Robust Adamantane-Based Membranes with Enhanced Conductivity for Vanadium Flow Battery Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the PAPEK Membranes
2.2. Characterization Methods
3. Results and Discussion
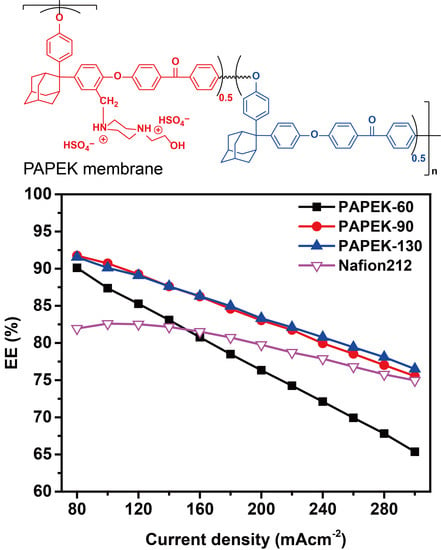
3.1. VFB Performance of PAPEK Membranes
3.2. Stability of PAPEK Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, M.; Ryu, J.; Wang, W.; Cho, J. Material design and engineering of next-generation flow-battery technologies. Nat. Rev. Mater. 2017, 2, 16080. [Google Scholar] [CrossRef]
- Tan, R.; Wang, A.; Malpass-Evans, R.; Williams, R.; Zhao, E.W.; Liu, T.; Ye, C.; Zhou, X.; Darwich, B.P.; Fan, Z.; et al. Hydrophilic microporous membranes for selective ion separation and flow-battery energy storage. Nat. Mater. 2019, 19, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Herve Bang, Y.; Di Noto, V. Hybrid inorganic-organic proton-conducting membranes based on SPEEK doped with WO3 nanoparticles for application in vanadium redox flow batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Wang, G.; Zou, H.; Zhu, X.; Ding, M.; Jia, C. Recent progress in zinc-based redox flow batteries: A review. J. Phys. D Appl. Phys. 2021, 55, 163001. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, C. Cost-effective iron-based aqueous redox flow batteries for large-scale energy storage application: A review. J. Power Sources 2021, 493, 229445. [Google Scholar] [CrossRef]
- Wang, W.; Luo, Q.; Li, B.; Wei, X.; Li, L.; Yang, Z. Recent Progress in Redox Flow Battery Research and Development. Adv. Funct. Mater. 2013, 23, 970–986. [Google Scholar] [CrossRef]
- Dai, Q.; Liu, Z.; Huang, L.; Wang, C.; Zhao, Y.; Fu, Q.; Zheng, A.; Zhang, H.; Li, X. Thin-film composite membrane breaking the trade-off between conductivity and selectivity for a flow battery. Nat. Commun. 2020, 11, 13. [Google Scholar] [CrossRef] [Green Version]
- Ding, C.; Zhang, H.; Li, X.; Liu, T.; Xing, F. Vanadium Flow Battery for Energy Storage: Prospects and Challenges. J. Phys. Chem. Lett. 2013, 4, 1281–1294. [Google Scholar] [CrossRef]
- Yu, L.; Yu, L.; Wang, L.; Wang, L.; Qiu, X.; Xi, J. Bilayer Designed Hydrocarbon Membranes for All-Climate Vanadium Flow Batteries To Shield Catholyte Degradation and Mitigate Electrolyte Crossover. ACS Appl. Mater. Interfaces 2019, 11, 13285–13294. [Google Scholar] [CrossRef]
- Mu, D.; Yu, L.; Yu, L.; Xi, J. Toward Cheaper Vanadium Flow Batteries: Porous Polyethylene Reinforced Membrane with Superior Durability. ACS Appl. Energy Mater. 2018, 1, 1641–1648. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, C.; Dai, Y.; Zheng, W.; Ruan, X.; He, G. A novel imidazolium-based amphoteric membrane for high-performance vanadium redox flow battery. J. Membr. Sci. 2017, 544, 98–107. [Google Scholar] [CrossRef]
- Yuan, Z.; Dai, Q.; Qiao, L.; Zhao, Y.; Zhang, H.; Li, X. Highly stable aromatic poly (ether sulfone) composite ion exchange membrane for vanadium flow battery. J. Membr. Sci. 2017, 541, 465–473. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, W.; Yuan, Z.; Qiao, L.; Li, X.; Zhang, H. Advanced charged porous membranes with flexible internal crosslinking structures for vanadium flow batteries. J. Mater. Chem. A 2017, 5, 6193–6199. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, J.; Lu, S.; Xiang, Y.; Jiang, S.P. Acid Pretreatment to Enhance Proton Transport of a Polysulfone-Polyvinylpyrrolidone Membrane for Application in Vanadium Redox Flow Batteries. ChemPlusChem 2018, 83, 909–914. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, F.; Guan, S.; Zhao, M.; Zhang, E.; Wang, G.; Zhang, Z.; Liu, X.; Zhang, S. Pyridinium functionalized, 2-adamantane containing poly(aryl ether ketone) membranes for use in vanadium redox flow batteries. J. Power Sources 2020, 477, 229011. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Q.; Guan, S.; Weng, Z.; Zhang, E.; Wang, G.; Zhang, Z.; Hu, J.; Zhang, S. High performance membranes based on new 2-adamantane containing poly(aryl ether ketone) for vanadium redox flow battery applications. J. Power Sources 2018, 399, 18–25. [Google Scholar] [CrossRef]
- Yu, L.; Wang, L.; Yu, L.; Mu, D.; Wang, L.; Xi, J. Aliphatic/aromatic sulfonated polyimide membranes with cross-linked structures for vanadium flow batteries. J. Membr. Sci. 2019, 572, 119–127. [Google Scholar] [CrossRef]
- Qiao, L.; Zhang, H.; Lu, W.; Dai, Q.; Li, X. Advanced Porous Membranes with Tunable Morphology Regulated by Ionic Strength of Nonsolvent for Flow Battery. ACS Appl. Mater. Interfaces 2019, 11, 24107–24113. [Google Scholar] [CrossRef]
- Chen, D.; Qi, H.; Sun, T.; Yan, C.; He, Y.; Kang, C.; Yuan, Z.; Li, X. Polybenzimidazole membrane with dual proton transport channels for vanadium flow battery applications. J. Membr. Sci. 2019, 586, 202–210. [Google Scholar] [CrossRef]
- Noh, C.; Serhiichuk, D.; Malikah, N.; Kwon, Y.; Henkensmeier, D. Optimizing the performance of meta-polybenzimidazole membranes in vanadium redox flow batteries by adding an alkaline pre-swelling step. Chem. Eng. J. 2021, 407, 126574. [Google Scholar] [CrossRef]
- Peng, S.; Wu, X.; Yan, X.; Gao, L.; Zhu, Y.; Zhang, D.; Li, J.; Wang, Q.; He, G. Polybenzimidazole membranes with nanophase-separated structure induced by non-ionic hydrophilic side chains for vanadium flow batteries. J. Mater. Chem. A 2018, 6, 3895–3905. [Google Scholar] [CrossRef]
- Lu, W.; Yuan, Z.; Zhao, Y.; Qiao, L.; Zhang, H.; Li, X. Advanced porous PBI membranes with tunable performance induced by the polymer-solvent interaction for flow battery application. Energy Storage Mater. 2018, 10, 40–47. [Google Scholar] [CrossRef]
- Chen, R.; Henkensmeier, D.; Kim, S.; Yoon, S.J.; Zinkevich, T.; Indris, S. Improved All-Vanadium Redox Flow Batteries using Catholyte Additive and a Cross-linked Methylated Polybenzimidazole Membrane. ACS Appl. Energy Mater. 2018, 1, 6047–6055. [Google Scholar] [CrossRef]
- Noh, C.; Jung, M.; Henkensmeier, D.; Nam, S.W.; Kwon, Y. Vanadium Redox Flow Batteries Using meta-Polybenzimidazole-Based Membranes of Different Thicknesses. ACS Appl. Mater. Interfaces 2017, 9, 36799–36809. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Zhang, H.; Li, M.; Yuan, Z.; Zhao, Y.; Li, X. A Venus-flytrap-inspired pH-responsive porous membrane with internal crosslinking networks. J. Mater. Chem. A 2017, 5, 25555–25561. [Google Scholar] [CrossRef]
- Yan, X.; Dong, Z.; Di, M.; Hu, L.; Zhang, C.; Pan, Y.; Zhang, N.; Jiang, X.; Wu, X.; Wang, J.; et al. A highly proton-conductive and vanadium-rejected long-side-chain sulfonated polybenzimidazole membrane for redox flow battery. J. Membr. Sci. 2020, 596, 117616. [Google Scholar] [CrossRef]
- Hu, L.; Gao, L.; Yan, X.; Zheng, W.; Dai, Y.; Hao, C.; Wu, X.; He, G. Proton delivery through a dynamic 3D H-bond network constructed from dense hydroxyls for advanced ion-selective membranes. J. Mater. Chem. A 2019, 7, 15137–15144. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzù, K.; Zawodzinski, T.A.; Meda, L.; Gambaro, C.; Di Noto, V. An efficient barrier toward vanadium crossover in redox flow batteries: The bilayer [Nafion/(WO3)x] hybrid inorganic-organic membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Mai, Z.; Zhang, H.; Vankelecom, I. Ion exchange membranes for vanadium redox flow battery (VRB) applications. Energy Environ. Sci. 2011, 4, 1147–1160. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, M.; Yuan, Z.; Li, X.; Zhang, H.; Vankelecom, I.F.J. Advanced Charged Sponge-Like Membrane with Ultrahigh Stability and Selectivity for Vanadium Flow Batteries. Adv. Funct. Mater. 2016, 26, 210–218. [Google Scholar] [CrossRef]
- Wu, C.; Lu, S.; Wang, H.; Xu, X.; Peng, S.; Tan, Q.; Xiang, Y. A novel polysulfone–polyvinylpyrrolidone membrane with superior proton to vanadium ion selectivity for vanadium redox flow batteries. J. Mater. Chem. A 2016, 4, 1174–1179. [Google Scholar] [CrossRef]
- Peng, S.; Yan, X.; Zhang, D.; Wu, X.; Luo, Y.; He, G. A H3PO4 preswelling strategy to enhance the proton conductivity of a H2SO4-doped polybenzimidazole membrane for vanadium flow batteries. RSC Adv. 2016, 6, 23479–23488. [Google Scholar] [CrossRef]
- Jang, J.; Kim, T.; Yoon, S.; Lee, J.; Lee, J.; Hong, Y. Highly proton conductive, dense polybenzimidazole membranes with low permeability to vanadium and enhanced H2SO4absorption capability for use in vanadium redox flow batteries. J. Mater. Chem. A 2016, 4, 14342–14355. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Zhang, F.; Li, X.; Li, Y.; Vankelecom, I. Advanced charged membranes with highly symmetric spongy structures for vanadium flow battery application. Energy Environ. Sci. 2013, 6, 776–781. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Li, X.; Mai, Z.; Zhang, J. Nanofiltration (NF) membranes: The next generation separators for all vanadium redox flow batteries (VRBs)? Energy Environ. Sci. 2011, 4, 1676–1679. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Z.; Ran, J.; Zhou, D.; Li, C.; Xu, T. Advances in proton-exchange membranes for fuel cells: An overview on proton conductive channels (PCCs). Phys. Chem. Chem. Phys. 2013, 15, 4870–4887. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, C.H.; Guiver, M.D.; Lee, Y.M. Sulfonated hydrocarbon membranes for medium-temperature and low-humidity proton exchange membrane fuel cells (PEMFCs). Prog. Polym. Sci. 2011, 36, 1443–1498. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Guiver, M.D. Ion transport by nanochannels in ion-containing aromatic copolymers. Macromolecules 2014, 47, 2175–2198. [Google Scholar] [CrossRef] [Green Version]
- Bengui, Z.; Zhao, M.; Liu, Q.; Zhang, X.; Fu, Y.; Zhang, E.; Wang, G.; Zhang, Z.; Zhang, S. High performance positively charged membranes with selective swelling-induced ion transport channels for vanadium flow battery application. J. Power Sources 2022, 526, 231140. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, M.; Liu, Q.; Zhang, X.; Fu, Y.; Zhang, E.; Wang, G.; Zhang, Z.; Zhang, S. Advanced anion exchange membranes with selective swelling-induced ion transport channels for vanadium flow battery application. J. Membr. Sci. 2022, 642, 119985. [Google Scholar] [CrossRef]
- Zhang, B.; Fu, Y.; Liu, Q.; Zhang, X.; Yang, Z.; Jiang, H.; Zhang, E.; Wang, K.; Wang, G.; Zhang, Z.; et al. Steric-hindrance benzimidazole constructed highly conductive and robust membrane for vanadium flow battery. J. Membr. Sci. 2022, 646, 120254. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, M.; Liu, Q.; Zhang, X.; Fu, Y.; Zhang, E.; Wang, G.; Zhang, Z.; Yuan, X.; Zhang, S. High performance membranes based on pyridine containing poly (aryl ether ketone ketone) for vanadium redox flow battery applications. J. Power Sources 2021, 506, 230128. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, S.; Weng, Z.; Wang, G.; Zhang, E.; Yu, P.; Chen, X.; Wang, X. Quaternized adamantane-containing poly(aryl ether ketone) anion exchange membranes for vanadium redox flow battery applications. J. Power Sources 2016, 325, 801–807. [Google Scholar] [CrossRef]
- Pixton, M.; Paul, D. Gas transport properties of adamantane based polysulfones. Polymer 1995, 36, 3165–3172. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, S.; Xing, D.; Han, R.; Yin, C.; Jian, X. Quaternized poly(phthalazinone ether ketone ketone) anion exchange membrane with low permeability of vanadium ions for vanadium redox flow battery application. J. Power Sources 2012, 217, 296–302. [Google Scholar] [CrossRef]
- Hossain, M.A.; Jang, H.; Sutradhar, S.C.; Ha, J.; Yoo, J.; Lee, C.; Lee, S.; Kim, W. Novel hydroxide conducting sulfonium-based anion exchange membrane for alkaline fuel cell applications. Int. J. Hydrogen Energy 2016, 41, 10458–10465. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, B.; Xing, D.; Jian, X. Poly(phthalazinone ether ketone ketone) anion exchange membranes with pyridinium as ion exchange groups for vanadium redox flow battery applications. J. Mater. Chem. A 2013, 1, 12246–12254. [Google Scholar] [CrossRef]
- Anthony, S.; Tisdale, R.; Disselkamp, R.; Tolber, M. FTIR studies of low temperature sulfuric acid aerosol. Geophys. Res. Lett. 1995, 22, 1105–1108. [Google Scholar] [CrossRef]
- Glipa, X.; Bonnet, B.; Mula, B.; Jones, D.; RozieÁre, J. Investigation of the conduction properties of phosphoric and sulfuric acid doped polybenzimidazole. J. Mater. Chem. 1999, 9, 3045–3049. [Google Scholar] [CrossRef]
- Aili, D.; Yang, J.; Jankova, K.; Henkensmeier, D.; Li, Q. From polybenzimidazoles to polybenzimidazoliums and polybenzimidazolides. J. Mater. Chem. A 2020, 8, 12854–12886. [Google Scholar] [CrossRef]
- Geng, K.; Li, Y.; Xing, Y.; Wang, L.; Li, N. A novel polybenzimidazole membrane containing bulky naphthalene group for vanadium flow battery. J. Membr. Sci. 2019, 586, 231–239. [Google Scholar] [CrossRef]
- Yuan, Z.; Duan, Y.; Zhang, H.; Li, X.; Zhang, H.; Vankelecom, I. Advanced porous membranes with ultra-high selectivity and stability for vanadium flow batteries. Energy Environ. Sci. 2016, 9, 441–447. [Google Scholar] [CrossRef]
- Dong, Z.; Di, M.; Hu, L.; Gao, L.; Yan, X.; Ruan, X.; Wu, X.; He, G. Hydrophilic/hydrophobic-bi-comb-shaped amphoteric membrane for vanadium redox flow battery. J. Membr. Sci. 2020, 608, 118179. [Google Scholar] [CrossRef]
- Palanisamy, G.; Sadhasivam, T.; Park, W.-S.; Bae, S.T.; Roh, S.-H.; Jung, H.-Y. Tuning the Ion Selectivity and Chemical Stability of a Biocellulose Membrane by PFSA Ionomer Reinforcement for Vanadium Redox Flow Battery Applications. ACS Sustain. Chem. Eng. 2020, 8, 2040–2051. [Google Scholar] [CrossRef]
- Hu, L.; Gao, L.; Zhang, C.; Yan, X.; Jiang, X.; Zheng, W.; Ruan, X.; Wu, X.; Yu, G.; He, G. “Fishnet-like” ion-selective nanochannels in advanced membranes for flow batteries. J. Mater. Chem. A 2019, 7, 21112–21119. [Google Scholar] [CrossRef]
- Ding, L.; Song, X.; Wang, L.; Zhao, Z.; He, G. Preparation of dense polybenzimidazole proton exchange membranes with different basicity and flexibility for vanadium redox flow battery applications. Electrochim. Acta 2018, 292, 10–19. [Google Scholar] [CrossRef]
- Kim, J.; Jeon, J.-D.; Kwak, S.-Y. Sulfonated poly(ether ether ketone) composite membranes containing microporous layered silicate AMH-3 for improved membrane performance in vanadium redox flow batteries. Electrochim. Acta 2017, 243, 220–227. [Google Scholar] [CrossRef]
- Ding, L.; Song, X.; Wang, L.; Zhao, Z. Enhancing proton conductivity of polybenzimidazole membranes by introducing sulfonate for vanadium redox flow batteries applications. J. Membr. Sci. 2019, 578, 126–135. [Google Scholar] [CrossRef]
- Prifti, H.; Parasuraman, A.; Winardi, S.; Lim, T.M.; Skyllas-Kazacos, M. Membranes for redox flow battery applications. Membranes 2012, 2, 275–306. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Wu, L.; Yu, L.; Qiu, X.; Xi, J. A comparative study of Nafion series membranes for vanadium redox flow batteries. J. Membr. Sci. 2016, 510, 18–26. [Google Scholar] [CrossRef]
- Geng, K.; Tang, H.; Li, Y.; Liu, L.; Li, N. A facile strategy for disentangling the conductivity and selectivity dilemma enables advanced composite membrane for vanadium flow batteries. J. Membr. Sci. 2020, 607, 118177. [Google Scholar] [CrossRef]
- Yuan, Z.; Dai, Q.; Zhao, Y.; Lu, W.; Li, X.; Zhang, H. Polypyrrole modified porous poly(ether sulfone) membranes with high performance for vanadium flow batteries. J. Mater. Chem. A 2016, 4, 12955–12962. [Google Scholar] [CrossRef]
- Lu, W.; Yuan, Z.; Zhao, Y.; Li, X.; Zhang, H.; Vankelecom, I.F.J. High-performance porous uncharged membranes for vanadium flow battery applications created by tuning cohesive and swelling forces. Energy Environ. Sci. 2016, 9, 2319–2325. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhu, X.; Li, M.; Lu, W.; Li, X.; Zhang, H. A Highly Ion-Selective Zeolite Flake Layer on Porous Membranes for Flow Battery Applications. Angew. Chem. 2016, 55, 3058–3062. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, H.; Hu, Z.; Li, L.; Hu, L.; Li, Z.; Gao, L.; Dai, Y.; Jian, X.; He, G. Amphoteric-Side-Chain-Functionalized “Ether-Free” Poly(arylene piperidinium) Membrane for Advanced Redox Flow Battery. ACS Appl. Mater. Interfaces 2019, 11, 44315–44324. [Google Scholar] [CrossRef]
- Du, Y.; Gao, L.; Hu, L.; Di, M.; Yan, X.; An, B.; He, G. The synergistic effect of protonated imidazole-hydroxyl-quaternary ammonium on improving performances of anion exchange membrane assembled flow batteries. J. Membr. Sci. 2020, 603, 118011. [Google Scholar] [CrossRef]
- Shi, M.; Dai, Q.; Li, F.; Li, T.; Hou, G.; Zhang, H.; Li, X. Membranes with Well-Defined Selective Layer Regulated by Controlled Solvent Diffusion for High Power Density Flow Battery. Adv. Energy Mater. 2020, 10, 2001382. [Google Scholar] [CrossRef]
- Mohammadi, T.; Kazacos, M.S. Evaluation of the chemical stability of some membranes in vanadium solution. J. Appl. Electrochem. 1997, 27, 153–158. [Google Scholar] [CrossRef]
- Choi, S.-W.; Kim, T.-H.; Jo, S.-W.; Lee, J.Y.; Cha, S.-H.; Hong, Y.T. Hydrocarbon membranes with high selectivity and enhanced stability for vanadium redox flow battery applications: Comparative study with sulfonated poly(ether sulfone)s and sulfonated poly(thioether ether sulfone)s. Electrochim. Acta 2018, 259, 427–439. [Google Scholar] [CrossRef]









| Solvents | APEK | CAPEK | PAPEK |
|---|---|---|---|
| Chloroform | + | + | − |
| N-Methyl pyrrolidone | + | + | + |
| Dichloromethane | + | + | − |
| Dimethyl sulfoxide | − | − | − |
| N-dimethyl acetamide | + | + | − |
| Toluene | − | − | − |
| Ethanol | − | − | − |
| Membrane | Young’s Modulus (GPa) |
|---|---|
| PAPEK-virgin | 1.55 |
| PAPEK-60 | 1.48 |
| PAPEK-90 | 1.40 |
| PAPEK-130 | 1.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Zhang, X.; Liu, Q.; Fu, Y.; Yang, Z.; Zhang, E.; Wang, K.; Wang, G.; Zhang, Z.; Zhang, S. Robust Adamantane-Based Membranes with Enhanced Conductivity for Vanadium Flow Battery Application. Polymers 2022, 14, 1552. https://doi.org/10.3390/polym14081552
Zhang B, Zhang X, Liu Q, Fu Y, Yang Z, Zhang E, Wang K, Wang G, Zhang Z, Zhang S. Robust Adamantane-Based Membranes with Enhanced Conductivity for Vanadium Flow Battery Application. Polymers. 2022; 14(8):1552. https://doi.org/10.3390/polym14081552
Chicago/Turabian StyleZhang, Bengui, Xueting Zhang, Qian Liu, Yanshi Fu, Zhirong Yang, Enlei Zhang, Kangjun Wang, Guosheng Wang, Zhigang Zhang, and Shouhai Zhang. 2022. "Robust Adamantane-Based Membranes with Enhanced Conductivity for Vanadium Flow Battery Application" Polymers 14, no. 8: 1552. https://doi.org/10.3390/polym14081552
APA StyleZhang, B., Zhang, X., Liu, Q., Fu, Y., Yang, Z., Zhang, E., Wang, K., Wang, G., Zhang, Z., & Zhang, S. (2022). Robust Adamantane-Based Membranes with Enhanced Conductivity for Vanadium Flow Battery Application. Polymers, 14(8), 1552. https://doi.org/10.3390/polym14081552