How Effective Are Antimicrobial Agents on Preventing the Adhesion of Candida albicans to Denture Base Acrylic Resin Materials? A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Polymers
3.1.1. Polymeric Biocides
3.1.2. Biocide-Releasing Polymers
Silver Nanoparticles
Silver Zeolites
Zinc Oxide
Zirconium Oxide
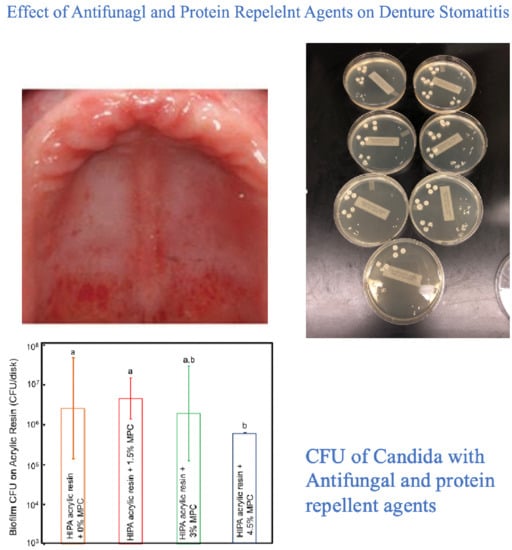
3.1.3. Biocidal Surface Coating (Protein Repellent Agent)
3.2. Nano-Diamonds and Fillers
3.2.1. Nano-Diamonds
3.2.2. Fluoridated Glass Fillers
3.3. Natural Antifungal Agents
3.3.1. Chitosan
3.3.2. Henna (Lawsonia inermis)
3.3.3. Neem Powder (Azadirachta indica)
3.4. Antifungal Medicaments
| Type of Material | Author | Date | ||
|---|---|---|---|---|
| Polymer | Polymeric Biocides | Pesci-Bardon et al. | 2006 | |
| Raj, P.A. and Dentino | 2011 | |||
| Rodriguez LS et al. | 2013 | |||
| da Silva Barboza et al. | 2021 | |||
| Biocide-releasing polymers | Silver nanoparticles | Sehajpal | 1989 | |
| Siedenbiedel F and Tiller | 2012 | |||
| Yadav et al. | 2012 | |||
| Asar et al. | 2013 | |||
| Gad M et al. 2017 | 2017 | |||
| Hamedi-Rad et al. | 2014 | |||
| Ghafari et al. | 2014 | |||
| Suganya et al. | 2014 | |||
| de Castro et al. | 2016 | |||
| Zhang et al. | 2017 | |||
| Hashim et al. | 2020 | |||
| Silver zeolites | Kawahara | 2000 | ||
| Casemiro et al. | 2008 | |||
| Zinc oxide | Kamonkhantikul et al. | 2017 | ||
| Anwander | 2017 | |||
| Zirconium oxide | Gad et al. | 2017 | ||
| Gad et al. | 2018 | |||
| Hamid et al. | 2021 | |||
| Abualsaud et al. | 2021 | |||
| Biocidal surface coatings (Protein Repellent Agent) | Bajunaid et al. | 2021 | ||
| Bajunaid et al. | 2022 | |||
| Nano-diamonds and Fillers | Nano-diamonds | Mangal et al. | 2019 | |
| Al-Harbi et al. | 2019 | |||
| Fouda et al. | 2019 | |||
| Fluoridated glass fillers | Kamijo et al. | 2009 | ||
| Tsutsumi et al. | 2016 | |||
| Natural Antifungal Agents | Chitosan | Song et al. | 2015 | |
| Ikono et al. | 2019 | |||
| Fakhri et al. | 2020 | |||
| Sonawane and Kamb | 2020 | |||
| Chander and Venkatraman | 2021 | |||
| Henna (Lawsonia inermis) | Nawasrah et al. | 2016 | ||
| Gad et al. | 2018 | |||
| Neem powder (Azadirachta indica) | Hamid et al. | 2019 | ||
| Chincholikar et al. | 2019 | |||
| Antifungal Medicaments | Darwish and Amin | 2011 | ||
| Salim et al. | 2013 | |||
| Maluf et al. | 2019 | |||
| Maluf et al. | 2020 | |||
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arweiler, N.B.; Netuschil, L. The oral microbiota. In Microbiota of the Human Body, 1st ed.; Implications in Health and Disease; Schwiertz, A., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 45–60. [Google Scholar]
- Coll, P.P.; Lindsay, A.; Meng, J.; Gopalakrishna, A.; Raghavendra, S.; Bysani, P.; O’Brien, D. The prevention of infections in older adults: Oral health. J. Am. Geriatr. Soc. 2020, 68, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Verma, R.; Murari, A.; Agrawal, A. Oral candidiasis: An overview. J. Oral. Maxillofac. Path. 2014, 18, 81–85. [Google Scholar] [CrossRef]
- Yarborough, A.; Cooper, L.; Duqum, I.; Mendonça, G.; McGraw, K.; Stoner, L. Evidence regarding the treatment of denture stomatitis. J. Prosth. 2016, 25, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.V. Denture sore mouth: A possible etiology. Br. Dent. J. 1962, 112, 357–360. [Google Scholar]
- Altarawneh, S.; Bencharit, S.; Mendoza, L.; Curran, A.; Barrow, D.; Barros, S.; Preisser, J.; Loewy, Z.G.; Gendreau, L.; Offenbacher, S. Clinical and histological findings of denture stomatitis as related to intraoral colonization patterns of Candida albicans, salivary flow, and dry mouth. J. Prosth. 2013, 22, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radford, D.R.; Sweet, S.P.; Challacombe, S.J.; Walter, J.D. Adherence of Candida albicans to denture base materials with different surface finishes. J. Dent. 1998, 26, 577–583. [Google Scholar] [CrossRef]
- Greenspan, D.; Greenspan, J.S. Management of the oral lesions of HIV infection. J. Am. Dent. Assoc. 1991, 122, 26–32. [Google Scholar] [CrossRef]
- Emami, E.; Taraf, H.; de Grandmont, P.; Gauthier, G.; de Koninck, L.; Lamarche, C.; de Souza, R.F. The association of denture stomatitis and partial removable dental prostheses: A systematic review. Int. J. Prosth. 2012, 25, 113–119. [Google Scholar]
- Anwander, M.; Rosentritt, M.; Schneider-Feyrer, S.; Hahnel, S. Biofilm formation on denture base resin including ZnO, CaO, and TiO2 nanoparticles. J. Adv. Prosth. 2017, 9, 482–485. [Google Scholar] [CrossRef] [Green Version]
- Gheorghe, D.C.; Niculescu, A.G.; Bîrcă, A.C.; Grumezescu, A.M. Biomaterials for the prevention of oral candidiasis development. Pharmaceut 2021, 13, 803. [Google Scholar] [CrossRef]
- Raj, P.A.; Dentino, A.R. New phosphated polymethyl methacrylate polymers for the prevention of denture-induced microbial infection: An in vitro study. Clin. Cosmet. Investig. Dent. 2011, 3, 25–32. [Google Scholar] [CrossRef] [Green Version]
- da Silva Barboza, A.; Fang, L.K.; Ribeiro, J.S.; Cuevas-Suárez, C.E.; Moraes, R.R.; Lund, R.G. Physico-mechanical, optical, and antifungal properties of polymethyl methacrylate modified with metal methacrylate monomers. J. Prosth. Dent. 2021, 125, 706. [Google Scholar] [CrossRef] [PubMed]
- Pesci-Bardon, C.; Fosse, T.; Serre, D.; Madinier, I. In vitro antiseptic properties of an ammonium compound combined with denture base acrylic resin. Gerodont 2006, 23, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.S.; Paleari, A.G.; Giro, G.; de Oliveira Junior, N.M.; Pero, A.C.; Compagnoni, M.A. Chemical characterization and flexural strength of a denture base acrylic resin with monomer 2- tert- butylaminoethyl methacrylate. J. Prosth. 2013, 22, 292–297. [Google Scholar] [CrossRef]
- Gad, M.M.; Fouda, S.M.; Al-Harbi, F.A.; Näpänkangas, R.; Raustia, A. PMMA denture base material enhancement: A review of fiber, filler, and nanofiller addition. Int. J. Nanomed. 2017, 12, 3801–3812. [Google Scholar] [CrossRef] [Green Version]
- Siedenbiedel, F.; Tiller, J.C. Antimicrobial polymers in solution and on surfaces: Overview and functional principles. Polymers 2012, 4, 46–71. [Google Scholar] [CrossRef] [Green Version]
- Yadav, P.; Mittal, R.; Sood, V.K.; Garg, R. Effect of incorporation of silane- treated silver and aluminum microparticles on strength and thermal conductivity of PMMA. J. Prosthodont. 2012, 21, 546–551. [Google Scholar] [CrossRef]
- Asar, N.V.; Albayrak, H.; Korkmaz, T.; Turkyilmaz, I. Influence of various metal oxides on mechanical and physical properties of heat-cured polymethylmethacrylate denture base resins. J. Adv. Prosthodont. 2013, 5, 241–247. [Google Scholar] [CrossRef] [Green Version]
- Sehajpal, S.B. Effect of metal fillers on some physical properties of acrylic resin. J. Prosthet. Dent. 1989, 61, 746–751. [Google Scholar] [CrossRef]
- Hashim, T.; Risan, M.H.; Kadhom, M.; Raheem, R.; Yousif, E. Antifungal, antiviral, and antibacterial activities of silver nanoparticles synthesized using fungi: A review. Lett. Appl. NanoBioSci. 2020, 9, 1307–1312. [Google Scholar]
- Hamedi-Rad, F.; Ghaffari, T.; Rezaii, F.; Ramazani, A. Effect of nanosilver on thermal and mechanical properties of acrylic base complete dentures. J. Dent. (Tehran) 2014, 11, 495–505. [Google Scholar]
- Ghafari, T.; Hamedi-Rad, F.; Ezzati, B. Does addition of silver nano-particles to denture base resin increase its thermal conductivity? J. Dent. Sch. 2014, 32, 139–144. [Google Scholar]
- Zhang, Y.; Chen, Y.Y.; Huang, L.; Chai, Z.G.; Shen, L.J.; Xiao, Y.H. The antifungal effects and mechanical properties of silver bromide/cationic polymer nano-composite-modified poly-methyl methacrylate-based dental resin. Sci. Rep. 2017, 7, 1547. [Google Scholar] [CrossRef] [PubMed]
- Suganya, S.; Ahila, S.C.; Kumar, B.M.; Kumar, M.V. Evaluation and comparison of anti-Candida effect of heat cure polymethylmethacrylate resin enforced with silver nanoparticles and conventional heat cure resins: An in vitro study. Ind. J. Dent. Res. 2014, 25, 204–207. [Google Scholar] [CrossRef]
- de Castro, D.T.; Valente, M.L.; da Silva, C.H.; Watanabe, E.; Siqueira, R.L.; Schiavon, M.A. Evaluation of antibiofilm and mechanical properties of new nanocomposites based on acrylic resins and silver vanadate nanoparticles. Arch. Oral Biol. 2016, 67, 46–53. [Google Scholar] [CrossRef]
- Kawahara, K.; Tsuruda, K.; Morishita, M.; Uchida, M. Antibacterial effect of silver–zeolite on oral bacteria under anaerobic condition. Dent. Mat. 2000, 16, 452–455. [Google Scholar] [CrossRef]
- Casemiro, L.A.; Martins, C.H.; Pires-de-Souza, F.D.; Panzeri, H. Antimicrobial and mechanical properties of acrylic resins with incorporated silver-zinc zeolite—Part I. Gerodont 2008, 25, 187–194. [Google Scholar] [CrossRef]
- Kamonkhantikul, K.; Arksornnukit, M.; Takahashi, H. Antifungal, optical, and mechanical properties of polymethylmethacrylate material incorporated with silanized zinc oxide nanoparticles. Int. J. Nanomed. 2017, 12, 2353–2360. [Google Scholar] [CrossRef] [Green Version]
- Hamid, S.K.; Alghamdi, L.A.; Alshahrani, F.A.; Khan, S.Q.; Matin, A.; Gad, M.M. In vitro assessment of artificial aging on the antifungal activity of PMMA denture base material modified with ZrO2 nanoparticles. Int. J. Dent. 2021, 13, 2–11. [Google Scholar] [CrossRef]
- Abualsaud, R.; Aleraky, D.M.; Akhtar, S.; Khan, S.; Gad, M. Antifungal activity of denture base resin containing nanozirconia: In vitro assessment of candida albicans biofilm. Sci. World J. 2021, 2021, 5556413. [Google Scholar] [CrossRef]
- Gad, M.M.; Abualsaud, R.; Rahoma, A.; Al-Thobity, A.M.; Al-Abidi, K.S.; Akhtar, S. Effect of zirconium oxide nanoparticles addition on the optical and tensile properties of polymethyl methacrylate denture base material. Int. J. Nanomed. 2018, 13, 283–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gad, M.M.; Al-Thobity, A.M.; Shahin, S.Y.; Alsaqer, B.T.; Ali, A.A. Inhibitory effect of zirconium oxide nanoparticles on Candida albicans adhesion to repaired polymethyl methacrylate denture bases and interim removable prostheses: A new approach for denture stomatitis prevention. Int. J. Nanomed. 2017, 12, 5409–5419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajunaid, S.O.; Baras, B.H.; Balhaddad, A.A.; Weir, M.D.; Xu, H.H.K. Antibiofilm and protein-repellent polymethylmethacrylate denture base acrylic resin for treatment of denture stomatitis. Materials 2021, 14, 1067. [Google Scholar] [CrossRef] [PubMed]
- Bajunaid, S.O.; Baras, B.H.; Weir, M.D.; Xu, H.H. Denture Acrylic Resin Material with Antibacterial and Protein-Repelling Properties for the Prevention of Denture Stomatitis. Polymers 2022, 14, 230. [Google Scholar] [CrossRef]
- Najeeb, S.; Khurshid, Z.; Agwan, A.S.; Zafar, M.S.; Alrahabi, M.; Qasim, S.B.; Sefat, F. Dental applications of nanodiamonds. Sci. Adv. Mat. 2016, 8, 2064–2070. [Google Scholar] [CrossRef] [Green Version]
- Szunerits, S.; Barras, A.; Boukherroub, R. Antibacterial applications of nanodiamonds. Int. J. Environ. Res. Public Health 2016, 13, 413. [Google Scholar] [CrossRef]
- Mangal, U.; Kim, J.Y.; Seo, J.Y.; Kwon, J.S.; Choi, S.H. Novel polymethyl methacrylate containing nanodiamonds to improve the mechanical properties and fungal resistance. Materials 2019, 12, 3438. [Google Scholar] [CrossRef] [Green Version]
- Al-Harbi, F.A.; Abdel-Halim, M.S.; Gad, M.M.; Fouda, S.M.; Baba, N.Z.; AlRumaih, H.S.; Akhtar, S. Effect of nanodiamond addition on flexural strength, impact strength, and surface roughness of PMMA denture base. J. Prosth. 2019, 1, 417–425. [Google Scholar] [CrossRef] [Green Version]
- Fouda, S.M.; Gad, M.M.; Ellakany, P.; Al-Thobity, A.M.; Al-Harbi, F.A.; Virtanen, J.I.; Raustia, A. The effect of nanodiamonds on candida albicans adhesion and surface characteristics of PMMA denture base material-an in vitro study. J. Appl. Oral Sci. 2019, 27, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kamijo, K.; Mukai, Y.; Tominaga, T.; Iwaya, I.; Fujino, F.; Hirata, Y.; Teranaka, T. Fluoride release and recharge characteristics of denture base resins containing surface pre-reacted glass-ionomer filler. Dent. Mater. J. 2009, 28, 227–233. [Google Scholar] [CrossRef]
- Tsutsumi, C.; Takakuda, K.; Wakabayashi, N. Reduction of Candida bio-film adhesion by incorporation of prereacted glass ionomer filler in denture base resin. J. Dent. Mater. 2016, 44, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, I.; Arunachalam, K.S.; Sajjan, S.; Ramaraju, A.V.; Rao, B.; Kamaraj, B. Incorporation of antimicrobial macromolecules in acrylic denture base resins: A research composition and update. J. Prosth. 2014, 23, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, E.; Eslami, H.; Maroufi, P.; Pakdel, F.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Mahmoudi, S.; et al. Chitosan biomaterials application in dentistry. Int. J. Biol. Macromol. 2020, 162, 956–974. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Zhong, Z.; Lin, L. Evaluation of chitosan quaternary ammonium salt-modified resin denture base material. Int. J. Biol. Macromol. 2015, 85, 102–110. [Google Scholar] [CrossRef]
- Ikono, R.; Vibriani, A.; Wibowo, I.; Saputro, K.E.; Muliawan, W.; Bachtiar, B.M.; Mardliyati, E.; Bachtiar, E.W.; Rochman, N.T.; Kagami, H.; et al. Nanochitosan antimicrobial activity against Streptococcus Mutans and Candida albicans dual-species biofilms. BMC Res. Notes 2019, 12, 383. [Google Scholar] [CrossRef] [Green Version]
- Chander, N.G.; Venkatraman, J. Mechanical properties and surface roughness of chitosan reinforced heat polymerized denture base resin. J. Prosth. Res. 2021, 66, 101–108. [Google Scholar] [CrossRef]
- Sonawane, A.; Kambala, S. Evaluating Salivary pH, Uric acid, & Preactive Protein levels in Completely Edentulous patients before and after wearing Complete dentures incorporated with and without 7.5% Chitosan nanoparticles–An Interventional Study. Eur. J. Mol. Clin. Med. 2020, 7, 2132–2137. [Google Scholar]
- Yusuf, M.; Ahmad, A.; Shahid, M.; Khan, M.I.; Khan, S.A.; Manzoor, N.; Mohammad, F. Assessment of colorimetric, antibacterial and antifungal properties of woollen yarn dyed with the extract of the leaves of henna (Lawsonia inermis). J. Clean. Prod. 2012, 27, 42–50. [Google Scholar] [CrossRef]
- Chaudhary, G.; Goyal, S.; Poonia, P. Lawsonia inermis linnaeus: A phytopharmacological review. Int. J. Pharm. Sci. Drug Res. 2010, 2, 91–98. [Google Scholar]
- Nawasrah, A.; AlNimr, A.; Ali, A.A. Antifungal effect of henna against candida albicans adhered to acrylic resin as a possible method for prevention of denture stomatitis. Int. J. Environ. Res. Public Health 2016, 13, 520. [Google Scholar] [CrossRef] [Green Version]
- Gad, M.; Rahoma, A.; Nawasra, A.; Ammar, M. Influence of henna addition on the flexural strength of acrylic denture base material: An in vitro study. Al-Azhar Dent. J. Girls 2018, 5, 277–2783. [Google Scholar] [CrossRef]
- Hamid, S.K.; Al-Dubayan, A.H.; Al-Awami, H.; Khan, S.Q.; Gad, M.M. In vitro assessment of the antifungal effects of neem powder added to polymethyl methacrylate denture base material. J. Clin. Exp. Dent. 2019, 11, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Chincholikar, S.; Sridevi, J.; Kalavathy, N.; Singh, S.; Kapoor, A.; Saumya, S. Comparative evaluation of two antifungal agents incorporated in auto polymerising denture base resin, heat polymerising denture base resin and permanent silicone soft liner-an in vitro study. J. Clin. Diag. Res. 2019, 13, 49–54. [Google Scholar] [CrossRef]
- Salim, N.; Silikas, N.; Satterthwaite, J.D.; Moore, C.; Ramage, G.; Rautemaa, R. Chlorhexidine-impregnated PEM/THFM polymer exhibits superior activity to fluconazole-impregnated polymer against Candida albicans biofilm formation. Int. J. Antimicrob Agents 2013, 41, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Darwish, R.; Amin, W. New form of administering Chlorhexidine for treatment of denture-induced stomatitis. Ther. Clin. Risk Manag. 2011, 7, 219–225. [Google Scholar]
- Maluf, C.V.; Fidalgo, T.K.D.S.; Valente, A.P.; Dos Anjos, M.J.; Hirata Junior, R.; Telles, D.M. Evaluation of polymethyl methacrylate containing Chlorhexidine: A randomized, controlled, split-mouth in situ study. Am. J. Dent. 2019, 32, 94–98. [Google Scholar]
- Maluf, C.V.; Peroni, L.V.; Menezes, L.R.; Coutinho, W.; Lourenço, E.J.V.; Telles, D.M. Evaluation of the physical and antifungal effects of Chlorhexidine diacetate incorporated into polymethyl methacrylate. J. Appl. Oral Sci. 2020, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, K.H.; Mayer, M.P.; Miyazima, T.Y.; Matsubara, V.H.; Silva, E.G.; Paula, C.R. A multispecies probiotic reduces oral Candida colonization in denture wearers. J. Prosth. 2015, 24, 194–199. [Google Scholar] [CrossRef]
- Chen, H.; Han, Q.; Zhou, X.; Zhang, K.; Wang, S.; Xu, H.H.K. Heat-Polymerized resin containing dimethylaminododecyl methacrylate inhibits candida albicans biofilm. Materials 2017, 10, 431. [Google Scholar] [CrossRef]
- Gad, M.M.; Abualsaud, R. Behavior of PMMA denture base materials containing titanium dioxide nanoparticles: A literature review. Int. J. Biomater. 2019, 2019, 6190610. [Google Scholar] [CrossRef]
- Abdulrazzaq Naji, S.; Jafarzadeh Kashi, T.S.; Pourhajibagher, M.; Behroozibakhsh, M.; Masaeli, R.; Bahador, A. Evaluation of antimicrobial properties of conventional polymethyl methacrylate denture base resin materials containing hydrothermally synthesized anatase TiO2 nanotubes against cariogenic bacteria and candida albicans. Iran. J. Pharm. Res. IJPR 2018, 17, 161–172. [Google Scholar] [PubMed]



| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Papers written in English | Qualitative and/or quantitative reviews |
| Papers published from 2010–2021 | Case series |
| Papers studied novel techniques of incorporating anti-fungal and protein repellent agents, both in-vitro or in-vivo | Case reports |
| Commentaries | |
| Letters to the editor | |
| Interviews | |
| Updates |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajunaid, S.O. How Effective Are Antimicrobial Agents on Preventing the Adhesion of Candida albicans to Denture Base Acrylic Resin Materials? A Systematic Review. Polymers 2022, 14, 908. https://doi.org/10.3390/polym14050908
Bajunaid SO. How Effective Are Antimicrobial Agents on Preventing the Adhesion of Candida albicans to Denture Base Acrylic Resin Materials? A Systematic Review. Polymers. 2022; 14(5):908. https://doi.org/10.3390/polym14050908
Chicago/Turabian StyleBajunaid, Salwa Omar. 2022. "How Effective Are Antimicrobial Agents on Preventing the Adhesion of Candida albicans to Denture Base Acrylic Resin Materials? A Systematic Review" Polymers 14, no. 5: 908. https://doi.org/10.3390/polym14050908
APA StyleBajunaid, S. O. (2022). How Effective Are Antimicrobial Agents on Preventing the Adhesion of Candida albicans to Denture Base Acrylic Resin Materials? A Systematic Review. Polymers, 14(5), 908. https://doi.org/10.3390/polym14050908