
| Sunflower | 34.1 | Russia, Ukraine, Argentina, USA, China, India and Turkey |
| Protein | Source | Total Protein Content (%) | Protein in Other Parts (%) | Top Global Producers | References |
|---|---|---|---|---|---|
| Cereals and Legumes | Barley (Hordeum vulgare L.) | 12.5 | Hay (10–15), Grain (11–15) | Fourth most widely grown in the world | [59,60,61,62] |
| Corn (Zea mays) | 40–50 | Kernel (7–12), Silage (8–11) | Latin America, Africa, Asia | ||
| Wheat (Triticum) | 7–22 | Flour (9–13), Bran crude (15.5) | Asia (China, India), Russia, United States | ||
| Rice (Oryza sativa) | 7 | Bran (13) | Asia (China, India, Bangladesh) | ||
| Soybean (Glycine max) | 42 | Hay (17), Defatted flour (50–59) | Brazil, United States, India | ||
| Mung bean (Vigna radiata) | 16–23 | Asia (India, China, Myanmar) | |||
| Sunflower (Helianthus) | 20–28 | Silage (11–12), Seeds (16.7), Hulls (6.2) | Russia, Ukraine, Argentina. EU-27 countries, China, USA | ||
| Peanut (Arachis hypogaea) | 38.11 | Asia (India, China) | |||
| Sorghum (Sorghum bicolor) | 22 | Hay (7), Stover (5) | Asia and Africa | ||
| Mustard (Vigna radiata) | 24–35 | Hay (10) | India | ||
| Vegetable and fruit wastes | Bottle guard (Spinacia oleracea) | Pulp (24.3) | Asia (India, Sri Lanka, Indonesia, Malaysia), South Africa | ||
| Citrus (Citrus limetta) | Pulp (without peels) (10.5) | Brazil, China, Mexico | |||
| Guava (Psidium guajava) | Seeds (7.6), Guava seed protein isolate (96.7) | India, China, Thailand | |||
| Peas (Pisum sativum) | Pea pods (19.8), Pea vine (11.8), Pea straw (5–10) | China, India, USA, France, Egypt | |||
| Snow peas (Pisum sativum saccharatum) | Culled (23.2) | Russia, China, Canada, Europe and fourth in worldwide | |||
| Sugar beet (Beta vulgaris) | Leaves (21.9), Pulp (10.0) | Russia, France, USA, Germany | |||
| Tomato (Solanum lycopersicum) | Pomace (19–22), Culled tomatoes (14–20), Seeds (24.5) | China, India, USA, Turkey, Egypt |
| Protein Source | Protein Percent in Grain (%) | Crude Protein in Other Parts (%) | References |
|---|---|---|---|
| Amaranth (Amaranthus sp.) | 14 | [63] | |
| Beet (Beta vulgaris) | 8.9 | Tops 12–15, root 7–10 | |
| Berseem clover (Trifolium alexandrinum L.) | 27–29 | ||
| Canola (Brassica napus) | 21 | Hay (16), silage (12), pasture (17), hull (15.2) | |
| Cereal rye (Secale cereale L.) | 14 | Straw (4) | |
| Chickpea (Cicer arietinum L.) | 22 | Straw (6) | |
| Chicory (Cichorium intybus L.) | 10–32 | ||
| Cowpea (Vigna unguiculata L.) | 19–24 | ||
| Field pea (Pisum satuvum arvense L.) | 24 | Silage (15) Hay (14) | |
| Kale (Brassica oleracea) | 30 | ||
| Lentil (Lens culinaris Medik) | 28 | Hay (14) Silage (15) | |
| Lupin (Lupinus L.) | Silage (15) | ||
| Medic (Medicago sp.) | Black medic 19–21 | ||
| Mustard (Brassica sp. L.) | 24–35 | Hay (10) | |
| Oats (Avena sativa L.) | 13–18 | Kernel (40–60), hay (9–15) | |
| Radish (Raphanus sativus) | 26–30 | ||
| Safflower (Carthamus tinctorius L.) | 18 | Hay (10–13) | |
| Spinach (Spinacia oleracea L.) | 20 | ||
| Sweetclover (Melilotussp. L.) | Hay (11–18) | ||
| Triticale (Triticale hexaploide Lart) | 17 | Hay (9–16) | |
| Turnip (Brassica rapa) | Tops (16), root (12–14) | ||
| Vetch (Viciasp.) | 13–20 | ||
| Wheat (Triticum aestivum L.) | 12–16 | Straw (4–10) | |
| White clover (Trifolium repens L.) | 24–30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senthilkumaran, A.; Babaei-Ghazvini, A.; Nickerson, M.T.; Acharya, B. Comparison of Protein Content, Availability, and Different Properties of Plant Protein Sources with Their Application in Packaging. Polymers 2022, 14, 1065. https://doi.org/10.3390/polym14051065
Senthilkumaran A, Babaei-Ghazvini A, Nickerson MT, Acharya B. Comparison of Protein Content, Availability, and Different Properties of Plant Protein Sources with Their Application in Packaging. Polymers. 2022; 14(5):1065. https://doi.org/10.3390/polym14051065
Chicago/Turabian StyleSenthilkumaran, Anupriya, Amin Babaei-Ghazvini, Michael T. Nickerson, and Bishnu Acharya. 2022. "Comparison of Protein Content, Availability, and Different Properties of Plant Protein Sources with Their Application in Packaging" Polymers 14, no. 5: 1065. https://doi.org/10.3390/polym14051065
APA StyleSenthilkumaran, A., Babaei-Ghazvini, A., Nickerson, M. T., & Acharya, B. (2022). Comparison of Protein Content, Availability, and Different Properties of Plant Protein Sources with Their Application in Packaging. Polymers, 14(5), 1065. https://doi.org/10.3390/polym14051065