An In Situ Experiment to Evaluate the Aging and Degradation Phenomena Induced by Marine Environment Conditions on Commercial Plastic Granules
Abstract
:1. Introduction
1.1. Bioplastic Case
1.2. Resin Pellets Case
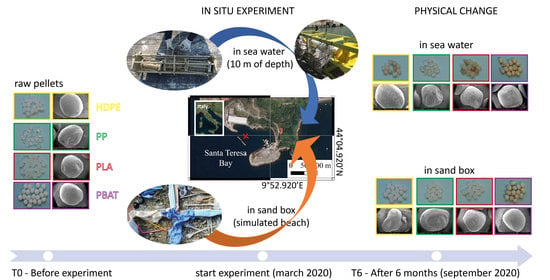
2. Materials and Methods
2.1. Materials
2.2. Experimental Set Up
2.3. Experimental Design
2.4. Instruments and Analysis Methods
3. Results
3.1. Environmental Analyses
3.2. Six-Months-Aged Standard Polymers
3.3. Six-Months-Aged Biodegradable Polymers
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergmann, M.; Gutow, L.; Klages, M. Marine Anthropogenic Litter; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–447. [Google Scholar]
- Crutzen, P.J. The “Anthropocene”; Journal De Physique. IV: JP.; Springer: Berlin/Heidelberg, Germany, 2002; pp. Pr10/1–Pr10/5. [Google Scholar]
- Thompson, R.C.; Swan, S.H.; Moore, C.J.; Vom Saal, F.S. Our plastic age. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1973–1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porta, R. Anthropocene, the plastic age and future perspectives. FEBS Open Bio 2021, 11, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Derraik, J.G. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef]
- Napper, I.E.; Thompson, R.C. Plastic Debris in the Marine Environment: History and Future Challenges. Glob. Chall. 2020, 4, 1900081. [Google Scholar] [CrossRef]
- Andrady, A.L. Persistence of Plastic Litter in the Oceans. In Marine Anthropogenic Litter; Springer International Publishing: Cham, Switzerland, 2015; pp. 57–72. [Google Scholar]
- Bhuyan, S.M.; Venkatramanan, S.; Selvam, S.; Szabo, S.; Maruf Hossain, M.; Rashed-Un-Nabi, M.; Paramasivam, C.R.; Jonathan, M.P.; Islam, S.M. Plastics in marine ecosystem: A review of their sources and pollution conduits. Reg. Stud. Mar. Sci. 2021, 41, 101539. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R Soc. Lond. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kliem, S.; Kreutzbruck, M.; Bonten, C. Review on the Biological Degradation of Polymers in Various Environments. Materials 2020, 13, 4586. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, E.J.; Smith, K.L., Jr. Plastics on the Sargasso sea surface. Science 1972, 175, 1240–1241. [Google Scholar] [CrossRef] [PubMed]
- Maes, T.; Barry, J.; Leslie, H.A.; Vethaak, A.D.; Nicolaus, E.E.M.; Law, R.J.; Lyons, B.P.; Martinez, R.; Harley, B.; Thain, J.E. Below the surface: Twenty-five years of seafloor litter monitoring in coastal seas of North West Europe (1992–2017). Sci. Total Environ. 2018, 630, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Cozar Cabañas, A.; Sanz-Martín, M.; Martí, E.; González-Gordillo, J.I.; Ubeda, B.; Gálvez, J.Á.; Irigoien, X.; Duarte, C.M. Concentrations of floating plastic debris in the Mediterranean Sea measured during MedSeA-2013 cruise. PLoS ONE 1972, 4062. [Google Scholar]
- Cózara, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Úbeda, B.; Hernández-León, S.; Palma, Á.T.; Navarro, S.; García-de-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senga Green, D.; Boots, B.; Blockley, D.J.; Rocha, C.; Thompson, R. Impacts of Discarded Plastic Bags on Marine Assemblages and Ecosystem Functioning. Environ. Sci. Technol. 2015, 49, 5380–5389. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Honorato-Zimmer, D.; Gatta-Rosemary, M.; Nuñez, P.; Hinojosa, I.A.; Thiel, M. Spatio-temporal variation of anthropogenic marine debris on Chilean beaches. Mar. Pollut. Bull. 2018, 126, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2008, 108, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Thiel, M. Distribution and abundance of small plastic debris on beaches in the SE Pacific (Chile): A study supported by a citizen science project. Mar. Environ. Res. 2013, 87–88, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Elsamahy, T.; Koutra, E.; Kornaros, M.; El-Sheekh, M.; Abdelkarim, E.A.; Zhu, D.; Sun, J. Degradation of conventional plastic wastes in the environment: A review on current status of knowledge and future perspectives of disposal. Sci. Total Environ. 2021, 771, 144719. [Google Scholar] [CrossRef] [PubMed]
- Suaria, G.; Avio, C.G.; Mineo, A.; Lattin, G.L.; Magaldi, M.G.; Belmonte, G.; Moore, C.J.; Regoli, F.; Aliani, S. The Mediterranean Plastic Soup: Synthetic polymers in Mediterranean surface waters. Sci. Rep. 2016, 6, 37551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Galgani, F.; Claro, F.; Depledge, M.; Fossi, C. Monitoring the impact of litter in large vertebrates in the Mediterranean Sea within the European Marine Strategy Framework Directive (MSFD): Constraints, specificities and recommendations. Mar. Environ. Res. 2014, 100, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Koelmans, A.A.; Bakir, A.; Burton, G.A.; Janssen, C.R. Microplastic as a Vector for Chemicals in the Aquatic Environment: Critical Review and Model-Supported Reinterpretation of Empirical Studies. Environ. Sci. Technol. 2016, 50, 3315–3326. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Besseling, E.; Shim, W.J. Nanoplastics in the Aquatic Environment. Critical Review. In Marine Anthropogenic Litter; Springer International Publishing: Cham, Switzerland, 2015; pp. 325–340. [Google Scholar]
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Buitrago, N.; Arroyo-Olarte, H.; Trilleras, J.; Arana, V.A.; Mantilla-Barbosa, E.; Gracia, C.A.; Mendoza, A.V.; Neal, W.J.; Williams, A.T.; Micallef, A. Microplastics pollution on Colombian Central Caribbean beaches. Mar. Pollut. Bull. 2021, 170, 112685. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Cho, S.-H.; Kim, K.-H.; Kwon, E.E. Progress in quantitative analysis of microplastics in the environment: A review. Chem. Eng. J. 2021, 422, 130154. [Google Scholar] [CrossRef]
- Liebezeit, G.; Dubaish, F. Microplastics in Beaches of the East Frisian Islands Spiekeroog and Kachelotplate. Bull. Environ. Contam. Toxicol. 2012, 89, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Devriese, L.; Galgani, F.S.; Robbens, J.; Janssen, C.R. Microplastics in sediments: A review of techniques, occurrence and effects. Mar. Environ. Res. 2015, 111, 5–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, L.; Fu, D.; Qi, H.; Lan, C.Q.; Yu, H.; Ge, C. Micro- and nano-plastics in marine environment: Source, distribution and threats—A review. Sci. Total Environ. 2020, 698, 134254. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Su, B.; Xu, X.; Di, D.; Huang, H.; Mei, K.; Dahlgren, R.A.; Zhang, M.; Shang, X. Preferential accumulation of small (<300 mm) microplastics in the sediments of a coastal plain river network in eastern China. Water Res. 2018, 144, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Frère, L.; Paul-Pont, I.; Rinnert, E.; Petton, S.; Jaffré, J.; Bihannic, I.; Soudant, P.; Lambert, C.; Huvet, A. Influence of environmental and anthropogenic factors on the composition, concentration and spatial distribution of microplastics: A case study of the Bay of Brest (Brittany, France). Environ. Pollut. 2017, 225, 211–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, L.K.; Bochow, M.; Imhof, H.K.; Oswald, S.E. Multi-temporal surveys for microplastic particles enabled by a novel and fast application of SWIR imaging spectroscopy—Study of an urban watercourse traversing the city of Berlin, Germany. Environ. Pollut. 2018, 239, 579–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zobkov, M.B.; Esiukova, E.E.; Zyubin, A.Y.; Samusev, I.G. Microplastic content variation in water column: The observations employing a novel sampling tool in stratified Baltic Sea. Mar. Pollut. Bull. 2019, 138, 193–205. [Google Scholar] [CrossRef] [PubMed]
- De-la-Torre, G.E.; Dioses-Salinas, D.C.; Castro, J.M.; Antay, R.; Fernándeza, N.Y.; Espinoza-Morriberónb, D.; Saldaña-Serrano, M. Abundance and distribution of microplastics on sandy beaches of Lima, Peru. Mar. Pollut. Bull. 2020, 151, 110877. [Google Scholar] [CrossRef] [PubMed]
- Giovacchini, A.; Merlino, S.; Locritani, M.; Stroobant, M. Spatial distribution of marine litter along italian coastal areas in the Pelagos sanctuary (Ligurian Sea—NW Mediterranean Sea): A focus on natural and urban beaches. Mar. Pollut. Bull. 2018, 130, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Merlino, S.; Locritani, M.; Bernardi, G.; Como, C.; Legnaioli, S.; Palleschi, V.; Abbate, M. Spatial and Temporal Distribution of Chemically Characterized Microplastics within the Protected Area of Pelagos Sanctuary (NW Mediterranean Sea): Focus on Natural and Urban Beaches. Water 2020, 12, 3389. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Wang, J.; Wang, Y.; Mu, J.; Wang, P.; Lin, X.; Ma, D. Microplastic pollution in the surface waters of the Bohai Sea, China. Environ. Pollut. 2017, 231, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Brach, L.; Deixonne, P.; Bernard, M.F.; Durand, E.; Desjean, M.C.; Perez, E.; van Sebille, E.; ter Halle, A. Anticyclonic eddies increase accumulation of microplastic in the North Atlantic subtropical gyre. Mar. Pollut. Bull. 2018, 126, 191–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massetti, L.; Rangel-Buitrago, N.; Pietrelli, L.; Merlino, S. Litter impacts on marine birds: The Mediterranean Northern gannet as case study. Mar. Pollut. Bull. 2021, 171, 112779. [Google Scholar] [CrossRef]
- van Franeker, J.A.; Blaize, C.; Danielsen, J.; Fairclough, K.; Gollan, J.; Guse, N.; Hansen, P.-L.; Heubeck, M.; Jensen, J.-K.; Le Guillou, G.; et al. Monitoring plastic ingestion by the northern fulmar Fulmarus glacialis in the North Sea. Mar. Environ. Res. 2011, 159, 2609–2615. [Google Scholar] [CrossRef] [PubMed]
- Mordecai, G.; Tyler, P.A.; Masson, D.G.; Huvenne, V.A.I. Litter in submarine canyons off the west coast of Portugal. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2011, 58, 2489–2496. [Google Scholar] [CrossRef]
- Knowlton, A.R.; Robbins, J.; Landry, S.; McKenna, H.A.; Kraus, S.D.; Werner, T.B. Effects of fishing rope strength on the severity of large whale entanglements. Conserv. Biol. 2016, 30, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Campani, T.; Baini, M.; Giannetti, M.; Cancelli, F.; Mancusi, C.; Serena, F.; Marsili, L.; Casini, S.; Fossi, M.C. Presence of plastic debris in loggerhead turtle stranded along the Tuscany coasts of the Pelagos Sanctuary for Mediterranean Marine Mammals (Italy). Mar. Pollut. Bull. 2013, 74, 225–230. [Google Scholar] [CrossRef] [PubMed]
- de Stephanis, R.; Giménez, J.; Carpinelli, E.; Gutierrez-Exposito, C.; Cañadas, A. As main meal for sperm whales: Plastics debris. Mar. Pollut. Bull. 2013, 69, 206–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waluda, C.M.; Staniland, I.J. Entanglement of Antarctic fur seals at Bird Island, South Georgia. Mar. Pollut. Bull. 2013, 74, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Kühn, S.; Bravo Rebolledo, E.L.; van Franeker, J.A. Deleterious Effects of Litter on Marine Life. In Marine Anthropogenic Litter; Springer International Publishing: Cham, Switzerland, 2015; pp. 75–116. [Google Scholar]
- Rodríguez, B.; Beneharo, J.; Rodríguez, A.; Arcos, J.M. Incidence of entanglements with marine debris by northern gannets (Morus bassanus) in the non-breeding grounds. Mar. Pollut. Bull. 2013, 75, 259–263. [Google Scholar] [CrossRef]
- Tetu, S.G.; Sarker, I.; Schrameyer, V.; Pickford, R.; Elbourne, L.D.H.; Moore, L.R.; Paulsen, I.T. Plastic leachates impair growth and oxygen production in Prochlorococcus, the ocean’s most abundant photosynthetic bacteria. Commun. Biol. 2019, 2, 184. [Google Scholar] [CrossRef] [Green Version]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.U.; Barlaz, M.A.; Jonsson, S.; Bjorn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. A Soc. Lond. B Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef] [Green Version]
- Browne, M.A.; Dissanayake, A.; Galloway, T.S.; Lowe, D.M.; Thompson, R.C. Ingested Microscopic Plastic Translocates to the Circulatory System of the Mussel, Mytilus edulis (L.). Environ. Sci. Technol. 2008, 42, 5026–5031. [Google Scholar] [CrossRef]
- Oehlmann, J.; Schulte-Oehlmann, U.; Kloas, W.; Jagnytsch, O.; Lutz, I.; Kusk, K.O.; Wollenberger, L.; Santos, E.M.; Paull, G.C.; Van Look, K.J.; et al. A critical analysis of the biological impacts of plasticizers on wildlife. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2047–2062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, H.M.; Calafat, A.M. Human body burdens of chemicals used in plastic manufacture. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2063–2078. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Sathyanarayana, S.; Swan, S.H. Phthalates and other additives in plastics: Human exposure and associated health outcomes. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2009, 364, 2097–2113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fossi, M.; Coppola, D.; Baini, M.; Giannetti, M.; Guerranti, C.; Marsili, L.; Panti, C.; de Sabata, E.; Clò, S. Large filter feeding marine organisms as indicators of microplastic in the pelagic environment: The case studies of the Mediterranean basking shark (Cetorhinus maximus) and fin whale (Balaenoptera physalus). Mar. Environ. Res. 2014, 100, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Fossi, M.; Panti, C.; Guerranti, C.; Coppola, D.; Giannetti, M.; Marsili, L.; Minutoli, R. Are baleen whales exposed to the threat of microplastics? A case study of the Mediterranean fin whale (Balaenoptera physalus). Mar. Pollut. Bull. 2012, 64, 2374–2379. [Google Scholar] [CrossRef] [PubMed]
- Fossi, M.C.; Panti, C.; Baini, M.; Lavers, J.L. A Review of Plastic-Associated Pressures: Cetaceans of the Mediterranean Sea and Eastern Australian Shearwaters as Case Studies. Front. Mar. Sci. 2018, 5, 173. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Takada, H.; Yamashita, R.; Mizukawa, K.; Fukuwaka, M.A.; Watanuki, Y. Accumulation of plastic-derived chemicals in tissues of seabirds ingesting marine plastics. Mar. Pollut. Bull. 2013, 69, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Galloway, T.S. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ. Sci. Technol. 2015, 49, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; d’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 1987, 198, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Romano, N.; Galloway, T.; Hamzah, H. Virgin microplastics cause toxicity and modulate the impacts of phenanthrene on biomarker responses in African catfish (Clarias gariepinus). Environ. Res. 2016, 151, 58–70. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Provencher, J.F.; Liboiron, M.; Borrelle, S.B.; Bond, A.L.; Rochman, C.; Lavers, J.L.; Avery-Gomm, S.; Yamashita, R.; Ryan, P.G.; Lusher, A.L.; et al. A Horizon Scan of research priorities to inform policies aimed at reducing the harm of plastic pollution to biota. Sci. Total Environ. 2020, 733, 139381. [Google Scholar] [CrossRef] [PubMed]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, E.R.; Thompson, J.T. Deposit- and suspension-feeding sea cucumbers (Echinodermata) ingest plastic fragments. J. Exp. Mar. Biol. Ecol. 2009, 368, 22–29. [Google Scholar] [CrossRef]
- Talsness, C.E.; Andrade, A.J.; Kuriyama, S.N.; Taylor, J.A.; vom Saal, F.S. Components of plastic: Experimental studies in animals and relevance for human health. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2079–2096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, E.; Nilsson, N.H.; Lithner, D.; Lassen, C. Hazardous Substances in Plastic Materials; COWI: Vejle, Denmark, 2013. [Google Scholar]
- Mato, Y.; Isobe, T.; Takada, H.; Kanehiro, H.; Ohtake, C.; Kaminuma, T. Plastic Resin Pellets as a Transport Medium for Toxic Chemicals in the Marine Environment. Environ. Sci. Technol. 2001, 35, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Takizawa, R.; Okuda, K.; Takada, H.; Chiba, K.; Kanehiro, H.; Ogi, H.; Yamashita, R.; Date, T. Concentration of polychlorinated biphenyls (PCBs) in beached resin pellets: Variability among individual particles and regional differences. Mar. Pollut. Bull. 2005, 50, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Rios, L.M.; Moore, C.; Jones, P.R. Persistent organic pollutants carried by synthetic polymers in the ocean environment. Mar. Pollut. Bull. 2007, 54, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Teuten, E.L.; Rowland, S.J.; Galloway, T.S.; Thompson, R.C. Potential for Plastics to Transport Hydrophobic Contaminants. Environ. Sci. Technol. 2007, 41, 7759–7764. [Google Scholar] [CrossRef] [PubMed]
- Lithner, D.; Larsson, A.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef] [PubMed]
- Hirai, H.; Takada, H.; Ogata, Y.; Yamashita, R.; Mizukawa, K.; Saha, M.; Kwan, C.; Moore, C.; Gray, H.; Laursen, D.; et al. Organic micropollutants in marine plastics debris from the open ocean and remote and urban beaches. Mar. Pollut. Bull. 2011, 62, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Bellas, J.; Martínez-Armental, J.; Martínez-Cámara, A.; Besada, V.; Martínez-Gómez, C. Ingestion of microplastics by demersal fish from the Spanish Atlantic and Mediterranean coasts. Mar. Pollut. Bull. 2016, 109, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qu, X.; Su, L.; Zhang, W.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in mussels along the coastal waters of China. Environ. Pollut. 2016, 214, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Gusmão, F.; Di Domenico, M.; Amaral, A.C.Z.; Martínez, A.; Gonzalez, B.C.; Worsaae, K.; Ivar do Sul, J.A.; da Cunha Lana, P. In situ ingestion of microfibres by meiofauna from sandy beaches. Environ. Pollut. 2016, 216, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.L.; Arueira, V.F.; da Costa, M.F.; Di Beneditto, A.P.M.; Zalmon, I.R. Can the Atlantic ghost crab be a potential biomonitor of microplastic pollution of sandy beaches sediment? Mar. Pollut. Bull. 2019, 145, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Wójcik-Fudalewska, D.; Normant-Saremba, M.; Anastácio, P. Occurrence of plastic debris in the stomach of the invasive crab Eriocheir sinensis. Mar. Pollut. Bull. 2016, 113, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Carlin, J.; Craig, C.; Little, S.; Donnelly, M.; Fox, D.; Zhai, L.; Walters, L. Microplastic accumulation in the gastrointestinal tracts in birds of prey in central Florida, USA. Environ. Pollut. 2020, 264, 114633. [Google Scholar] [CrossRef] [PubMed]
- Fazey, F.M.C.; Ryan, P.G. Biofouling on buoyant marine plastics: An experimental study into the effect of size on surface longevity. Environ. Pollut. 2016, 210, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Lobelle, D.; Cunliffe, M. Early microbial biofilm formation on marine plastic debris. Mar. Pollut. Bull. 2011, 62, 197–200. [Google Scholar] [CrossRef]
- Da Costa, J.P.; Nunes, A.R.; Santos, P.S.M.; Girão, A.V.; Duarte, A.C.; Rocha-Santos, T. Degradation of polyethylene microplastics in seawater: Insights into the environmental degradation of polymers. J. Environ. Sci. Health A 2018, 53, 866–875. [Google Scholar] [CrossRef]
- O’Brine, T.; Thompson, R.C. Degradation of plastic carrier bags in the marine environment. Mar. Pollut. Bull. 2010, 60, 2279–2283. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.R.; Laforsch, C.; Greiner, A.; Agarwal, S. Fate of So-Called Biodegradable Polymers in Seawater and Freshwater. Glob. Chall. 2017, 1, 1700048. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Paiano, R.B.; Lourenço, R.V.; Bittante, A.; Sobral, P.J.A. Biodegradability in aquatic system of thin materials based on chitosan, PBAT and HDPE polymers: Respirometric and physical-chemical analysis. Int. J. Biol. Macromol. 2020, 164, 1399–1412. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, E.J.; Anderson, S.J.; Harvey, G.R.; Miklas, H.P.; Peck, B.B. Polystyrene spherules in coastal waters. Science 1972, 178, 749–750. [Google Scholar] [CrossRef] [PubMed]
- Abelouah, M.R.; Ben-Haddad, M.; Alla, A.A.; Rangel-Buitrago, N. Marine litter in the central Atlantic coast of Morocco. Ocean. Coast. Manag. 2021, 214, 105940. [Google Scholar] [CrossRef]
- Gündogdu, S.; Çevik, C. Mediterranean dirty edge: High level of meso and macroplastics pollution on the Turkish coast. Environ. Pollut. 2019, 255, 113351. [Google Scholar] [CrossRef] [PubMed]
- Camacho, M.; Herrera, A.; Gómez, M.; Acosta-Dacal, A.; Martínez, I.; Henríquez-Hernández, L.A.; Luzardo, O.P. Organic pollutants in marine plastic debris from Canary Islands beaches. Sci. Total Environ. 2019, 662, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Ogata, Y.; Takada, H.; Mizukawa, K.; Hirai, H.; Iwasa, S.; Endo, S.; Mato, Y.; Saha, M.; Okuda, K.; Nakashima, A.; et al. International Pellet Watch: Global monitoring of persistent organic pollutants (POPs) in coastal waters. 1. Initial phase data on PCBs, DDTs, and HCHs. Mar. Pollut. Bull. 2009, 58, 1437–1446. [Google Scholar] [CrossRef]
- Karapanagioti, H.K.; Klontza, I. Testing phenanthrene distribution properties of virgin plastic pellets and plastic eroded pellets found on Lesvos island beaches (Greece). Mar. Environ. Res. 2008, 65, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Heskett, M.; Takada, H.; Yamashita, R.; Yuyama, M.; Ito, M.; Geok, Y.B.; Ogata, Y.; Kwan, C.; Heckhausen, A.; Taylor, H.; et al. Measurement of persistent organic pollutants (POPs) in plastic resin pellets from remote islands: Toward establishment of background concentrations for International Pellet Watch. Mar. Pollut. Bull. 2012, 64, 445–448. [Google Scholar] [CrossRef]
- Lohmann, R. Critical Review of Low-Density Polyethylene Partitioning and Diffusion Coefficients for Trace Organic Contaminants and Implications for Its Use As a Passive Sampler. Environ. Sci. Technol. 2012, 46, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Karapanagioti, H.K.; Endo, S.; Ogata, Y.; Takada, H. Diffuse pollution by persistent organic pollutants as measured in plastic pellets sampled from various beaches in Greece. Mar. Pollut. Bull. 2011, 62, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Karapanagioti, H.; Ogata, Y.; Takada, H. Eroded plastic pellets as monitoring tools for polycyclic aromatic hydrocarbons (PAH):Laboratory and field studies. Glob. Nest J. 2010, 12, 327–334. [Google Scholar]
- Karkanorachaki, K.; Kiparissis, S.; Kalogerakis, G.C.; Yiantzi, E.; Psillakis, E.; Kalogerakis, N. Plastic pellets, meso- and microplastics on the coastline of Northern Crete: Distribution and organic pollution. Mar. Pollut. Bull. 2018, 133, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, S.; Colabuono, F.I.; Dias, P.S.; Oliveira, R.; Fisner, M.; Turra, A.; Izar, G.M.; Abessa, D.M.; Saha, M.; Hosoda, J.; et al. Spatial variability in persistent organic pollutants and polycyclic aromatic hydrocarbons found in beach-stranded pellets along the coast of the state of São Paulo, southeastern Brazil. Mar. Pollut. Bull. 2016, 106, 87–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, S.; Yuyama, M.; Takada, H. Desorption kinetics of hydrophobic organic contaminants from marine plastic pellets. Mar. Pollut. Bull. 2013, 74, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Hoh, E.; Hentschel, B.T.; Kaye, S. Long-Term Field Measurement of Sorption of Organic Contaminants to Five Types of Plastic Pellets: Implications for Plastic Marine Debris. Environ. Sci. Technol. 2013, 47, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.F.; Manomanii, K.; Mortimer, M.R.; McLachlan, M.S. Partitioning of polycyclic aromatic hydrocarbons in the polyethylene/water system. Fresenius J. Anal. Chem. 2001, 371, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Smedes, F.; Geertsma, R.W.; van der Zande, T.; Booij, K. Polymer-water partition coefficients of hydrophobic compounds for passive sampling: Application of cosolvent models for validation. Environ. Sci. Technol. 2009, 43, 7047–7054. [Google Scholar] [CrossRef] [PubMed]
- Accustandard Accustandard Plastic Additive Guide. Available online: https://www.accustandard.com/plastic-additive-catalog-2nd-edition (accessed on 7 December 2021).
- Quero, E.; Müller, A.J.; Signori, F.; Coltelli, M.-B.; Bronco, S. Isothermal Cold-Crystallization of PLA/PBAT Blends With and Without the Addition of Acetyl Tributyl Citrate. Macromol. Chem. Phys. 2012, 213, 36–48. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Selke, S.E.M.; Matuana, L.M. Effect of the high-density polyethylene melt index on the microcellular foaming of high-density polyethylene/polypropylene blends. J. Appl. Polym. Sci. 2004, 93, 364–371. [Google Scholar] [CrossRef]
- Xiao, H.; Lu, W.; Yeh, J.-T. Crystallization behavior of fully biodegradable poly(lactic acid)/poly(butylene adipate-co-terephthalate) blends. J. Appl. Polym. Sci. 2009, 112, 3754–3763. [Google Scholar] [CrossRef]
- Brandon, J.; Goldstein, M.; Ohman, M.D. Long-term aging and degradation of microplastic particles: Comparing in situ oceanic and experimental weathering patterns. Mar. Pollut. Bull. 2016, 110, 299–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Bioplastics. Available online: https://www.european-bioplastics.org/bioplastics (accessed on 9 December 2021).
- Corcoran, P.; Biesinger, M.; Grifi, M. Plastics and beaches: A degrading relationship. Mar. Pollut. Bull. 2009, 58, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, P.; Trivedi, K.; Stoklasa, K.; Svobodova, D.; Ougizawa, T. Study of crystallization behaviour of electron beam irradiated polypropylene and high-density polyethylene. R. Soc. Open Sci. 2021, 8, 202250. [Google Scholar] [CrossRef] [PubMed]
- Contat-Rodrigo, L. Thermal characterization of the oxo-degradation of polypropylene containing a pro-oxidant/pro-degradant additive. Polym. Degrad. Stab. 2013, 98, 2117–2124. [Google Scholar] [CrossRef]
- Itävaara, M.; Karjomaa, S.; Selin, J.-F. Biodegradation of polylactide in aerobic and anaerobic thermophilic conditions. Chemosphere 2002, 46, 879–885. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Andres-Puche, R.; Dominguez, E.; Verdejo, E.; Monreal, L.; Ribes-Greus, A. Influence of substrate and temperature on the biodegradation of polyester-based materials: Polylactide and poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) as model cases. Polym. Degrad. Stab. 2020, 180, 109288. [Google Scholar] [CrossRef]
- Kalita, N.K.; Bhasney, S.M.; Mudenur, C.; Kalamdhad, A.; Katiyar, V. End-of-life evaluation and biodegradation of Poly(lactic acid) (PLA)/Polycaprolactone (PCL)/Microcrystalline cellulose (MCC) polyblends under composting conditions. Chemosphere 2020, 247, 125875. [Google Scholar] [CrossRef] [PubMed]
- Kalita, N.K.; Hazarika, D.; Kalamdhad, A.; Katiyar, V. Biodegradation of biopolymeric composites and blends under different environmental conditions: Approach towards end-of-life panacea for crop sustainability. Bioresour. Technol. Rep. 2021, 15, 100705. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Preziosi, R.; Robson, G.D. Abiotic and biotic environmental degradation of the bioplastic polymer poly(lactic acid): A review. Polym. Degrad. Stab. 2017, 137, 122–130. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, H.; Suzuyoshi, K. Environmental degradation of biodegradable polyesters 1. Poly(ε-caprolactone), poly[(R)-3-hydroxybutyrate], and poly(L-lactide) films in controlled static seawater. Polym. Degrad. Stab. 2002, 75, 347–355. [Google Scholar] [CrossRef]
- Palsikowski, P.; Kuchnier, C.; Pinheiro, I.; Morales, A. Biodegradation in Soil of PLA/PBAT Blends Compatibilized with Chain Extender. J. Polym. Environ. 2018, 26, 1–12. [Google Scholar] [CrossRef]








| First Heating (from/to) | Hold (min) | Cooling (from/to) | Hold (min) | Second Heating (from/to) | Hold (min) | |
|---|---|---|---|---|---|---|
| HDPE/PP | 20−220 °C | 2 | 220/−80 °C | 5 | −80/220 | 2 |
| PLA | 20−180 °C | 1 | 180/−80 °C | 1 | −80/180 | 1 |
| PBAT | 20−200 °C | 1 | 200/−80 °C | 1 | −80/200 | 1 |
| Average Temperaures (°C) | Standard Deviation * (°C) | Standard Deviation ** (°C) | Tmin (°C) | Tmax (°C) | |
|---|---|---|---|---|---|
| March 2020 | 13.70 | 0.077 | 0.32 | 12.93 | 14.32 |
| April 2020 | 15.07 | 0.055 | 0.78 | 13.69 | 17.33 |
| May 2020 | 18.20 | 0.064 | 0.72 | 16.93 | 20.16 |
| June 2020 | 20.88 | 0.072 | 0.85 | 18.97 | 23.19 |
| July 2020 | 21.25 | 0.11 | 0.59 | 19.75 | 23.35 |
| August 2020 | 23.68 | 0.20 | 2.20 | 22.12 | 25.24 |
| Average Temperaures (°C) | Standard Deviation * (°C) | Standard Deviation ** (°C) | Tmin (°C) | Tmax (°C) | |
|---|---|---|---|---|---|
| March 2021 | 12.88 | 2.94 | 7.15 | 1.95 | 36.16 |
| April 2021 | 14.98 | 2.53 | 6.45 | 0.123 | 36.21 |
| May 2021 | 18.64 | 2.43 | 6.05 | 8.28 | 42.08 |
| June 2021 | 25.64 | 2.15 | 7.48 | 11.79 | 45.60 |
| July 2021 | 27.69 | 1.99 | 6.82 | 17.84 | 48.69 |
| August 2021 | 29.06 | 2.09 | 7.19 | 18.81 | 51.63 |
| Sarzana | Average Temperaures (°C) | Standard Deviation (°C) | Tmin (°C) | Tmax (°C) | Pisa | Average Temperaures (°C) | Standard Deviation (°C) | Tmin (°C) | Tmax (°C) |
|---|---|---|---|---|---|---|---|---|---|
| March 2020 | 11.09 | 2.22 | 2 | 20 | March 2021 | 9.65 | 1.85 | −1 | 22 |
| April 2020 | 14.48 | 2.58 | 2 | 21 | April 2021 | 11.70 | 2.15 | −2 | 21 |
| May 2020 | 19.35 | 1.98 | 12 | 25 | May 2021 | 15.39 | 1.67 | 5 | 24 |
| June 2020 | 20.67 | 2.67 | 14 | 30 | June 2021 | 21.93 | 1.86 | 10 | 32 |
| July 2020 | 24.65 | 1.53 | 18 | 30 | July 2021 | 24.23 | 1.89 | 16 | 33 |
| August 2020 | 25.19 | 1.86 | 18 | 32 | August 2021 | 24.74 | 1.97 | 15 | 36 |
| TGA Results | DSC Results | |||||
|---|---|---|---|---|---|---|
| Sample | Tonset (°C) | Tmax (°C) | Residue at 700 °C (%) | Tm (°C) | ΔHm (J/g) | χ% * |
| HDPE | 255.5 | 464.3 | 0.8 | 135.1 | 228 | 77.8 |
| HDPE_6SW | 249.1 | 444.7 | 0.0 | 130.9 | 183 | 62.5 |
| HDPE_6S | 242.1 | 465.0 | 0.0 | 132.8 | 207 | 70.6 |
| TGA Results | DSC Results | |||||
|---|---|---|---|---|---|---|
| Sample | Tonset (°C) | Tmax (°C) | Residue at 700 °C (%) | Tm (°C) | ΔHm (J/g) | χ% * |
| PP | 261.5 | 355.7 | 0.3 | 162.5 | 108.0 | 52.2 |
| PP_6SW | 261.4 | 340.6 | 0.9 | 160.6 | 102.0 | 49.3 |
| PP_6S | 255.0 | 339.4 | 2.2 | 156.3 (162.4) | 98.6 | 47.6 |
| TGA Results | DSC Results | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Tonset (°C) | Tmax (°C) | Residue at 700 °C (%) | Tg (°C) | ΔCp (J/g°C) | Tcc (°C) | ΔHcc (J/g) | Tm (°C) | ΔHm (J/g) | χ% * |
| PLA | 323.1 | 367.7 | 0.0 | 58.3 | 0.55 | 122.7 | −0.19 | 150.9 | 0.21 | 0.022 |
| PLA_6SW | 320.2 | 365.4 | 0.6 | 57.5 | 0.57 | - | - | 147.9 | 0.16 | 0.17 |
| PLA_6S | 315.0 | 359.2 | 0.0 | 58.5 | 0.51 | 117.6 | −1.89 | 147.5 (152.4) | 2.04 | 0.16 |
| TGA Results | DSC Results | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample | Tonset (°C) | Tmax (°C) | Residue at 700 °C (%) | Tg (°C) | ΔCp (J/g°C) | Tm (°C) | ΔHm (J/g) | χ% * |
| PBAT | 369.9 | 407.1 | 0.5 | −35.4 | 0.40 | 120.6 | 18.4 | 16.1 |
| PBAT_6SW | 359.3 | 403.6 | 0.6 | −35.0 | 0.41 | 122.1 | 18.2 | 16.0 |
| PBAT_6S | 357.2 | 412.6 | 5.6 | −36.0 | 0.44 | 119.5 | 18.7 | 16.4 |
| Sample | (KDa) | (KDa) | PDI |
|---|---|---|---|
| PLA | 84.6 | 146.3 | 1.7 |
| PLA_6SW | 82.2 | 145.2 | 1.8 |
| PLA_6S | 88.9 | 149.7 | 1.7 |
| PBAT | 21.5 | 47.3 | 2.2 |
| PBAT_6SW | 19.8 | 45.2 | 2.3 |
| PBAT_6S | 12.6 | 35.8 | 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Monte, C.; Locritani, M.; Merlino, S.; Ricci, L.; Pistolesi, A.; Bronco, S. An In Situ Experiment to Evaluate the Aging and Degradation Phenomena Induced by Marine Environment Conditions on Commercial Plastic Granules. Polymers 2022, 14, 1111. https://doi.org/10.3390/polym14061111
De Monte C, Locritani M, Merlino S, Ricci L, Pistolesi A, Bronco S. An In Situ Experiment to Evaluate the Aging and Degradation Phenomena Induced by Marine Environment Conditions on Commercial Plastic Granules. Polymers. 2022; 14(6):1111. https://doi.org/10.3390/polym14061111
Chicago/Turabian StyleDe Monte, Cristina, Marina Locritani, Silvia Merlino, Lucia Ricci, Agnese Pistolesi, and Simona Bronco. 2022. "An In Situ Experiment to Evaluate the Aging and Degradation Phenomena Induced by Marine Environment Conditions on Commercial Plastic Granules" Polymers 14, no. 6: 1111. https://doi.org/10.3390/polym14061111
APA StyleDe Monte, C., Locritani, M., Merlino, S., Ricci, L., Pistolesi, A., & Bronco, S. (2022). An In Situ Experiment to Evaluate the Aging and Degradation Phenomena Induced by Marine Environment Conditions on Commercial Plastic Granules. Polymers, 14(6), 1111. https://doi.org/10.3390/polym14061111