Towards a Circular Economy of Plastics: An Evaluation of the Systematic Transition to a New Generation of Bioplastics
Abstract
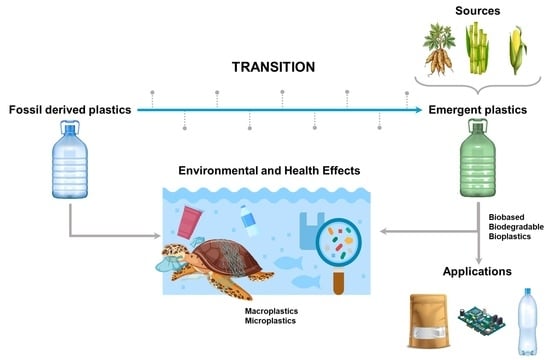
:1. Introduction
2. Historical Development of Plastic Materials: A Brief Timeline
3. Environmental Impact and Health Effects of Synthetic Plastics and Bioplastics
3.1. Plastic Degradation and Insertion into the Environment: An Overview
3.2. Macroplastics
3.3. Microplastics
3.4. Other Plastic-Derived Pollutants
4. New Generations of Plastics
4.1. Biodegradable Plastics from Fossil Resources
4.2. Biobased Non-Biodegradable Plastics
4.3. Biobased and Biodegradable Plastics
4.3.1. Starch
4.3.2. Cellulose
4.3.3. Polybutylene Succinate
4.3.4. Polylactic Acid
4.3.5. Polyhydroxyalkanoates
| Biopolymer | Microorganism | Production Scale | Employed Substrate | Productivity | Reference |
|---|---|---|---|---|---|
| Bacterial cellulose | Gluconacetobacter xylinum BC-11 | Wastewater | 1.77 g/L | [147] | |
| Gluconacetobacter xylinus | Wastewater | 0.659 g/L | [145] | ||
| Komagataeibacter saccharivorans | Static production in flasks | Crude distillery effluent | 1.24 g/L | [142] | |
| Gluconacetobacter oboediens | 1 L | Crude distillery effluent | 0.85 g/100 mL | [164] | |
| Gluconacetobacter sucrofermentans B-11267 | Flask | Whey | 5.45 g/L | [143] | |
| Gluconaceter xylinus BNKC19 | Pineapple peel | 12.3 g/L | [141] | ||
| Gluconacetobacter xylinum CGMCC No.2955 | Wastewater of candied jujube-processing industry | 2.25 g/L | [146] | ||
| Bacillus cabrialesii | Grass straw, grass husk, wheat husk, and corn cobs | [144] | |||
| PHA 1 | Pseudomonas putida KT2440 | 4 BB (3 L) | Waste vegetable oil | 1.91 g/L | [160] |
| Pseudomonas chlororaphis 555 | Pulse-fed batch fermentation (5 L) | Waste cooking oil | 13.87 g/L | [161] | |
| Pseudomonas resinovorans | 4 BB (15 L) | Grease-trap waste | 0.41 g/g maximum mcl-PHA2 61.8% | [165] | |
| Pseudomonas chlororaphis subsp. Aurantiaca | 4 BB (2 L) | Diluted fruit pulp waste | 0.15 g/g maximum mcl-PHA2 49% | [166] | |
| Halomonas campisalis MCM B-1027 | 5 SF 250 mL | Banana and orange peel | 0.329 g/L (banana) 0.11 g/L (orange) | [162] | |
| PHB 3 | Bacillus cereus | 5 SF 250 mL | Grape peel | 0.53 g/L | [159] |
| Bacillus subtilis | 5 SF | Papaya and orange peels | 11.65 g/L (papaya) 9.68 g/L (orange) | [167] | |
| Klebsiella pneumoniae | 5 SF 125 mL | Watermelon, papaya, orange, and banana peels | 22.61 g/L 23.72 g/L 23.38 g/L 25.11 g/L | [163] | |
| PHB 3 and mcl-PHA 2 | Cupriavidus necator, Pseudomonas citronellolis | 4 BB (10 L) | Apple pulp waste | 3.03 g/L | [168] |
| L-lactic | Bacillus coagulans | Sugarcane bagasse | 1.7 g/L·h | [152] | |
| Bacillus coagulans | Corn cob residue | 79 g/L | [169] | ||
| Enteroccus mundtii | 350 mL flask | Spent sulfite liquor | 56.3 g/L | [170] | |
| Lactic acid | Bacillus subtillis and Lactobacillus buchneri | Alfalfa silage | 44.2 g/L | [153] | |
| Lactobacillus bulgaricus, Strepto- coccus thermophilus, Lactobacillus acidophilus, Lactobacillus plantarum, and Lactobacillus casei. | 1000 mL bottles | Swine manure with apple waste | 28 g/L | [151] | |
| Lactobacillus rhamnosus B103 | Dairy industry waste | 143.7 g/L | [171] | ||
| D-lactic acid | Lactobacillus delbrueckii ssp. delbrueckii CECT286 | 4 BB (1 L) | Orange peel wastes | 6.72 g/L·h | [149] |
| Saccharomyces cerevisiae | Spent coffee grounds | 13.4 g/L | [150] |
4.3.6. Other Natural Sources
5. Green Industry of Plastics
5.1. Global Market, Business Cases, and Applications
5.2. Strategies for Plastic Reinsertion and Environmental Impact Mitigation
6. Challenges and Opportunities for Biodegradable Plastics from Production to Degradation, and Further Perspectives under Circular Economy
7. Legislation and Certifications for Biodegradable Plastics
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, S.; Chaudhary, J.; Sharma, B.; Verma, A.; Tamulevicius, S.; Thakur, V.K. Sustainability of bioplastics: Opportunities and challenges. Curr. Opin. Green Sustain. Chem. 2018, 13, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Ramkumar, M.; Balasubramani, K.; Santosh, M.; Nagarajan, R. The plastisphere: A morphometric genetic classification of plastic pollutants in the natural environment. Gondwana Res. 2021; in press. [Google Scholar] [CrossRef]
- Nagalakshmaiah, M.; Afrin, S.; Malladi, R.P.; Elkoun, S.; Robert, M.; Ansari, M.A.; Svedberg, A.; Karim, Z. Biocomposites: Present trends and challenges for the future. In Green Composites for Automotive Applications; Koronis, G., Silva, A., Eds.; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Cambridge, UK, 2019; pp. 197–215. ISBN 978-0-08-102177-4. [Google Scholar]
- Chen, Y.; Awasthi, A.K.; Wei, F.; Tan, Q.; Li, J. Single-use plastics: Production, usage, disposal, and adverse impacts. Sci. Total Environ. 2021, 752, 141772. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Mittal, D.; Aggarwal, N.K. Plastic accumulation during COVID-19: Call for another pandemic; bioplastic a step towards this challenge? Environ. Sci. Pollut. Res. 2022, 29, 11039–11053. [Google Scholar] [CrossRef]
- de Sousa, F.D.B. Plastic and its consequences during the COVID-19 pandemic. Environ. Sci. Pollut. Res. 2021, 28, 46067–46078. [Google Scholar] [CrossRef]
- Ali, S.S.; Elsamahy, T.; Koutra, E.; Kornaros, M.; El-Sheekh, M.; Abdelkarim, E.A.; Zhu, D.; Sun, J. Degradation of conventional plastic wastes in the environment: A review on current status of knowledge and future perspectives of disposal. Sci. Total Environ. 2021, 771, 144719. [Google Scholar] [CrossRef]
- William, R. Goal 14. Conserve and Sustainably Use the Oceans, Seas, and Marine Resources for Sustainable Development. In A New Era in Global Health Nursing and the United Nations 2030 Agenda for Sustainable Development; William, R., Ed.; Springer Publishing: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Walker, T.R. (Micro)plastics and the UN Sustainable Development Goals. Curr. Opin. Green Sustain. Chem. 2021, 30, 100497. [Google Scholar] [CrossRef]
- Arikan, E.B.; Ozsoy, H.D. A review: Investigation of bioplastics. J. Civ. Eng. Arch. 2015, 9, 188–192. [Google Scholar]
- Narancic, T.; Cerrone, F.; Beagan, N.; O’Connor, K.E. Recent advances in bioplastics: Application and biodegradation. Polymers 2020, 12, 920. [Google Scholar] [CrossRef] [Green Version]
- Bhagwat, G.; Gray, K.; Wilson, S.P.; Muniyasamy, S.; Vincent, S.G.T.; Bush, R.; Palanisami, T. Benchmarking Bioplastics: A Natural Step Towards a Sustainable Future. J. Polym. Environ. 2020, 28, 3055–3075. [Google Scholar] [CrossRef]
- Pellis, A.; Malinconico, M.; Guarneri, A.; Gardossi, L. Renewable polymers and plastics: Performance beyond the green. New Biotechnol. 2021, 60, 146–158. [Google Scholar] [CrossRef] [PubMed]
- The BPF—A History. Available online: https://www.bpf.co.uk/about_the_bpf/The_BPF_A_History.aspx (accessed on 3 February 2022).
- Gilbert, M. Plastics Materials: Introduction and Historical Development. In Brydson's Plastics Materials, 8th ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 2–18. [Google Scholar] [CrossRef]
- Zhang, G.; Yin, T.; Nian, G.; Suo, Z. Fatigue-resistant polyurethane elastomer composites. Extrem. Mech. Lett. 2021, 48, 101434. [Google Scholar] [CrossRef]
- Das, A.; Mahanwar, P. A brief discussion on advances in polyurethane applications. Adv. Ind. Eng. Polym. Res. 2020, 3, 93–101. [Google Scholar] [CrossRef]
- Jassal, M.; Agrawal, A.K.; Gupta, D.; Panwar, K. Aramid Fibers. In Handbook of Fibrous Materials; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2020; pp. 207–231. [Google Scholar]
- Jethy, B.; Paul, S.; Das, S.K.; Adesina, A.; Mustakim, S.M. Critical review on the evolution, properties, and utilization of plasticwastes for construction applications. J. Mater. Cycles Waste Manag. 2022, 24, 435–451. [Google Scholar] [CrossRef]
- Kalai Thendral, K.; Abraham Antony, D. Ballistic Impact analysis of 2D woven Kevlar/Basalt reinforced composite. IOP Conf. Ser. Mater. Sci. Eng. 2020, 912, 052023. [Google Scholar] [CrossRef]
- Pathak, S.; Sneha, C.; Mathew, B.B. Bioplastics: Its Timeline Based Scenario & Challenges. J. Polym. Biopolym. Phys. Chem. 2014, 2, 84–90. [Google Scholar] [CrossRef]
- Kabasci, S. Bio-Based Plastics Materials and Applications; Wiley: Hoboken, NJ, USA, 2014; ISBN 9781118676646. [Google Scholar]
- Bakar, N.F.A.; Othman, S.A. Corn Bio-plastics for Packaging Application. J. Des. Sustain. Environ. 2019, 1, 1–3. [Google Scholar]
- Plasctic Europe—Association of Plastics Manufactures; Plastic Europe: Brussels, Belgium, 2020; pp. 1–64.
- da Costa, J.P. The 2019 global pandemic and plastic pollution prevention measures: Playing catch-up. Sci. Total Environ. J. 2021, 774, 145806. [Google Scholar] [CrossRef]
- Li, P.; Wang, X.; Su, M.; Zou, X.; Duan, L.; Zhang, H. Characteristics of Plastic Pollution in the Environment: A Review. Bull. Environ. Contam. Toxicol. 2021, 107, 577–584. [Google Scholar] [CrossRef]
- Liang, Y.; Tan, Q.; Song, Q.; Li, J. An analysis of the plastic waste trade and management in Asia. Waste Manag. 2021, 119, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zuo, J.; Duan, X.; Wang, S.; Hu, K.; Chang, R. Impacts and mitigation measures of plastic waste: A critical review. Environ. Impact Assess. Rev. 2021, 90, 106642. [Google Scholar] [CrossRef]
- Zhang, K.; Hamidian, A.H.; Tubić, A.; Zhang, Y.; Fang, J.K.H.; Wu, C.; Lam, P.K.S. Understanding plastic degradation and microplastic formation in the environment: A review. Environ. Pollut. 2021, 274, 116554. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.A.; Corcoran, P. Effects of Chemical and Mechanical Weathering Processes on the Degradation of Plastic Debris on Marine Beaches Graduate Program in Geology. Ph.D. Thesis, Western University, London, ON, Canada, 2012. [Google Scholar]
- Ahmed, T.; Shahid, M.; Azeem, F.; Rasul, I.; Shah, A.A.; Noman, M.; Hameed, A.; Manzoor, N.; Manzoor, I.; Muhammad, S. Biodegradation of plastics: Current scenario and future prospects for environmental safety. Environ. Sci. Pollut. Res. 2018, 25, 7287–7298. [Google Scholar] [CrossRef] [PubMed]
- Blettler, M.C.M.; Ulla, M.A.; Rabuffetti, A.P.; Garello, N. Plastic pollution in freshwater ecosystems: Macro-, meso-, and microplastic debris in a floodplain lake. Environ. Monit. Assess. 2017, 189, 581. [Google Scholar] [CrossRef]
- Helm, P.A. Occurrence, Sources, Transport, and Fate of Microplastics in the Great Lakes–St. Lawrence River Basin. In Contaminants in the Great Lakes: An Introduction; Springer: Cham, Switzerland, 2020; pp. 15–47. [Google Scholar]
- Pojar, I.; Stănică, A.; Stock, F.; Kochleus, C.; Schultz, M.; Bradley, C. Sedimentary microplastic concentrations from the Romanian Danube River to the Black Sea. Sci. Rep. 2021, 11, 2000. [Google Scholar] [CrossRef] [PubMed]
- Napper, I.E.; Baroth, A.; Barrett, A.C.; Bhola, S.; Chowdhury, G.W.; Davies, B.F.R.; Duncan, E.M.; Kumar, S.; Nelms, S.E.; Hasan Niloy, M.N.; et al. The abundance and characteristics of microplastics in surface water in the transboundary Ganges River. Environ. Pollut. 2021, 274, 116348. [Google Scholar] [CrossRef]
- Whitehead, P.G.; Bussi, G.; Hughes, J.M.R.; Castro-Castellon, A.T.; Norling, M.D.; Jeffers, E.S.; Rampley, C.P.N.; Read, D.S.; Horton, A.A. Modelling microplastics in the river thames: Sources, sinks and policy implications. Water 2021, 13, 861. [Google Scholar] [CrossRef]
- Chae, Y.; Kim, D.; Kim, S.W.; An, Y.J. Trophic transfer and individual impact of nano-sized polystyrene in a four-species freshwater food chain. Sci. Rep. 2018, 8, 284. [Google Scholar] [CrossRef]
- Shen, M.; Zeng, Z.; Wen, X.; Ren, X.; Zeng, G.; Zhang, Y.; Xiao, R. Presence of microplastics in drinking water from freshwater sources: The investigation in Changsha, China. Environ. Sci. Pollut. Res. 2021, 28, 42313–42324. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, E.G.; Li, J.; Chen, Q.; Ma, L.; Zeng, E.Y.; Shi, H. A Review of Microplastics in Table Salt, Drinking Water, and Air: Direct Human Exposure. Environ. Sci. Technol. 2020, 54, 3740–3751. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Pathak, V.M.; Bagheri, A.R.; Bilal, M. Microplastic contaminants in the aqueous environment, fate, toxicity consequences, and remediation strategies. Environ. Res. 2021, 200, 111762. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, L.; Slat, B.; Ferrari, F.; Sainte-Rose, B.; Aitken, J.; Marthouse, R.; Hajbane, S.; Cunsolo, S.; Schwarz, A.; Levivier, A.; et al. Evidence that the Great Pacific Garbage Patch is rapidly accumulating plastic. Sci. Rep. 2018, 8, 4666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provencher, J.F.; Ammendolia, J.; Rochman, C.M.; Mallory, M.L. Assessing plastic debris in aquatic food webs: What we know and don’t know about uptake and trophic transfer. Environ. Rev. 2019, 27, 304–317. [Google Scholar] [CrossRef]
- Mbachu, O.; Jenkins, G.; Kaparaju, P.; Pratt, C. The rise of artificial soil carbon inputs: Reviewing microplastic pollution effects in the soil environment. Sci. Total Environ. 2021, 780, 146569. [Google Scholar] [CrossRef]
- Rillig, M.C.; Ziersch, L.; Hempel, S. Microplastic transport in soil by earthworms. Sci. Rep. 2017, 7, 1362. [Google Scholar] [CrossRef]
- Huerta Lwanga, E.; Mendoza Vega, J.; Ku Quej, V.; Chi, J.D.L.A.; Sanchez del Cid, L.; Chi, C.; Escalona Segura, G.; Gertsen, H.; Salánki, T.; van der Ploeg, M.; et al. Field evidence for transfer of plastic debris along a terrestrial food chain. Sci. Rep. 2017, 7, 14071. [Google Scholar] [CrossRef]
- Sun, Y.; Duan, C.; Cao, N.; Li, X.; Li, X.; Chen, Y.; Huang, Y.; Wang, J. Effects of microplastics on soil microbiome: The impacts of polymer type, shape, and concentration. Sci. Total Environ. 2022, 806, 150516. [Google Scholar] [CrossRef]
- Webb, H.K.; Arnott, J.; Crawford, R.J.; Ivanova, E.P. Plastic degradation and its environmental implications with special reference to poly(ethylene terephthalate). Polymers 2013, 5, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Verma, R.; Vinoda, K.S.; Papireddy, M.; Gowda, A.N.S. Toxic Pollutants from Plastic Waste—A Review. Procedia Environ. Sci. 2016, 35, 701–708. [Google Scholar] [CrossRef]
- Wright, S.L.; Ulke, J.; Font, A.; Chan, K.L.A.; Kelly, F.J. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ. Int. 2020, 136, 105411. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yan, N. A brief overview of renewable plastics. Mater. Today Sustain. 2020, 7–8, 100031. [Google Scholar] [CrossRef]
- Huang, W.; Song, B.; Liang, J.; Niu, Q.; Zeng, G.; Shen, M.; Deng, J.; Luo, Y.; Wen, X.; Zhang, Y. Microplastics and associated contaminants in the aquatic environment: A review on their ecotoxicological effects, trophic transfer, and potential impacts to human health. J. Hazard. Mater. 2021, 405, 124187. [Google Scholar] [CrossRef] [PubMed]
- Fanini, L.; Defeo, O.; Elliott, M.; Paragkamian, S.; Pinna, M.; Salvo, V.-S. Coupling beach ecology and macroplastics litter studies: Current trends and the way ahead. Mar. Pollut. Bull. 2021, 173, 112951. [Google Scholar] [CrossRef]
- Parolini, M.; De Felice, B.; Lamonica, C.; Cioccarelli, S.; Crosta, A.; Diolaiuti, G.; Ortenzi, M.A.; Ambrosini, R. Macroplastics contamination on glaciers from Italian Central-Western Alps. Environ. Adv. 2021, 5, 100084. [Google Scholar] [CrossRef]
- Pietz, O.; Augenstein, M.; Georgakakos, C.B.; Singh, K.; McDonald, M.; Walter, M.T. Macroplastic accumulation in roadside ditches of New York State’s Finger Lakes region (USA) across land uses and the COVID-19 pandemic. J. Environ. Manag. 2021, 298, 113524. [Google Scholar] [CrossRef]
- Patrício Silva, A.L.; Prata, J.C.; Walker, T.R.; Duarte, A.C.; Ouyang, W.; Barcelò, D.; Rocha-Santos, T. Increased plastic pollution due to COVID-19 pandemic: Challenges and recommendations. Chem. Eng. J. 2021, 405, 126683. [Google Scholar] [CrossRef]
- Pascuta, M.S.; Vodnar, D.C. Nanocarriers for Sustainable Active Packaging: An Overview during and Post COVID-19. Coatings 2022, 12, 102. [Google Scholar] [CrossRef]
- Blettler, M.C.M.; Mitchell, C. Dangerous traps: Macroplastic encounters affecting freshwater and terrestrial wildlife. Sci. Total Environ. 2021, 798, 149317. [Google Scholar] [CrossRef]
- Law, K.L.; Narayan, R. Reducing environmental plastic pollution by designing polymer materials for managed end-of-life. Nat. Rev. Mater. 2021, 7, 104–116. [Google Scholar] [CrossRef]
- Rech, S. Marine Plastic Pollution as a Vector for Non-Native Species Transport. Los Plásticos Contaminantes Marinos Como Vector de Transporte para Especies Exóticas. Ph.D. Thesis, Universidad de Oviedo, Oviedo, Spain, 2018. Available online: https://digibuo.uniovi.es/dspace/handle/10651/50378 (accessed on 11 March 2022).
- Naidoo, T.; Rajkaran, A.; Sershen. Impacts of plastic debris on biota and implications for human health: A South African perspective. S. Afr. J. Sci. 2020, 116, 1–8. [Google Scholar] [CrossRef]
- Machovsky-Capuska, G.E.; Amiot, C.; Denuncio, P.; Grainger, R.; Raubenheimer, D. A nutritional perspective on plastic ingestion in wildlife. Sci. Total Environ. 2019, 656, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.G.; Machovsky-Capuska, G.E.; Andrades, R. Plastic ingestion as an evolutionary trap: Toward a holistic understanding. Science 2021, 373, 56–60. [Google Scholar] [CrossRef]
- Honingh, D.; van Emmerik, T.; Uijttewaal, W.; Kardhana, H.; Hoes, O.; van de Giesen, N. Urban River Water Level Increase Through Plastic Waste Accumulation at a Rack Structure. Front. Earth Sci. 2020, 8, 28. [Google Scholar] [CrossRef]
- Umer, M.; Abbasi, B.N.; Sohail, A.; Tang, J.; Ullah, I.; Abbasi, H. Determinants of the Usage of Plastic Bags. Int. J. Bus. Econ. Manag. Works 2018, 5, 8–22. [Google Scholar]
- Superio, M.D.A.; Abreo, N.A.S. Plastic in Freshwater Ecosytems: A looming crisis in the Philippines. Philipp. Sci. Lett. 2020, 13, 1–5. [Google Scholar]
- Olofinnade, O.; Chandra, S.; Chakraborty, P. Recycling of high impact polystyrene and low-density polyethylene plastic wastes in lightweight based concrete for sustainable construction. Mater. Today Proc. 2020, 38, 2151–2156. [Google Scholar] [CrossRef]
- Piehl, S.; Leibner, A.; Löder, M.G.J.; Dris, R.; Bogner, C.; Laforsch, C. Identification and quantification of macro- and microplastics on an agricultural farmland. Sci. Rep. 2018, 8, 17950. [Google Scholar] [CrossRef] [Green Version]
- Suyadi; Manullang, C.Y. Distribution of plastic debris pollution and it is implications on mangrove vegetation. Mar. Pollut. Bull. 2020, 160, 111642. [Google Scholar] [CrossRef]
- Leonov, V.D.; Tiunov, A.V. Interaction of Invertebrates and Synthetic Polymers in Soil: A Review. Russ. J. Ecol. 2020, 51, 503–517. [Google Scholar] [CrossRef]
- Lehmann, A.; Leifheit, E.F.; Gerdawischke, M.; Rillig, M.C. Microplastics have shape- and polymer-dependent effects on soil aggregation and organic matter loss—An experimental and meta-analytical approach. Microplast. Nanoplast. 2021, 1, 1–14. [Google Scholar] [CrossRef]
- Rochman, C.M.; Brookson, C.; Bikker, J.; Djuric, N.; Earn, A.; Bucci, K.; Athey, S.; Huntington, A.; McIlwraith, H.; Munno, K.; et al. Rethinking microplastics as a diverse contaminant suite. Environ. Toxicol. Chem. 2019, 38, 703–711. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.; Cui, T.; Wu, H.; LeBlanc, G.A.; Wang, F.; An, L. A proposed nomenclature for microplastic contaminants. Mar. Pollut. Bull. 2021, 172, 112960. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, J.; Xing, B. Environmental source, fate, and toxicity of microplastics. J. Hazard. Mater. 2021, 407, 124357. [Google Scholar] [CrossRef] [PubMed]
- Hidayaturrahman, H.; Lee, T.-G. A study on characteristics of microplastic in wastewater of South Korea: Identification, quantification, and fate of microplastics during treatment process. Mar. Pollut. Bull. 2019, 146, 696–702. [Google Scholar] [CrossRef]
- Hebner, T.S.; Maurer-Jones, M.A. Characterizing microplastic size and morphology of photodegraded polymers placed in simulated moving water conditions. Environ. Sci. Process. Impacts 2020, 22, 398–407. [Google Scholar] [CrossRef]
- Burrows, S.D.; Frustaci, S.; Thomas, K.V.; Galloway, T. Expanding exploration of dynamic microplastic surface characteristics and interactions. TrAC-Trends Anal. Chem. 2020, 130, 115993. [Google Scholar] [CrossRef]
- Rosal, R. Morphological description of microplastic particles for environmental fate studies. Mar. Pollut. Bull. 2021, 171, 112716. [Google Scholar] [CrossRef]
- Vivekanand, A.C.; Mohapatra, S.; Tyagi, V.K. Microplastics in aquatic environment: Challenges and perspectives. Chemosphere 2021, 282, 131151. [Google Scholar] [CrossRef]
- Xiang, Y.; Jiang, L.; Zhou, Y.; Luo, Z.; Zhi, D.; Yang, J.; Lam, S.S. Microplastics and environmental pollutants: Key interaction and toxicology in aquatic and soil environments. J. Hazard. Mater. 2022, 422, 126843. [Google Scholar] [CrossRef]
- Muñiz-González, A.B.; Silva, C.J.M.; Patricio Silva, A.L.; Campos, D.; Pestana, J.L.T.; Martínez-Guitarte, J.L. Suborganismal responses of the aquatic midge Chironomus riparius to polyethylene microplastics. Sci. Total Environ. 2021, 783, 146981. [Google Scholar] [CrossRef] [PubMed]
- Zandaryaa, S. Freshwater Microplastic Pollution: The State of Knowledge and Research. In Plastics in the Aquatic Environment—Part I: Current Status and Challenges; Stock, F., Reifferscheid, G., Brennholt, N., Kostianaia, E., Eds.; The Handbook of Environmental Chemistry; Springer International Publishing: Cham, Switzerland, 2022; pp. 255–272. ISBN 978-3-030-84118-8. [Google Scholar]
- Fajardo, C.; Martín, C.; Costa, G.; Sánchez-Fortún, S.; Rodríguez, C.; de Lucas Burneo, J.J.; Nande, M.; Mengs, G.; Martín, M. Assessing the role of polyethylene microplastics as a vector for organic pollutants in soil: Ecotoxicological and molecular approaches. Chemosphere 2022, 288, 132460. [Google Scholar] [CrossRef] [PubMed]
- Godoy, V.; Blázquez, G.; Calero, M.; Quesada, L.; Martín-Lara, M.A. The potential of microplastics as carriers of metals. Environ. Pollut. 2019, 255, 113363. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, Y.; Chen, Y.; Zhang, W.; Zhao, J.; He, S.; Yang, C.; Zhang, T.; Tang, C.; Zhang, C.; et al. A review: Research progress on microplastic pollutants in aquatic environments. Sci. Total Environ. 2021, 766, 142572. [Google Scholar] [CrossRef] [PubMed]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Transport of persistent organic pollutants by microplastics in estuarine conditions. Estuar. Coast. Shelf Sci. 2014, 140, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A detailed review study on potential effects of microplastics and additives of concern on human health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef] [Green Version]
- Prata, J.C. Airborne microplastics: Consequences to human health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef]
- Vianello, A.; Jensen, R.L.; Liu, L.; Vollertsen, J. Simulating human exposure to indoor airborne microplastics using a Breathing Thermal Manikin. Sci. Rep. 2019, 9, 8670. [Google Scholar] [CrossRef]
- Carr, S.A. Sources and dispersive modes of micro-fibers in the environment. Integr. Environ. Assess. Manag. 2017, 13, 466–469. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Briou, B.; Caillol, S.; Robin, J.; Lapinte, V.; Briou, B.; Caillol, S.; Robin, J.; Non-endocrine, V.L. Non-endocrine disruptor effect for cardanol based plasticizer. Ind. Crops Prod. 2019, 130, 1–8. [Google Scholar] [CrossRef]
- Jamarani, R.; Erythropel, H.C.; Nicell, J.A.; Leask, R.L.; Marić, M. How green is your plasticizer? Polymers 2018, 10, 834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, M.; Xu, Y.; Dong, Y.; Song, Z.; Liu, Y. Accumulation and metabolism of di(n-butyl) phthalate (DBP) and di(2-ethylhexyl) phthalate (DEHP) in mature wheat tissues and their effects on detoxification and the antioxidant system in grain. Sci. Total Environ. 2019, 697, 133981. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.O.; Abrantes, N.; Gonçalves, F.J.M.; Nogueira, H.; Marques, J.C.; Gonçalves, A.M.M. Impacts of plastic products used in daily life on the environment and human health: What is known? Environ. Toxicol. Pharmacol. 2019, 72, 103239. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, S.; Tan, T.; Lee, S.T.; Cheng, S.H.; Lee, F.W.F.; Xu, S.J.L.; Ho, K.C. Toxicity and estrogenic endocrine disrupting activity of phthalates and their mixtures. Int. J. Environ. Res. Public Health 2014, 11, 3156–3168. [Google Scholar] [CrossRef]
- Sandeep, S.; Rowdhwal, S. Toxic Effects of Di-2-ethylhexyl Phthalate: An Overview. Biomed Res. Int. 2018, 2018, 1750368. [Google Scholar]
- Kotowska, U.; Kapelewska, J.; Sawczuk, R. Occurrence, removal, and environmental risk of phthalates in wastewaters, landfill leachates, and groundwater in Poland. Environ. Pollut. 2020, 267, 115643. [Google Scholar] [CrossRef]
- Xia, B.; Zhu, Q.; Zhao, Y.; Ge, W.; Zhao, Y.; Song, Q.; Zhou, Y.; Shi, H.; Zhang, Y. Phthalate exposure and childhood overweight and obesity: Urinary metabolomic evidence. Environ. Int. 2018, 121, 159–168. [Google Scholar] [CrossRef]
- Wen, Y.; Rattan, S.; Flaws, J.A.; Irudayaraj, J. Multi and transgenerational epigenetic effects of di-(2-ethylhexyl) phthalate (DEHP) in liver. Toxicol. Appl. Pharmacol. 2020, 402, 115123. [Google Scholar] [CrossRef]
- Fred-Ahmadu, O.H.; Bhagwat, G.; Oluyoye, I.; Benson, N.U.; Ayejuyo, O.O.; Palanisami, T. Interaction of chemical contaminants with microplastics: Principles and perspectives. Sci. Total Environ. 2020, 706, 135978. [Google Scholar] [CrossRef]
- Alp, A.C.; Yerlikaya, P. Phthalate ester migration into food: Effect of packaging material and time. Eur. Food Res. Technol. 2020, 246, 425–435. [Google Scholar] [CrossRef]
- Novotna, K.; Cermakova, L.; Pivokonska, L.; Cajthaml, T.; Pivokonsky, M. Microplastics in drinking water treatment—Current knowledge and research needs. Sci. Total Environ. 2019, 667, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Abdelmoez, W.; Dahab, I.; Ragab, E.M.; Abdelsalam, O.A.; Mustafa, A. Bio- and oxo-degradable plastics: Insights on facts and challenges. Polym. Adv. Technol. 2021, 32, 1981–1996. [Google Scholar] [CrossRef]
- Schiavo, S.; Oliviero, M.; Chiavarini, S.; Manzo, S. Adverse effects of oxo-degradable plastic leachates in freshwater environment. Environ. Sci. Pollut. Res. 2020, 27, 8586–8595. [Google Scholar] [CrossRef] [PubMed]
- Markowicz, F.; Szymańska-Pulikowska, A. Analysis of the possibility of environmental pollution by composted biodegradable and oxobiodegradable plastics. Geosciences 2019, 9, 460. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Morillas, A.; Beltrán-Villavicencio, M.; Alvarez-Zeferino, J.C.; Osada-Velázquez, M.H.; Moreno, A.; Martínez, L.; Yañez, J.M. Biodegradation and Ecotoxicity of Polyethylene Films Containing Pro-Oxidant Additive. J. Polym. Environ. 2016, 24, 221–229. [Google Scholar] [CrossRef]
- Foschi, E.; Bonoli, A. The commitment of packaging industry in the framework of the european strategy for plastics in a circular economy. Adm. Sci. 2019, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Goel, V.; Luthra, P.; Kapur, G.S.; Ramakumar, S.S.V. Biodegradable/Bio-plastics: Myths and Realities. J. Polym. Environ. 2021, 29, 3079–3104. [Google Scholar] [CrossRef]
- Tabba, H.D.; Hijji, Y.M.; Abu-Surrah, A.S. Olefin Polymerization. In Polyolefin Compounds and Materials: Fundamentals and Industrial Applications; AlMa’adeed, M.A.-A., Krupa, I., Eds.; Springer Series on Polymer and Composite Materials; Springer: Cham, Switzerland, 2016; pp. 51–77. ISBN 978-3-319-25982-6. [Google Scholar]
- Zeenat; Elahi, A.; Bukhari, D.A.; Shamim, S.; Rehman, A. Plastics degradation by microbes: A sustainable approach. J. King Saud Univ.-Sci. 2021, 33, 101538. [Google Scholar] [CrossRef]
- Eyheraguibel, B.; Traikia, M.; Fontanella, S.; Sancelme, M.; Bonhomme, S.; Fromageot, D.; Lemaire, J.; Lauranson, G.; Lacoste, J.; Delort, A.M. Characterization of oxidized oligomers from polyethylene films by mass spectrometry and NMR spectroscopy before and after biodegradation by a Rhodococcus rhodochrous strain. Chemosphere 2017, 184, 366–374. [Google Scholar] [CrossRef]
- Dang, T.C.H.; Nguyen, D.T.; Thai, H.; Nguyen, T.C.; Hien Tran, T.T.; Le, V.H.; Nguyen, V.H.; Tran, X.B.; Thao Pham, T.P.; Nguyen, T.G.; et al. Plastic degradation by thermophilic Bacillus sp. BCBT21 isolated from composting agricultural residual in Vietnam. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 015014. [Google Scholar] [CrossRef]
- Rujnić-Sokele, M.; Pilipović, A. Challenges and opportunities of biodegradable plastics: A mini review. Waste Manag. Res. 2017, 35, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, V.; Blanco, I. Polymers Analogous to Petroleum-Derived Ones for Packaging and Engineering Applications. Polymers 2020, 12, 1641. [Google Scholar] [CrossRef]
- Thiruchelvi, R.; Das, A.; Sikdar, E. Bioplastics as better alternative to petro plastic. Mater. Today Proc. 2021, 37, 1634–1639. [Google Scholar] [CrossRef]
- European Bioplastics. Available online: https://www.european-bioplastics.org (accessed on 12 September 2021).
- Sid, S.; Mor, R.S.; Kishore, A.; Sharanagat, V.S. Bio-sourced polymers as alternatives to conventional food packaging materials: A review. Trends Food Sci. Technol. 2021, 115, 87–104. [Google Scholar] [CrossRef]
- Garrido, R.; Cabeza, L.F.; Falguera, V. An overview of bioplastic research on its relation to national policies. Sustainability 2021, 13, 7848. [Google Scholar] [CrossRef]
- Fredi, G.; Dorigato, A. Recycling of bioplastic waste: A review. Adv. Ind. Eng. Polym. Res. 2021, 4, 159–177. [Google Scholar] [CrossRef]
- Jian, J.; Xiangbin, Z.; Xianbo, H. An overview on synthesis, properties and applications of poly(butylene-adipate-co-terephthalate)-PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Kim, S.Y. Application of the three-dimensionally printed biodegradable polycaprolactone (PCL) mesh in repair of orbital wall fractures. J. Cranio-Maxillofac. Surg. 2019, 47, 1065–1071. [Google Scholar] [CrossRef]
- Atiwesh, G.; Mikhael, A.; Parrish, C.C.; Banoub, J.; Le, T.A.T. Environmental impact of bioplastic use: A review. Heliyon 2021, 7, e07918. [Google Scholar] [CrossRef]
- Montava-Jordà, S.; Torres-Giner, S.; Ferrandiz-Bou, S.; Quiles-Carrillo, L.; Montanes, N. Development of sustainable and cost-competitive injection-molded pieces of partially bio-based polyethylene terephthalate through the valorization of cotton textile waste. Int. J. Mol. Sci. 2019, 20, 1378. [Google Scholar] [CrossRef] [Green Version]
- Shafqat, A.; Tahir, A.; Mahmood, A.; Tabinda, A.B.; Yasar, A.; Pugazhendhi, A. A review on environmental significance carbon foot prints of starch based bio-plastic: A substitute of conventional plastics. Biocatal. Agric. Biotechnol. 2020, 27, 101540. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Starch-based films: Major factors affecting their properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Zoungranan, Y.; Lynda, E.; Dobi-Brice, K.K.; Tchirioua, E.; Bakary, C.; Yannick, D.D. Influence of natural factors on the biodegradation of simple and composite bioplastics based on cassava starch and corn starch. J. Environ. Chem. Eng. 2020, 8, 104396. [Google Scholar] [CrossRef]
- Srinivasa Rao, L.; Naidu, C.D.; Tiwari, S. Investigation on synthesis, structure and degradability of starch based bioplastics. Mater. Today Proc. 2022, 49, 257–261. [Google Scholar] [CrossRef]
- Asrofi, M.; Sapuan, S.M.; Ilyas, R.A.; Ramesh, M. Characteristic of composite bioplastics from tapioca starch and sugarcane bagasse fiber: Effect of time duration of ultrasonication (Bath-Type). Mater. Today Proc. 2020, 46, 1626–1630. [Google Scholar] [CrossRef]
- Astuti, P.; Erprihana, A.A. Antimicrobial Edible Film from Banana Peels as Food Packaging. Abstract. Am. J. Oil Chem. Technol. 2014, 2, 65–70. [Google Scholar]
- Sujuthi, R.A.F.M.; Liew, K.C. Properties of bioplastic sheets made from different types of starch incorporated with recycled newspaper pulp. Trans. Sci. Technol. 2016, 3, 257–264. [Google Scholar]
- Maulida; Siagian, M.; Tarigan, P. Production of Starch Based Bioplastic from Cassava Peel Reinforced with Microcrystalline Celllulose Avicel PH101 Using Sorbitol as Plasticizer. J. Phys. Conf. Ser. 2016, 710, 012012. [Google Scholar] [CrossRef] [Green Version]
- Tsou, C.H.; Suen, M.C.; Yao, W.H.; Yeh, J.T.; Wu, C.S.; Tsou, C.Y.; Chiu, S.H.; Chen, J.C.; Wang, R.Y.; Lin, S.M.; et al. Preparation and characterization of Bioplastic-Based green renewable composites from tapioca with acetyl tributyl citrate as a plasticizer. Materials 2014, 7, 5617–5632. [Google Scholar] [CrossRef] [Green Version]
- Fabra, M.J.; Martínez-Sanz, M.; Gómez-Mascaraque, L.G.; Gavara, R.; López-Rubio, A. Structural and physicochemical characterization of thermoplastic corn starch films containing microalgae. Carbohydr. Polym. 2018, 186, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, C.; Cao, G.; Wang, D.; Ho, S.H. A sustainable solution to plastics pollution: An eco-friendly bioplastic film production from high-salt contained Spirulina sp. residues. J. Hazard. Mater. 2020, 388, 121773. [Google Scholar] [CrossRef] [PubMed]
- Mathiot, C.; Ponge, P.; Gallard, B.; Sassi, J.F.; Delrue, F.; Le Moigne, N. Microalgae starch-based bioplastics: Screening of ten strains and plasticization of unfractionated microalgae by extrusion. Carbohydr. Polym. 2019, 208, 142–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostafa, N.A.; Farag, A.A.; Abo-dief, H.M.; Tayeb, A.M. Production of biodegradable plastic from agricultural wastes. Arab. J. Chem. 2018, 11, 546–553. [Google Scholar] [CrossRef] [Green Version]
- Nigam, S.; Das, A.K.; Patidar, M.K. Synthesis, characterization and biodegradation of bioplastic films produced from Parthenium hysterophorus by incorporating a plasticizer (PEG600). Environ. Chall. 2021, 5, 100280. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Tye, Y.Y.; Saurabh, C.K.; Leh, C.P.; Lai, T.K.; Chong, E.W.N.; Nurul Fazita, M.R.; Hafiidz, J.M.; Banerjee, A.; Syakir, M.I. Biodegradable polymer films from seaweed polysaccharides: A review on cellulose as a reinforcement material. Express Polym. Lett. 2017, 11, 244–265. [Google Scholar] [CrossRef]
- Velásquez-Riaño, M.; Bojacá, V. Production of bacterial cellulose from alternative low-cost substrates. Cellulose 2017, 24, 2677–2698. [Google Scholar] [CrossRef]
- Soemphol, W.; Hongsachart, P.; Tanamool, V. Production and characterization of bacterial cellulose produced from agricultural by-product by Gluconacetobacter strains. Mater. Today Proc. 2018, 5, 11159–11168. [Google Scholar] [CrossRef]
- Gayathri, G.; Srinikethan, G. Bacterial Cellulose production by K. saccharivorans BC1 strain using crude distillery effluent as cheap and cost effective nutrient medium. Int. J. Biol. Macromol. 2019, 138, 950–957. [Google Scholar] [CrossRef]
- Revin, V.; Liyaskina, E.; Nazarkina, M.; Bogatyreva, A.; Shchankin, M. Cost-effective production of bacterial cellulose using acidic food industry by-products. Braz. J. Microbiol. 2018, 49, 151–159. [Google Scholar] [CrossRef]
- Sadalage, P.S.; Pawar, K.D. Production of microcrystalline cellulose and bacterial nanocellulose through biological valorization of lignocellulosic biomass wastes. J. Clean. Prod. 2021, 327, 129462. [Google Scholar] [CrossRef]
- Huang, C.; Guo, H.J.; Xiong, L.; Wang, B.; Shi, S.L.; Chen, X.F.; Lin, X.Q.; Wang, C.; Luo, J.; Chen, X. De Using wastewater after lipid fermentation as substrate for bacterial cellulose production by Gluconacetobacter xylinus. Carbohydr. Polym. 2016, 136, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, L.; Hua, J.; Jia, S.; Zhang, J.; Liu, H. Production of nano bacterial cellulose from waste water of candied jujube-processing industry using Acetobacter xylinum. Carbohydr. Polym. 2015, 120, 115–119. [Google Scholar] [CrossRef]
- Zhao, H.; Xia, J.; Wang, J.; Yan, X.; Wang, C.; Lei, T.; Xian, M.; Zhang, H. Production of bacterial cellulose using polysaccharide fermentation wastewater as inexpensive nutrient sources. Biotechnol. Biotechnol. Equip. 2018, 32, 350–356. [Google Scholar] [CrossRef] [Green Version]
- Vytejčková, S.; Vápenka, L.; Hradecký, J.; Dobiáš, J.; Hajšlová, J.; Loriot, C.; Vannini, L.; Poustka, J. Testing of polybutylene succinate based films for poultry meat packaging. Polym. Test. 2017, 60, 357–364. [Google Scholar] [CrossRef]
- de la Torre, I.; Acedos, M.G.; Ladero, M.; Santos, V.E. On the use of resting L. delbrueckii spp. delbrueckii cells for D-lactic acid production from orange peel wastes hydrolysates. Biochem. Eng. J. 2019, 145, 162–169. [Google Scholar] [CrossRef]
- Kim, J.W.; Jang, J.H.; Yeo, H.J.; Seol, J.; Kim, S.R.; Jung, Y.H. Lactic Acid Production from a Whole Slurry of Acid-Pretreated Spent Coffee Grounds by Engineered Saccharomyces cerevisiae. Appl. Biochem. Biotechnol. 2019, 189, 206–216. [Google Scholar] [CrossRef]
- Lian, T.; Zhang, W.; Cao, Q.; Wang, S.; Dong, H. Enhanced lactic acid production from the anaerobic co-digestion of swine manure with apple or potato waste via ratio adjustment. Bioresour. Technol. 2020, 318, 124237. [Google Scholar] [CrossRef]
- Alves de Oliveira, R.; Schneider, R.; Vaz Rossell, C.E.; Maciel Filho, R.; Venus, J. Polymer grade L-lactic acid production from sugarcane bagasse hemicellulosic hydrolysate using Bacillus coagulans. Bioresour. Technol. Rep. 2019, 6, 26–31. [Google Scholar] [CrossRef]
- Bai, J.; Xu, D.; Xie, D.; Wang, M.; Li, Z.; Guo, X. Effects of antibacterial peptide-producing Bacillus subtilis and Lactobacillus buchneri on fermentation, aerobic stability, and microbial community of alfalfa silage. Bioresour. Technol. 2020, 315, 123881. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, P.; Tao, F. L-Lactic acid production by Bacillus coagulans through simultaneous saccharification and fermentation of lignocellulosic corncob residue. Bioresour. Technol. Rep. 2019, 6, 131–137. [Google Scholar] [CrossRef]
- Mulla, M.Z.; Rahman, M.R.T.; Marcos, B.; Tiwari, B.; Pathania, S. Poly lactic acid (Pla) nanocomposites: Effect of inorganic nanoparticles reinforcement on its performance and food packaging applications. Molecules 2021, 26, 1967. [Google Scholar] [CrossRef] [PubMed]
- Rajesh Banu, J.; Kavitha, S.; Yukesh Kannah, R.; Poornima Devi, T.; Gunasekaran, M.; Kim, S.H.; Kumar, G. A review on biopolymer production via lignin valorization. Bioresour. Technol. 2019, 290, 121790. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.B.S.; Malafaia, C.B. Perspectives on the production, structural characteristics and potential applications of bioplastics derived from polyhydroxyalkanoates. Int. J. Biol. Macromol. 2018, 107, 615–625. [Google Scholar] [CrossRef]
- Rahman, A.; Miller, C.D. Microalgae as a Source of Bioplastics; Elsevier B.V.: Amsterdam, The Netherlands, 2017; ISBN 9780444640413. [Google Scholar]
- Andler, R.; Valdés, C.; Urtuvia, V.; Andreeßen, C.; Díaz-Barrera, A. Fruit residues as a sustainable feedstock for the production of bacterial polyhydroxyalkanoates. J. Clean. Prod. 2021, 307, 127236. [Google Scholar] [CrossRef]
- Borrero-de Acuña, J.M.; Aravena-Carrasco, C.; Gutierrez-Urrutia, I.; Duchens, D.; Poblete-Castro, I. Enhanced synthesis of medium-chain-length poly(3-hydroxyalkanoates) by inactivating the tricarboxylate transport system of Pseudomonas putida KT2440 and process development using waste vegetable oil. Process Biochem. 2019, 77, 23–30. [Google Scholar] [CrossRef]
- Ruiz, C.; Kenny, S.T.; Narancic, T.; Babu, R.; Connor, K.O. Conversion of waste cooking oil into medium chain polyhydroxyalkanoates in a high cell density fermentation. J. Biotechnol. 2019, 306, 9–15. [Google Scholar] [CrossRef]
- Kulkarni, S.O.; Kanekar, P.P.; Jog, J.P.; Sarnaik, S.S.; Nilegaonkar, S.S. Production of copolymer, poly (hydroxybutyrate-co-hydroxyvalerate) by Halomonas campisalis MCM B-1027 using agro-wastes. Int. J. Biol. Macromol. 2015, 72, 784–789. [Google Scholar] [CrossRef]
- Valdez-Calderón, A.; Barraza-Salas, M.; Quezada-Cruz, M.; Islas-Ponce, M.A.; Angeles-Padilla, A.F.; Carrillo-Ibarra, S.; Rodríguez, M.; Rojas-Avelizapa, N.G.; Garrido-Hernández, A.; Rivas-Castillo, A.M. Production of polyhydroxybutyrate (PHB) by a novel Klebsiella pneumoniae strain using low-cost media from fruit peel residues. Biomass Convers. Biorefin. 2020. [Google Scholar] [CrossRef]
- Jahan, F.; Kumar, V.; Saxena, R.K. Distillery effluent as a potential medium for bacterial cellulose production: A biopolymer of great commercial importance. Bioresour. Technol. 2018, 250, 922–926. [Google Scholar] [CrossRef]
- Acedos, M.G.; Moreno-Cid, J.; Verdú, F.; González, J.A.; Tena, S.; López, J.C. Exploring the potential of slaughterhouse waste valorization: Development and scale-up of a new bioprocess for medium-chain length polyhydroxyalkanoates production. Chemosphere 2022, 287, 132401. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.R.; Araújo, D.; Freitas, P.; Marques, A.C.; Alves, V.D.; Sevrin, C.; Grandfils, C.; Fortunato, E.; Reis, M.A.M.; Freitas, F. Production of medium-chain-length polyhydroxyalkanoates by Pseudomonas chlororaphis subsp. aurantiaca: Cultivation on fruit pulp waste and polymer characterization. Int. J. Biol. Macromol. 2021, 167, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Haque, S.; El-Enshasy, H.A.; Singh, V.; Mishra, B.N. RSM–GA based optimization of bacterial PHA production and In Silico modulation of citrate synthase for enhancing PHA production. Biomolecules 2019, 9, 872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebocho, A.T.; Pereira, J.R.; Neves, L.A.; Alves, V.D.; Sevrin, C.; Grandfils, C.; Freitas, F.; Reis, M.A.M. Preparation and characterization of films based on a natural p(3hb)/mcl-pha blend obtained through the co-culture of cupriavidus necator and pseudomonas citronellolis in apple pulp waste. Bioengineering 2020, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Li, B.; Zhou, L.; Yu, S.; Su, Z.; Song, J.; Sun, Q.; Sha, O.; Wang, X.; Jiang, W.; et al. Prodigiosin inhibits Wnt/β-catenin signaling and exerts anticancer activity in breast cancer cells. Proc. Natl. Acad. Sci. USA 2016, 113, 13150–13155. [Google Scholar] [CrossRef] [Green Version]
- Hoheneder, R.; Fitz, E.; Bischof, R.H.; Russmayer, H.; Ferrero, P.; Peacock, S.; Sauer, M. Efficient conversion of hemicellulose sugars from spent sulfite liquor into optically pure L-lactic acid by Enterococcus mundtii. Bioresour. Technol. 2021, 333, 125215. [Google Scholar] [CrossRef]
- Bernardo, M.P.; Coelho, L.F.; Sass, D.C.; Contiero, J. L-(+)-Lactic acid production by Lactobacillus rhamnosus B103 from dairy industry waste. Braz. J. Microbiol. 2016, 47, 640–646. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.; Yusoff, S.; Ng, C.G.; Lim, P.E.; Ching, Y.C. Bioplastic made from seaweed polysaccharides with green production methods. J. Environ. Chem. Eng. 2021, 9, 105895. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Hermosilla, J.; Acevedo, F.; Navia, R. The influences of solvents on the electrospun of whole Scenedesmus almeriensis and poly(ethylene oxide) for the preparation of composite nanofibers. Compos. Commun. 2018, 10, 18–24. [Google Scholar] [CrossRef]
- Kim, Y.; Ruedy, D. Mushroom Packages an Ecovative Approach in Packaging Industry. In Handbook of Engaged Sustainability; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–25. [Google Scholar]
- Wu, J. Extraction of Chitin Nanofibers and Utilization for Sustainable Composites and Foams. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2014. [Google Scholar]
- Nandakumar, A.; Chuah, J.A.; Sudesh, K. Bioplastics: A boon or bane? Renew. Sustain. Energy Rev. 2021, 147, 111237. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Takada, K.; Elkasaby, T.; Pangestu, R.; Toyoshima, M.; Kahar, P.; Ogino, C.; Kaneko, T.; Kondo, A. Recent advances in lignocellulosic biomass white biotechnology for bioplastics. Bioresour. Technol. 2022, 344, 126165. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Fontaine, G.; Bourbigot, S. Recent developments in fire retardancy of polybutylene succinate. Polym. Degrad. Stab. 2021, 183, 109466. [Google Scholar] [CrossRef]
- QMILK. The Material of the Future. Available online: https://www.qmilkfiber.eu/?lang=en (accessed on 3 February 2022).
- Bioplástico–Biofase. Available online: https://biofase.com.mx/bioplastico (accessed on 3 February 2022).
- NatureWorks|What Is Ingeo. Available online: https://www.natureworksllc.com/What-is-Ingeo (accessed on 3 February 2022).
- Thiounn, T.; Smith, R.C. Advances and approaches for chemical recycling of plastic waste. J. Polym. Sci. 2020, 58, 1347–1364. [Google Scholar] [CrossRef] [Green Version]
- Meys, R.; Frick, F.; Westhues, S.; Sternberg, A.; Klankermayer, J.; Bardow, A. Towards a circular economy for plastic packaging wastes—The environmental potential of chemical recycling. Resour. Conserv. Recycl. 2020, 162, 105010. [Google Scholar] [CrossRef]
- Wei, R.; Tiso, T.; Bertling, J.; O’Connor, K.; Blank, L.M.; Bornscheuer, U.T. Possibilities and limitations of biotechnological plastic degradation and recycling. Nat. Catal. 2020, 3, 867–871. [Google Scholar] [CrossRef]
- Hatti-Kaul, R.; Nilsson, L.J.; Zhang, B.; Rehnberg, N.; Lundmark, S. Designing Biobased Recyclable Polymers for Plastics. Trends Biotechnol. 2020, 38, 50–67. [Google Scholar] [CrossRef]
- Tuominen, J.; Kylma, J.; Kapanen, A.; Venelampi, O.; Itävaara, M.; Seppälä, J. Biodegradation of lactic acid based polymers under controlled composting conditions and evaluation of the ecotoxicological impact. Biomacromolecules 2002, 3, 445–455. [Google Scholar] [CrossRef]
- Hoffmann, J.; Řezníčková, I.; Kozáková, J.; Růžička, J.; Alexy, P.; Bakoš, D.; Precnerová, L. Assessing biodegradability of plastics based on poly(vinyl alcohol) and protein wastes. Polym. Degrad. Stab. 2003, 79, 511–519. [Google Scholar] [CrossRef]
- Muenmee, S.; Chiemchaisri, W.; Chiemchaisri, C. Microbial consortium involving biological methane oxidation in relation to the biodegradation of waste plastics in a solid waste disposal open dump site. Int. Biodeterior. Biodegrad. 2015, 102, 172–181. [Google Scholar] [CrossRef]
- Massardier-Nageotte, V.; Pestre, C.; Cruard-Pradet, T.; Bayard, R. Aerobic and anaerobic biodegradability of polymer films and physico-chemical characterization. Polym. Degrad. Stab. 2006, 91, 620–627. [Google Scholar] [CrossRef]
- Tournier, V.; Topham, C.M.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.L.; Texier, H.; Gavalda, S.; et al. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 2020, 580, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Breite, D.; Song, C.; Gräsing, D.; Ploss, T.; Hille, P.; Schwerdtfeger, R.; Matysik, J.; Schulze, A.; Zimmermann, W. Biocatalytic Degradation Efficiency of Postconsumer Polyethylene Terephthalate Packaging Determined by Their Polymer Microstructures. Adv. Sci. 2019, 6, 1900491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcilla, A.; Beltrán, M.I.; Navarro, R. Evolution of products during the degradation of polyethylene in a batch reactor. J. Anal. Appl. Pyrolysis 2009, 86, 14–21. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Insura, N.; Williams, P.T. Composition of products from the pyrolysis of polyethylene and polystyrene in a closed batch reactor: Effects of temperature and residence time. J. Anal. Appl. Pyrolysis 2009, 86, 293–303. [Google Scholar] [CrossRef]
- Artetxe, M.; Lopez, G.; Amutio, M.; Barbarias, I.; Arregi, A.; Aguado, R.; Bilbao, J.; Olazar, M. Styrene recovery from polystyrene by flash pyrolysis in a conical spouted bed reactor. Waste Manag. 2015, 45, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Ismail Khan, M.; Khan, H.; Ishaq, M.; Tariq, R.; Gul, K.; Ahmad, W. Pyrolysis study of polypropylene and polyethylene into premium oil products. Int. J. Green Energy 2015, 12, 663–671. [Google Scholar] [CrossRef]
- López-Mondéjar, R.; Zühlke, D.; Becher, D.; Riedel, K.; Baldrian, P. Cellulose and hemicellulose decomposition by forest soil bacteria proceeds by the action of structurally variable enzymatic systems. Sci. Rep. 2016, 6, 25279. [Google Scholar] [CrossRef]
- Nawaz, A.; Hasan, F.; Shah, A.A. Degradation of poly(ε-caprolactone) (PCL) by a newly isolated Brevundimonas sp. strain MRL-AN1 from soil. FEMS Microbiol. Lett. 2015, 362, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Dobrucka, R. Bioplastic packaging materials in circular economy. Logforum 2019, 15, 129–137. [Google Scholar] [CrossRef]
- Galgani, L.; Loiselle, S.A. Plastic pollution impacts on marine carbon biogeochemistry. Environ. Pollut. 2021, 268, 115598. [Google Scholar] [CrossRef]
- Alves de Oliveira, R.; Komesu, A.; Vaz Rossell, C.E.; Maciel Filho, R. Challenges and opportunities in lactic acid bioprocess design—From economic to production aspects. Biochem. Eng. J. 2018, 133, 219–239. [Google Scholar] [CrossRef]
- Otoni, C.G.; Azeredo, H.M.C.; Mattos, B.D.; Beaumont, M.; Correa, D.S.; Rojas, O.J. The Food–Materials Nexus: Next Generation Bioplastics and Advanced Materials from Agri-Food Residues. Adv. Mater. 2021, 33, 2102520. [Google Scholar] [CrossRef]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef]
- Briassoulis, D.; Pikasi, A.; Hiskakis, M. Recirculation potential of post-consumer /industrial bio-based plastics through mechanical recycling—Techno-economic sustainability criteria and indicators. Polym. Degrad. Stab. 2021, 183, 109217. [Google Scholar] [CrossRef]
- Jõgi, K.; Bhat, R. Valorization of food processing wastes and by-products for bioplastic production. Sustain. Chem. Pharm. 2020, 18, 100326. [Google Scholar] [CrossRef]
- Dilkes-Hoffman, L.; Ashworth, P.; Laycock, B.; Pratt, S.; Lant, P. Public attitudes towards bioplastics—Knowledge, perception and end-of-life management. Resour. Conserv. Recycl. 2019, 151, 104479. [Google Scholar] [CrossRef]
- Geueke, B.; Groh, K.; Muncke, J. Food packaging in the circular economy: Overview of chemical safety aspects for commonly used materials. J. Clean. Prod. 2018, 193, 491–505. [Google Scholar] [CrossRef]
- Pelamatti, T.; Rios-Mendoza, L.M.; Hoyos-Padilla, E.M.; Galván-Magaña, F.; De Camillis, R.; Marmolejo-Rodríguez, A.J.; González-Armas, R. Contamination knows no borders: Toxic organic compounds pollute plastics in the biodiversity hotspot of Revillagigedo Archipelago National Park, Mexico. Mar. Pollut. Bull. 2021, 170, 112623. [Google Scholar] [CrossRef]
- Meereboer, K.W.; Misra, M.; Mohanty, A.K. Review of recent advances in the biodegradability of polyhydroxyalkanoate (PHA) bioplastics and their composites. Green Chem. 2020, 22, 5519–5558. [Google Scholar] [CrossRef]
- Di Bartolo, A.; Infurna, G.; Dintcheva, N.T. A review of bioplastics and their adoption in the circular economy. Polymers 2021, 13, 1229. [Google Scholar] [CrossRef] [PubMed]
- Talan, A.; Pokhrel, S.; Tyagi, R.D.; Drogui, P. Biorefinery strategies for microbial bioplastics production: Sustainable pathway towards Circular Bioeconomy. Bioresour. Technol. Rep. 2022, 17, 100875. [Google Scholar] [CrossRef]
- Devadas, V.V.; Khoo, K.S.; Chia, W.Y.; Chew, K.W.; Munawaroh, H.S.H.; Lam, M.K.; Lim, J.W.; Ho, Y.C.; Lee, K.T.; Show, P.L. Algae biopolymer towards sustainable circular economy. Bioresour. Technol. 2021, 325, 124702. [Google Scholar] [CrossRef] [PubMed]
- Teleky, B.E.; Vodnar, D.C. Recent advances in biotechnological itaconic acid production, and application for a sustainable approach. Polymers 2021, 13, 3574. [Google Scholar] [CrossRef]
- Filiciotto, L.; Rothenberg, G. Biodegradable Plastics: Standards, Policies, and Impacts. ChemSusChem 2021, 14, 56–72. [Google Scholar] [CrossRef] [PubMed]
- OK Compost & Seedling. Available online: https://www.tuv-at.be/green-marks/certifications/ok-compost-seedling/ (accessed on 3 February 2022).
- OK Biodegradable. Available online: https://www.tuv-at.be/green-marks/certifications/ok-biodegradable/ (accessed on 3 February 2022).
- OK Biobased. Available online: https://www.tuv-at.be/green-marks/certifications/ok-biobased/ (accessed on 3 February 2022).
- NEN Bio-Based Content. Available online: https://www.tuv-at.be/green-marks/certifications/nen-bio-based-content/ (accessed on 3 February 2022).






| Biopolymer | Source | Reinforcement | Plasticizer | Reference |
|---|---|---|---|---|
| Starch | Corn and cassava | Cola cordifolia | Glycerol | [127] |
| Rice and corn | Ethanol, rice, and olive oil | Sorbitol | [128] | |
| Tapioca | Sugarcane bagasse fiber | Glycerol | [129] | |
| Banana peel | Glycerol | [130] | ||
| Corn, potato, and cassava | Recycled newspaper pulp fiber | Glycerol | [131] | |
| Cassava | Microcrystalline cellulose | Sorbitol | [132] | |
| Tapioca | Acetyl Tributyl Citrate | [133] | ||
| Corn | Microalgae Nannochloropsis | Glycerol | [134] | |
| Microalgae Spirulina sp. | Poly(vinyl alcohol) | [135] | ||
| Microalgae Chlamydomonas reinhardtii 11-32A | Glycerol | [136] | ||
| Cellulose acetate | Cotton linters | Polyethylene glycol 600 | [137] | |
| Flax fibers | Polyethylene glycol 600 | [137] | ||
| Parthenium hysterophorus weed | Polyethylene glycol 600 | [138] |
| Biopolymer | Applications | Properties | Cost USD/kg | Reference |
|---|---|---|---|---|
| Starch | Translucent film, net packaging, bags, containers, egg boxes, sandwich bags, capsules, carrier bags, drinking straws, drug-release films | Sealable, durable, fine finishing, barrier for water | 0.5–2.0 | [118] |
| Cellulose | Packaging films, films, transparent films, barrier films, cups for cold drinks, plates and dishes, cups for hot drinks, labels | Sealable, barrier for water, transparent, approved for direct food contact | 1.8–4.0 | [118] |
| PLA 1 | Bottles, cups, transparent films, containers, dishes, fruit nets, top-covering films, trays, tea bags, ice cream cups, carrier bags | Approved for direct contact, transparent, sealable, durable, barrier for water and oxygen | 4.0–6.0 | [118] |
| PHA 2 | Disposable cups, plates, and cutlery; Tetra Pak covers; tubes to produce vegetable seedlings; agrochemical packaging; textile fibers; electronic equipment components | Physical properties like conventional plastics; insoluble in water, nontoxic, and biocompatible; present piezoelectric properties; some PHA films exhibit gas-barrier properties | 2.4–5.5 | [157] |
| Bio-PE 3 | Food packaging, cosmetics, personal care, automotive and toy applications | Equal in its chemical, physical, and mechanical properties to fossil-based PE | 2.3 | [115] |
| PBS 4 | Biopackaging, tissue-engineering, and medical materials; agriculture mulch film; plant pots; hygiene products | High processability, good mechanical properties, thermal properties | 4.0–10.0 | [120,178] |
| PLC 5 | Drug delivery systems and tissue-engineering scaffolds | High toughness and flexibility, biocompatibility, and slow degradation in in vivo conditions | 4.5–10.0 | [120] |
| PBAT 6 | Compostable organic waste bags, agricultural mulch films, packaging (wrapping) films, disposable tableware | Excellent toughness, improved wear and fracture resistance, good chemical resistance to water and oils, high strain at break | 3.8–5.8 | [120] |
| Company | Bioplastic | Applications | Properties | Country |
|---|---|---|---|---|
| Plantic®® 1 | Starch | Food and goods packing, agricultural plastics | Biodegradable and compostable | Australia |
| Mater-Bi®®- Novamont 2 | Starch | Bags, toys, food, and cosmetic containers | Biodegradable and compostable | Italy |
| BIOPAR®® 3 | Starch | Bags and flexible packaging | Biodegradable | Portugal |
| Biofase®® 4 | Starch-based | Cutlery | Biodegradable | Mexico |
| Solany®® 5 | Starch-derived | Flowerpots, tomato clips, cultivation tubes, promotional items, toys, CD and DVD trays, protection covers for packaging, cup holders, plant stakes, golf tees | Biodegradable and compostable | Canada |
| Bionolle StarclaTM -Showa Denko 6 | Starch- and PLA-based | Bioplastics | Biodegradable and compostable | Japan |
| BIOFRONT-Teijin 7 | Stereocomplex PLA 13 | Automotive, films and packaging, molded parts for civil engineering and construction, parts for electronic devices | Biodegradable | Japan |
| IngeoTM-Nature Works 8 | PLA 13 | Bottles, gift cards, durable goods, films, layers of paper, cups and containers for food, fabrics, clothing, disposables, and base material for many compounds | Biodegradable and compostable | USA |
| WeforYou 9 | PLA 13 | Reusable bags | Biodegradable and compostable | Austria |
| Total-Corbion 10 | PLA 13 | Biopolymer | Biodegradable and compostable | Netherlands/ Thailand |
| Danimer Scientific 11 | PHA 14 | Straws, cups, lids, bottles, produce bags, shopping bags, cutlery, diaper linings, plates, wipes, toys, trash bags, seals, labels, glues, and much more | Biodegradable and compostable | USA 15 |
| Qmilk 12 | Milk protein | Textile fibers | Compostable | Germany |
| Plastic | Biodegradation Conditions (Chemical/Biological) | Biodegradation | Reference |
|---|---|---|---|
| Cassava-based bioplastic | Burial-soil pH measurement, 14 days (using microorganisms) | [127] | |
| Starch (TPS)–PLA 1 | Ulomoides dermestoides, 5 days | TPS biodigestion—biodegradation (80%) and PLA biodisintegration (50%) | [186] |
| PHA 2 | Alluvial-type soil, 35% soil moisture, 60 days | 35% | [187] |
| HDPE 3 | Incubation with microbial consortium, 357 days | 15% | [188] |
| LDPE 4 | 4.96% | ||
| PP 5 | 6.7% | ||
| PS 6 | 5.29% | ||
| Incubation under standard test aerobic and anaerobic conditions | Aerobic conditions, 117 days | [189] | |
| PHB 7 | PHB 7 83% | ||
| PBHV 8 87.4% | |||
| PHBV 8 | PCL 10 77.6% | ||
| PBS 9 | Anaerobic, 77 days | ||
| PCL 10 | PHB 7 83.9% | ||
| PLA 1 | PBHV 8 81.2% | ||
| PET 11 | PET7 hydrolase enzyme, 10 h | 90% | [190] |
| PET 11 | Recombinant bacterial polyester hydro- lase TfCut2, expressed in Bacillus subtilis, 70 °C, 96 h | 50% | [191] |
| LDPE 2 and HDPE 3 | Thermal degradation (pyrolysis), 30 to 550 °C at 5 °C min−1 | 1-oleofins and n-paraffins if C2–C6 were the major products | [192] |
| LDPE 2 and PS 6 | Pyrolysis, 300–500 °C, nitrogen pressure of 0.3 MPa | LDPE 2 was degraded to oil at 425 °C PS 6 was degraded at around 350 °C | [193] |
| PS 6 | Pyrolysis, room temperature 800 °C under inert atmosphere | 70% | [194] |
| Polyethylene (HDPE) 3 pellets | Thermal pyrolysis, 350 °C | 81%; the oil consisted mainly of paraffinic hydrocarbons, most of which contained between 6 and 16 carbon atoms | [195] |
| Cellulose | Enzymatic degradation (endoglucanases, β-glucosidases, endoxylanases, β-xylosidases, mannosidases), 7 days | 0.5% (w/v) | [196] |
| PCL 10 | Enzymatic degradation (external PCL 5 depolymerase), 10 days | >80% | [197] |
| Country | Nomenclature of the Standard | Title of Standard |
|---|---|---|
| Mexico | NMX-E-273-NYCE-2019 | Plastic Industry—Compostable plastics—Specifications and essay methods |
| NMX-E-267-CNCP-2016 | Plastic industry—Biobased plastics—Essay methods | |
| USA | ASTM D5071-06(2013) | Standard practice for exposure of photodegradable plastics in a xenon arc apparatus |
| ASTM D5208-14 | Standard practice for fluorescent ultraviolet (UV) exposure of photodegradable plastics | |
| ASTM D5272-08(2013) | Standard practice for outdoor exposure testing of photodegradable plastics | |
| ASTM D5338-15 | Standard test method for determining aerobic biodegradation of plastic materials under controlled composting conditions, incorporating thermophilic temperatures | |
| ASTM D5511-18 | Standard test method for determining anaerobic biodegradation of plastic materials under high-solids anaerobic-digestion conditions | |
| ASTM D5526-18 | Standard test method for determining anaerobic biodegradation of plastic materials under accelerated landfill conditions | |
| ASTM D5988-18 | Standard test method for determining aerobic biodegradation of plastic materials in soil | |
| ASTM D6400-19 | Standard specification for labeling of plastics designed to be aerobically composted in municipal or industrial facilities | |
| ASTM D6691-17 | Standard test method for determining aerobic biodegradation of plastic materials in the marine environment by a defined microbial consortium or natural sea water inoculum | |
| ASTM D6866-21 | Standard test methods for determining the biobased content of solid, liquid, and gaseous samples using radiocarbon analysis | |
| ASTM D6868-21 | Standard specification for labeling of end items that incorporate plastics and polymers as coatings or additives with paper and other substrates designed to be aerobically composted in municipal or industrial facilities | |
| ASTM D6954-18 | Standard guide for exposing and testing plastics that degrade in the environment by a combination of oxidation and biodegradation | |
| ASTM D7444-18a | Standard practice for heat and humidity aging of oxidatively degradable plastics | |
| ASTM D7475-20 | Standard test method for determining the aerobic degradation and anaerobic biodegradation of plastic materials under accelerated bioreactor landfill conditions | |
| ASTM D7991-15 | Standard test method for determining aerobic biodegradation of plastics buried in sandy marine sediment under controlled laboratory conditions | |
| UK | BS 8472:2011 | Methods for the assessment of the oxo-biodegradation of plastics and of the phyto-toxicity of the residues in controlled laboratory conditions |
| BS ISO 16620-1:2015 | Plastics. Biobased content General principles | |
| BS ISO 16620-2:2019 | Plastics. Biobased content Determination of biobased carbon content | |
| PD CEN/TR 16721:2014 | Biobased products. Overview of methods to determine the biobased content (British standard) | |
| BS ISO 16620-3:2015 | Plastics. Biobased content Determination of biobased synthetic polymer content | |
| BS ISO 22526-3:2020 | Plastics. Carbon and environmental footprint of biobased plastics Process carbon footprint, requirements, and guidelines for quantification | |
| BS ISO 23517:2021 | Plastics. Soil biodegradable materials for mulch films for use in agriculture and horticulture. Requirements and test methods regarding biodegradation, ecotoxicity and control of constituents | |
| BS ISO 5412 | Biodegradable plastic shopping bags for industrial composting | |
| EU | CSN EN ISO 10210 | Plastics—Methods for the preparation of samples for biodegradation testing of plastic materials |
| DIN EN 13432 | Requirements for packaging recoverable through composting and biodegradation—Test scheme and evaluation criteria for the final acceptance of packaging | |
| CSN EN ISO 14851 | Determination of the ultimate aerobic biodegradability of plastic materials in an aqueous medium—Method by measuring the oxygen demand in a closed respirometer | |
| CSN EN ISO 14852 | Determination of the ultimate aerobic biodegradability of plastic materials in an aqueous medium—Method by analysis of evolved CO2 | |
| CSN EN ISO 14853 | Plastics—Determination of the ultimate anaerobic biodegradation of plastic materials in an aqueous system—Method by measurement of biogas production | |
| CSN EN ISO 14855-1 | Determination of the ultimate aerobic biodegradability of plastic materials under controlled composting conditions—Method by analysis of evolved CO2—Part 1: General method | |
| CSN EN ISO 14855-2 | Determination of the ultimate aerobic biodegradability of plastic materials under controlled composting conditions—Method by analysis of evolved CO2—Part 2: Gravimetric measurement of CO2 evolved in a laboratory-scale test | |
| CSN EN 14995 | Plastics—Evaluation of compostability—Test scheme and specifications | |
| CSN EN ISO 15985 | Plastics—Determination of the ultimate anaerobic biodegradation under high-solids anaerobic-digestion conditions—Method by analysis of released biogas | |
| CSN EN 16640 | Biobased products—Biobased carbon content—Determination of the biobased carbon content using the radiocarbon method | |
| CSN EN 16760 | Biobased products—Life Cycle Assessment | |
| EN 16785-1 | Biobased products—Biobased content—Part 1: Determination of the biobased content using the radiocarbon analysis and elemental analysis | |
| CSN EN 16785-2 | Biobased products—Biobased content—Part 2: Determination of the biobased content using the material balance method | |
| CSN EN ISO 16929 | Plastics—Determination of the degree of disintegration of plastic materials under defined composting conditions in a pilot-scale test | |
| CSN EN ISO 17556 | Plastics—Determination of the ultimate aerobic biodegradability of plastic materials in soil by measuring the oxygen demand in a respirometer or the amount of CO2 evolved | |
| CSN EN 17417 | Determination of the ultimate biodegradation of plastics materials in an aqueous system under anoxic (denitrifying) conditions—Method by measurement of pressure increase | |
| CSN EN ISO 18830 | Plastics—Determination of aerobic biodegradation of nonfloating plastic materials in a seawater/sandy sediment interface—Method by measuring the oxygen demand in closed respirometer | |
| CSN EN ISO 19679 | Plastics—Determination of aerobic biodegradation of nonfloating plastic materials in a seawater/sediment interface—Method by analysis of evolved CO2 | |
| International | ISO 14851 | Determination of the ultimate aerobic biodegradability of plastic materials in an aqueous medium—Method by measuring the oxygen demand in a closed respirometer |
| ISO 14852 | Determination of the ultimate aerobic biodegradability of plastic materials in an aqueous medium—Method by analysis of evolved CO2 | |
| ISO 14853 | Plastics—Determination of the ultimate anaerobic biodegradation of plastic materials in an aqueous system—Method by measurement of biogas production |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melchor-Martínez, E.M.; Macías-Garbett, R.; Alvarado-Ramírez, L.; Araújo, R.G.; Sosa-Hernández, J.E.; Ramírez-Gamboa, D.; Parra-Arroyo, L.; Alvarez, A.G.; Monteverde, R.P.B.; Cazares, K.A.S.; et al. Towards a Circular Economy of Plastics: An Evaluation of the Systematic Transition to a New Generation of Bioplastics. Polymers 2022, 14, 1203. https://doi.org/10.3390/polym14061203
Melchor-Martínez EM, Macías-Garbett R, Alvarado-Ramírez L, Araújo RG, Sosa-Hernández JE, Ramírez-Gamboa D, Parra-Arroyo L, Alvarez AG, Monteverde RPB, Cazares KAS, et al. Towards a Circular Economy of Plastics: An Evaluation of the Systematic Transition to a New Generation of Bioplastics. Polymers. 2022; 14(6):1203. https://doi.org/10.3390/polym14061203
Chicago/Turabian StyleMelchor-Martínez, Elda M., Rodrigo Macías-Garbett, Lynette Alvarado-Ramírez, Rafael G. Araújo, Juan Eduardo Sosa-Hernández, Diana Ramírez-Gamboa, Lizeth Parra-Arroyo, Abraham Garza Alvarez, Rosina Paola Benavides Monteverde, Karen Aleida Salazar Cazares, and et al. 2022. "Towards a Circular Economy of Plastics: An Evaluation of the Systematic Transition to a New Generation of Bioplastics" Polymers 14, no. 6: 1203. https://doi.org/10.3390/polym14061203
APA StyleMelchor-Martínez, E. M., Macías-Garbett, R., Alvarado-Ramírez, L., Araújo, R. G., Sosa-Hernández, J. E., Ramírez-Gamboa, D., Parra-Arroyo, L., Alvarez, A. G., Monteverde, R. P. B., Cazares, K. A. S., Reyes-Mayer, A., Yáñez Lino, M., Iqbal, H. M. N., & Parra-Saldívar, R. (2022). Towards a Circular Economy of Plastics: An Evaluation of the Systematic Transition to a New Generation of Bioplastics. Polymers, 14(6), 1203. https://doi.org/10.3390/polym14061203