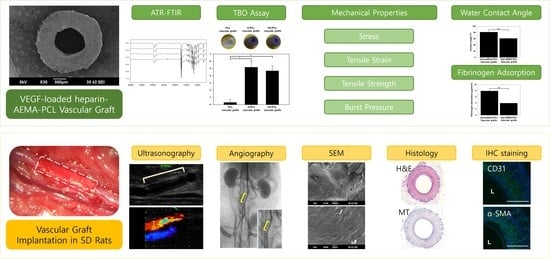
In Vivo Evaluation of Gamma-Irradiated and Heparin-Immobilized Small-Diameter Polycaprolactone Vascular Grafts with VEGF in Aged Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Heparin-Immobilized and VEGF-Loaded Methacrylated PCL Vascular Grafts
2.3. Characterization of Nanofibers
2.3.1. Scanning Electron Microscopy
2.3.2. Attenuated Total Reflection–Fourier Transform Infrared Spectroscopy
2.3.3. Mechanical Properties
2.3.4. Toluidine Blue O Assay
2.3.5. Water Contact Angle
2.3.6. Fibrinogen Adsorption
2.4. Implantation of Vascular Grafts into Aged Rats
2.4.1. Animals
2.4.2. Vascular Graft Implantation
2.5. In Vivo Evaluation of Vascular Grafts in Aged Rats
2.5.1. Ultrasonography
2.5.2. Angiography
2.5.3. Scanning Electron Microscopy for Harvested Vascular Grafts
2.5.4. Histological Evaluation of Harvested Vascular Grafts
2.5.5. Immunofluorescence Staining
2.5.6. Statistical Evaluation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janairo, R.R.R.; Henry, J.J.D.; Lee, B.L.-P.; Hashi, C.K.; Derugin, N.; Lee, R.; Li, S. Heparin-modified small-diameter nanofibrous vascular grafts. IEEE Trans. Nanobiosci. 2012, 11, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Magnaudeix, A.; Usseglio, J.; Lasgorceix, M.; Lalloue, F.; Damia, C.; Brie, J.; Pascaud-Mathieu, P.; Champion, E. Quantitative analysis of vascular colonisation and angio-conduction in porous silicon-substituted hydroxyapatite with various pore shapes in a chick chorioallantoic membrane (CAM) model. Acta Biomater. 2016, 38, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.; Montezano, A.C.; Lopes, R.A.; Rios, F.; Touyz, R.M. Vascular fibrosis in aging and hypertension: Molecular mechanisms and clinical implications. Can. J. Cardiol. 2016, 32, 659–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocco, K.A.; Maxfield, M.W.; Best, C.A.; Dean, E.W.; Breuer, C.K. In vivo applications of electrospun tissue-engineered vascular grafts: A review. Tissue Eng. Part B Rev. 2014, 20, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Kuwano, H.; Amano, J.; Yokomise, H. Thoracic and cardiovascular surgery in Japan during 2010: Annual report by The Japanese Association for Thoracic Surgery. Gen. Thorac. Cardiovasc. Surg. 2012, 60, 680–708. [Google Scholar] [CrossRef] [PubMed]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R.; TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease (TASC II). J. Vasc. Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diehm, C.; Allenberg, J.R.; Pittrow, D.; Mahn, M.; Tepohl, G.; Haberl, R.L.; Darius, H.; Burghaus, I.; Trampisch, H.J.; German Epidemiological Trial on Ankle Brachial Index Study Group. German epidemiological trial on ankle brachial index study group, mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation 2009, 120, 2053–2061. [Google Scholar] [CrossRef] [Green Version]
- Pektok, E.; Nottelet, B.; Tille, J.-C.; Gurny, R.; Kalangos, A.; Moeller, M.; Walpoth, B.H. Degradation and healing characteristics of small-diameter poly(epsilon-caprolactone) vascular grafts in the rat systemic arterial circulation. Circulation 2008, 118, 2563–2570. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Lin, P.; Yao, Q.; Chen, C. Development of small-diameter vascular grafts. World J. Surg. 2007, 31, 682–689. [Google Scholar] [CrossRef]
- Malm, C.J.; Risberg, B.; Bodin, A.; Bäckdahl, H.; Johansson, B.R.; Gatenholm, P.; Jeppsson, A. Small calibre biosynthetic bacterial cellulose blood vessels: 13-months patency in a sheep model. Scand. Cardiovasc. J. 2012, 46, 57–62. [Google Scholar] [CrossRef]
- Pawlowski, K.J.; Rittgers, S.E.; Schmidt, S.P.; Bowlin, G.L. Endothelial cell seeding of polymeric vascular grafts. Front. Biosci. 2014, 9, 1412–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Xu, Y.; Li, Q.; Turng, L.-S. Artificial small-diameter blood vessels: Materials, fabrication, surface modification, mechanical properties, and bioactive functionalities. J. Mater. Chem. B 2020, 8, 1801–1822. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.I.; Jeon, O.; Krebs, M.D.; Hill, M.C.; Alsberg, E. Biodegradable photo-crosslinked alginate nanofibre scaffolds with tuneable physical properties, cell adhesivity and growth factor release. Eur. Cell Mater. 2012, 24, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Shi, Q.; Guo, W.; Terrell, L.; Qureshi, A.T.; Hayes, D.J.; Wu, Q. Electrospun bio-nanocomposite scaffolds for bone tissue engineering by cellulose nanocrystals reinforcing maleic anhydride grafted PLA. ACS Appl. Mater. Interfaces 2013, 5, 3847–3854. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Memic, A.; Annabi, N.; Hossain, M.; Paul, A.; Dokmeci, M.R.; Dehghani, F.; Khademhosseini, A. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2014, 10, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Bucci, R.; Vaghi, F.; Erba, E.; Romanelli, A.; Gelmi, M.L.; Clerici, F. Peptide grafting strategies before and after electrospinning of nanofibers. Acta Biomater. 2021, 122, 82–100. [Google Scholar] [CrossRef]
- Jeong, J.-O.; Jeong, S.I.; Park, J.-S.; Gwon, H.-J.; Ahn, S.-J.; Shin, H.; Lee, J.Y.; Lim, Y.-M. Development and characterization of heparin-immobilized polycaprolactone nanofibrous scaffolds for tissue engineering using gamma-irradiation. RSC Adv. 2017, 7, 8963–8972. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.I.; Park, S.-C.; Park, S.-J.; Kim, E.-J.; Heo, H.; Park, J.-S.; Gwon, H.-J.; Lim, Y.-M.; Jang, M.-K. One-step synthesis of gene carrier via gamma irradiation and its application in tumor gene therapy. Int. J. Nanomed. 2018, 13, 525–536. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.M.; Lim, J.-Y.; Park, J.-S.; Gwon, H.J.; Jeon, S.I.; Lim, Y.-M. Radiation-induced biomimetic modification of dual-layered nano/microfibrous scaffolds for vascular tissue engineering. Biotechnol. Bioprocess Eng. 2014, 19, 118–125. [Google Scholar] [CrossRef]
- Lim, Y.-M.; Jeong, S.I.; Shin, Y.M.; Park, J.-S.; Gwon, H.-J.; Nho, Y.-C.; An, S.-J.; Choi, J.-B.; Jeong, J.-O.; Choi, J.-W. Physicochemical characterization of gelatin-immobilized, acrylic acid-bacterial cellulose nanofibers as cell scaffolds using gamma-irradiation. Biotechnol. Bioprocess Eng. 2015, 20, 942–947. [Google Scholar] [CrossRef]
- Baldwin, A.D.; Kiick, K.L. Polysaccharide-modified synthetic polymeric biomaterials. Biopolymers 2010, 94, 128–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.G.; Shin, H.; Lim, D.W. Biomimetic scaffolds for tissue engineering. Adv. Funct. Mater. 2012, 22, 2446–2468. [Google Scholar] [CrossRef]
- Kiritani, S.; Kaneko, J.; Ito, D.; Morito, M.; Ishizawa, T.; Akamatsu, N.; Tanaka, M.; Iida, T.; Tanaka, T.; Tanaka, R.; et al. Silk fibroin vascular graft: A promising tissue-engineered scaffold material for abdominal venous system replacement. Sci. Rep. 2020, 10, 21041. [Google Scholar] [CrossRef] [PubMed]
- Sevostyanova, V.V.; Antonova, L.V.; Velikanova, E.A.; Matveeva, V.G.; Krivkina, E.O.; Glushkova, T.V.; Mironov, A.V.; Burago, A.Y.; Barbarash, L.S. Endothelialization of polycaprolactone vascular graft under the action of locally applied vascular endothelial growth factor. Bull. Exp. Biol. Med. 2018, 165, 264–268. [Google Scholar] [CrossRef]
- Bergmeister, H.; Seyidova, N.; Schreiber, C.; Strobl, M.; Grasl, C.; Walter, I.; Messner, B.; Baudis, S.; Fröhlich, S.; Marchetti-Deschmann, M.; et al. Biodegradable, thermoplastic polyurethane grafts for small diameter vascular replacements. Acta Biomater. 2015, 11, 104–113. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, X.; Jiang, L.; Zhang, L.; Dong, Y.; Midgley, A.C.; Kong, D.; Wang, S. Regulation of the inflammatory response by vascular grafts modified with Aspirin-Triggered Resolvin D1 promotes blood vessel regeneration. Acta Biomater. 2019, 97, 360–373. [Google Scholar] [CrossRef]
- Yang, X.; Wei, J.; Lei, D.; Liu, Y.; Wu, W. Appropriate density of PCL nano-fiber sheath promoted muscular remodeling of PGS/PCL grafts in arterial circulation. Biomaterials 2016, 88, 34–47. [Google Scholar] [CrossRef]
- Sengupta, P. The laboratory rat: Relating its age with human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Tang, Y.; Wang, L.; Wang, J.; Lin, X.; Wang, Y.; Jin, K.; Yang, G.-Y. Ischemia-induced Angiogenesis is Attenuated in Aged Rats. Aging Dis. 2015, 7, 326–335. [Google Scholar] [CrossRef] [Green Version]
- Jin, B.-F.; Xue, Y.-Y.; Zhang, X.-D.; Xia, G.-S.; Ye, J.; Sun, D.-L.; Gao, Y.-J.; Xu, F.-S. Effects of Yixin Kangtai capsule on spermatogenesis and the levels of superoxide dismutase and malondialdehyde in aged SD rats. Zhonghua Nan Ke Xue 2012, 18, 851–855. [Google Scholar]
- Tanabe, T.; Maeda, S.; Miyauchi, T.; Iemitsu, M.; Takanashi, M.; Irukayama-Tomobe, Y.; Yokota, T.; Ohmori, H.; Matsuda, M. Exercise training improves ageing-induced decrease in eNOS expression of the aorta. Acta Physiol. Scand. 2003, 178, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Antonova, L.V.; Sevostyanova, V.V.; Kutikhin, A.G.; Mironov, A.V.; Krivkina, E.O.; Shabaev, A.R.; Matveeva, V.G.; Velikanova, E.A.; Sergeeva, E.A.; Burago, A.Y.; et al. Vascular endothelial growth factor improves physico-mechanical properties and enhances endothelialization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/Poly(ε-caprolactone) small-diameter vascular grafts in vivo. Front. Pharmacol. 2016, 7, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pashneh-Tala, S.; MacNeil, S.; Claeyssens, F. The tissue-engineered vascular graft-past, present, and future. Tissue Eng. Part B Rev. 2015, 22, 68–100. [Google Scholar] [CrossRef]
- Chandra, P.; Atala, A. Engineering blood vessels and vascularized tissues: Technology trends and potential clinical applications. Clin. Sci. 2019, 133, 1115–1135. [Google Scholar] [CrossRef] [PubMed]
- Freed, L.E.; Engelmayr, G.C., Jr.; Borenstein, J.T.; Moutos, F.T.; Guilak, F. Advanced material strategies for tissue engineering scaffolds. Adv. Mater. 2009, 21, 3410–3418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soletti, L.; Hong, Y.; Guan, J.; Stankus, J.J.; El-Kurdi, M.S.; Wagner, W.R.; Vorp, D.A. A bilayered elastomeric scaffold for tissue engineering of small diameter vascular grafts. Acta Biomater. 2010, 6, 110–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stekelenburg, M.; Rutten, M.C.M.; Snoeckx, L.H.E.H.; Baaijens, F.P.T. Dynamic straining combined with fibrin gel cell seeding improves strength of tissue-engineered small-diameter vascular grafts. Tissue Eng. Part A 2009, 15, 1081–1089. [Google Scholar] [CrossRef]
- Desmet, W.; Vanhaecke, J.; Vrolix, M.; Van de Werf, F.; Piessens, J.; Willems, J.; de Geest, H. Isolated single coronary artery: A review of 50,000 consecutive coronary angiographies. Eur. Heart J. 1992, 13, 1637–1640. [Google Scholar] [CrossRef]
- Park, G.E.; Pattison, M.A.; Park, K.; Webster, T.J. Accelerated chondrocyte functions on NaOH-treated PLGA scaffolds. Biomaterials 2005, 26, 3075–3082. [Google Scholar] [CrossRef]
- Bolbasova, E.N.; Rybachukb, M.; Golovkinc, A.S.; Antonovac, L.V.; Shesterikova, E.V.; Malchikhinaa, A.I.; Novikovd, V.A.; Anissimove, Y.G.; Tverdokhlebova, S.I. Surface modification of poly(l-lactide) and polycaprolactone bioresorbable polymers using RF plasma discharge with sputter deposition of a hydroxyapatite target. Mater. Lett. 2014, 132, 281–284. [Google Scholar] [CrossRef] [Green Version]
- Grøndahl, L.; Chandler-Temple, A.; Trau, M. Polymeric grafting of acrylic acid onto poly(3-hydroxybutyrate-co-3-hydroxyvalerate): Surface functionalization for tissue engineering applications. Biomacromolecules 2005, 6, 2197–2203. [Google Scholar] [CrossRef] [PubMed]
- Luk, J.Z.; Cooper-White, J.; Rintoul, L.; Taran, E.; Grøndahl, L. Functionalised polycaprolactone films and 3D scaffolds via gamma irradiation-induced grafting. J. Mater. Chem. B 2013, 1, 4171–4181. [Google Scholar] [CrossRef] [PubMed]
- Eilenberg, M.; Enayati, M.; Ehebruster, D.; Grasl, C.; Walter, I.; Messner, B.; Baudis, S.; Potzmann, P.; Kaun, C.; Podesser, B.K.; et al. Long Term Evaluation of Nanofibrous, Bioabsorbable polycarbonate urethane grafts for small diameter vessel replacement in rodents. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, J.; Cui, Y.; Xu, R.; Wang, Z.; Zhang, Z.; Wang, K.; Li, Y.; Zhao, Q.; Kong, D. Effect of sustained heparin release from PCL/chitosan hybrid small-diameter vascular grafts on anti-thrombogenic property and endothelialization. Acta Biomater. 2014, 10, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Lei, D.; Li, S.; Huang, P.; Qi, Q.; Sun, Y.; Zhang, Y.; Wang, Z.; You, Z.; Ye, X.; et al. Hybrid small-diameter vascular grafts: Anti-expansion effect of electrospun poly ε-caprolactone on heparin-coated decellularized matrices. Biomaterials 2016, 76, 359–370. [Google Scholar] [CrossRef]
- Wang, X.-N.; Chen, C.-Z.; Yang, M.; Gu, Y.J. Implantation of decellularized small-caliber vascular xenografts with and without surface heparin treatment. Artif. Organs 2007, 31, 99–104. [Google Scholar] [CrossRef]
- Quiñones-Baldrich, W.J.; Prego, A.; Ucelay-Gomez, R.; Vescera, C.L.; Moore, W.S. Failure of PTFE infrainguinal revascularization: Patterns, management alternatives, and outcome. Ann. Vasc. Surg. 1991, 5, 163–169. [Google Scholar] [CrossRef]
- Rensen, S.S.M.; Doevendans, P.A.F.M.; van Eys, G.J.J.M. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth. Heart J. 2007, 15, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Chan-Park, M.B.; Shen, J.Y.; Cao, Y.; Xiong, Y.; Liu, Y.; Rayatpisheh, S.; Kang, G.C.-W.; Greisler, H.P. Biomimetic control of vascular smooth muscle cell morphology and phenotype for functional tissue-engineered small-diameter blood vessels. J. Biomed. Mater. Res. A 2009, 88, 1104–1121. [Google Scholar] [CrossRef]
- de Valence, S.; Tille, J.-C.; Mugnai, D.; Mrowczynski, W.; Gurny, R.; Möller, M.; Walpoth, B.H. Long term performance of polycaprolactone vascular grafts in a rat abdominal aorta replacement model. Biomaterials 2012, 33, 38–47. [Google Scholar] [CrossRef]
- Walpoth, B.H.; Zammaretti, P.; Cikirikcioglu, M.; Khabiri, E.; Djebaili, M.K.; Pache, J.-C.; Tille, J.-C.; Aggoun, Y.; Morel, D.; Kalangos, A.; et al. Enhanced intimal thickening of expanded polytetrafluoroethylene grafts coated with fibrin or fibrin-releasing vascular endothelial growth factor in the pig carotid artery interposition model. J. Thorac. Cardiovasc. Surg. 2007, 133, 1163–1170. [Google Scholar] [CrossRef]
- Randone, B.; Cavallaro, G.; Polistena, A.; Cucina, A.; Coluccia, P.; Graziano, P.; Cavallaro, A. Dual role of VEGF in pretreated experimental ePTFE arterial grafts. J. Surg. Res. 2005, 127, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Rothuizen, T.C.; Damanik, F.F.R.; Anderson, J.M.; Lavrijsen, T.; Cox, M.A.J.; Rabelink, T.J.; Moroni, L.; Rotmans, J.I. Tailoring the foreign body response for in situ vascular tissue engineering. Tissue Eng. Part C Methods 2015, 21, 436–446. [Google Scholar] [CrossRef]
- Lakatta, E.G.; Levy, D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: A “set up” for vascular disease. Circulation 2003, 107, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakatta, E.G. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part III: Cellular and molecular clues to heart and arterial aging. Circulation 2003, 107, 490–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marutama, Y. Aging and arterial-cardiac interactions in the elderly. Int. J. Cardiol. 2012, 155, 14–19. [Google Scholar] [CrossRef]
- Minamino, T.; Komuro, I. Vascular aging: Insights from studies on cellular senescence, stem cell aging, and progeroid syndromes. Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, 637–648. [Google Scholar] [CrossRef]
- Brown, K.A.; Didion, S.P.; Andresen, J.J.; Faraci, F.M. Effect of aging, MnSOD deficiency, and genetic background on endothelial function: Evidence for MnSOD haploinsufficiency. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1941–1946. [Google Scholar] [CrossRef] [Green Version]
- Nisoli, E.; Clementi, E.; Carruba, M.O.; Moncada, S. Defective mitochondrial biogenesis: A hallmark of the high cardiovascular risk in the metabolic syndrome? Circ. Res. 2007, 100, 795–806. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-E.; Jeong, S.-I.; Shim, K.-M.; Jang, K.; Park, J.-S.; Lim, Y.-M.; Kang, S.-S. In Vivo Evaluation of Gamma-Irradiated and Heparin-Immobilized Small-Diameter Polycaprolactone Vascular Grafts with VEGF in Aged Rats. Polymers 2022, 14, 1265. https://doi.org/10.3390/polym14061265
Kim S-E, Jeong S-I, Shim K-M, Jang K, Park J-S, Lim Y-M, Kang S-S. In Vivo Evaluation of Gamma-Irradiated and Heparin-Immobilized Small-Diameter Polycaprolactone Vascular Grafts with VEGF in Aged Rats. Polymers. 2022; 14(6):1265. https://doi.org/10.3390/polym14061265
Chicago/Turabian StyleKim, Se-Eun, Sung-In Jeong, Kyung-Mi Shim, Kwangsik Jang, Jong-Seok Park, Youn-Mook Lim, and Seong-Soo Kang. 2022. "In Vivo Evaluation of Gamma-Irradiated and Heparin-Immobilized Small-Diameter Polycaprolactone Vascular Grafts with VEGF in Aged Rats" Polymers 14, no. 6: 1265. https://doi.org/10.3390/polym14061265
APA StyleKim, S. -E., Jeong, S. -I., Shim, K. -M., Jang, K., Park, J. -S., Lim, Y. -M., & Kang, S. -S. (2022). In Vivo Evaluation of Gamma-Irradiated and Heparin-Immobilized Small-Diameter Polycaprolactone Vascular Grafts with VEGF in Aged Rats. Polymers, 14(6), 1265. https://doi.org/10.3390/polym14061265