Effectiveness and Applications of a Metal-Coated HNT/Polylactic Acid Antimicrobial Filtration System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication Design
2.2. Inner and Outer Fabric Layers
2.3. Material Preparation and Filament Extrusion
2.3.1. Metal HNT Preparation
2.3.2. HNT and mHNT PLA Filament Preparation
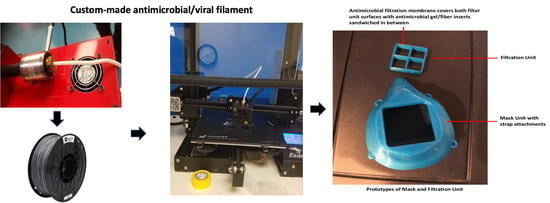
2.4. Three-Dimensional Printing of Masks
2.5. Three-Dimensional Printing of Testing Discs
2.6. Antibacterial Testing
2.6.1. Muller–Hinton Broth
2.6.2. Muller–Hinton Agar
2.7. Solution Blow Spinning of mHNT Fibers
2.8. Statistical Analysis
3. Results
3.1. Liquid Growth Inhibition Studies
3.2. Agar Plate Growth Inhibition Studies
3.3. Fabrication of the N95 Mask and Filter Assembly
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Coronavirus Disease (COVID-19) Pandemic. 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 1 May 2020).
- WHO. Coronavirus Disease 2019 (COVID-19) Situation Report. 2020. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200318-sitrep-58-covid-19.pdf?sfvrsn=20876712_2 (accessed on 1 May 2020).
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Ostrikov, K.K. Future antiviral surfaces: Lessons from COVID-19 pandemic. Sustain. Mater. Technol. 2020, 25, e00203. [Google Scholar] [CrossRef]
- National Public Radio. Can the U.S. Crowdsource Its Way Out of a Mask Shortage? Available online: https://www.npr.org/2020/03/25/820795727/can-the-u-s-crowdsource-its-way-out-of-a-mask-shortage-no-but-it-still-helps (accessed on 2 May 2020).
- Zhipeng, L.; Yang, J. Computer-aided customized shape design of an N95 filtering facepiece respirator. In International Design Engineering Technical Conferences and Computers and Information in Engineering Conference; American Society of Mechanical Engineers: New York, NY, USA, 2013; Volume 55898, p. V03BT03A020. [Google Scholar]
- 3M Personal Safety Division. Surgical N95 vs. Standard N95—Which to Consider? Technical Bulletin for 3M: Saint Paul, MN, USA, March 2020; Available online: https://multimedia.3m.com/mws/media/1839703O/surgical-n95-vs-standard-n95-which-to-consider.pdf (accessed on 5 May 2020).
- Singh, R.; Nalwa, H.S. Medical applications of nanoparticles in biological imaging, cell labeling, antimicrobial agents, and anticancer nanodrugs. J. Biomed. Nanotechnol. 2011, 7, 489–503. [Google Scholar] [CrossRef]
- Qazi, U.Y.; Javaid, R. A Review on Metal Nanostructures: Preparation Methods and Their Potential Applications. Adv. Nanoparticles 2016, 5, 27–43. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Quinn, J.; Pinsky, B.; Shah, N.H.; Brown, I. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA 2020, 323, 2085–2086. [Google Scholar] [CrossRef] [Green Version]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Del Pozo, C.H.; Prosper, F.; et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 2020, 181, 905–913. [Google Scholar] [CrossRef]
- Zuniga, J.M.; Cortes, A. The role of additive manufacturing and antimicrobial polymers in the COVID-19 pandemic. Expert Rev. Med Devices 2020, 17, 477–481. [Google Scholar] [CrossRef]
- How Are People Being Infected with COVID-19? Available online: https://www.livescience.com/how-covid-19-spreads-transmission-routes.html (accessed on 10 July 2020).
- World Health Organization. Infection Prevention and Control During Health Care When COVID-19 Is Suspected. Available online: https://www.who.int/publications-detail/infection-prevention-and-control-during-health-care-when-novel-coronavirus-(ncov)-infection-is-suspected-20200125 (accessed on 15 August 2020).
- Shortage of Personal Protective Equipment Endangering Health Workers Worldwide. 2020. Available online: https://www.who.int/news-room/detail/03-03-2020-shortage-of-personal-protective-equipment-endangering-health-workers-worldwide (accessed on 2 May 2020).
- How Not to Get Sick. Available online: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html (accessed on 13 April 2022).
- US Food and Drug Administration. FAQs on Shortages of Surgical Masks and Gowns. Available online: https://www.fda.gov/medical-devices/personal-protective-equipment-infection-control/faqs-shortages-surgical-masks-and-gowns-during-covid-19-pandemic (accessed on 1 May 2020).
- Woo, P.C.; Lau, S.K.; Chu, C.M.; Chan, K.H.; Tsoi, H.W.; Huang, Y.; Wong, B.H.; Poon, R.W.; Cai, J.J.; Luk, W.K.; et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 2005, 79, 884–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarano, A.; Inchingolo, F.; Rapone, B.; Festa, F.; Tari, S.R.; Lorusso, F. Protective face masks: Effect on the oxygenation and heart rate status of oral surgeons during surgery. Int. J. Environ. Res. Public Health 2021, 18, 2363. [Google Scholar] [CrossRef] [PubMed]
- Elisheva, R. Adverse effects of prolonged mask use among healthcare professionals during COVID-19. J. Infect. Dis. Epidemiology 2020, 6, 130. [Google Scholar] [CrossRef]
- León-Buitimea, A.; Garza-Cárdenas, C.R.; Garza-Cervantes, J.A.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R. The demand for new antibiotics: Antimicrobial peptides, nanoparticles, and combinatorial therapies as future strategies in antibacterial agent design. Front. Microbiol. 2020, 11, 1669. [Google Scholar] [CrossRef]
- Makowski, M.; Silva, Í.C.; Pais do Amaral, C.; Gonçalves, S.; Santos, N.C. Advances in lipid and metal nanoparticles for antimicrobial peptide delivery. Pharmaceutics 2019, 11, 588. [Google Scholar] [CrossRef] [Green Version]
- Turner, R.J. Metal-based antimicrobial strategies. Microb. Biotechnol. 2017, 10, 1062–1065. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [Green Version]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Hwang, I.-S.; Hwang, J.H.; Choi, H.; Kim, K.-J.; Lee, D.G. Synergistic effects between silver nanoparticles and antibiotics and the mechanisms involved. J. Med. Microbiol. 2012, 61, 1719–1726. [Google Scholar] [CrossRef] [Green Version]
- Katva, S.; Das, S.; Moti, H.S.; Jyoti, A.; Kaushik, S. Antibacterial synergy of silver nanoparticles with gentamicin and chloramphenicol against Enterococcus faecalis. Pharmacogn. Mag. 2018, 13, S828–S833. [Google Scholar] [CrossRef]
- OrganicNANO—Ignite the Night. Available online: https://livestream.com/nasaitech/cycle2forum/videos/212425482 (accessed on 20 May 2020).
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, E.; Gilmore, B.F.; Larrañeta, E.; Lamprou, D.A. Antimicrobial 3D printed objects in the fight against pandemics. 3D Print. Addit. Manuf. 2021, 8, 79–86. [Google Scholar] [CrossRef]
- Aimar, A.; Palermo, A.; Innocenti, B. The role of 3D printing in medical applications: A state of the art. J. Health Eng. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tappa, K.; Jammalamadaka, U. Novel biomaterials used in medical 3D printing techniques. J. Funct. Biomater. 2018, 9, 17. [Google Scholar] [CrossRef] [Green Version]
- Tack, P.; Victor, J.; Gemmel, P.; Annemans, L. 3D-printing techniques in a medical setting: A systematic literature review. Biomed. Eng. Online 2016, 15, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Cornel, G.; Tănase, A. Dimitrie Cantemir. Christ. Univ. Knowl. Horiz. -Econ. 2016, 8, 32–39. [Google Scholar]
- Dey, D.; Srinivas, D.; Panda, B.; Suraneni, P.; Sitharam, T. Use of industrial waste materials for 3D printing of sustainable concrete: A review. J. Clean. Prod. 2022, 340, 130749. [Google Scholar] [CrossRef]
- Lee, J.-Y.; An, J.; Chua, C.K. Fundamentals and applications of 3D printing for novel materials. Appl. Mater. Today 2017, 7, 120–133. [Google Scholar] [CrossRef]
- Layani, M.; Wang, X.; Magdassi, S. Novel Materials for 3D Printing by Photopolymerization. Adv. Mater. 2018, 30, e1706344. [Google Scholar] [CrossRef]
- Clifton, W.; Damon, A.; Martin, A.K. Considerations and cautions for three-dimensional-printed personal protective equipment in the COVID-19 crisis. 3D Print. Addit. Manuf. 2020, 7, 97–99. [Google Scholar] [CrossRef]
- The Research Committee of the Society of Healthcare. Epidemiology of America enhancing patient safety by reducing healthcare-associated infections: The role of discovery and dissemination. Infect. Control Hosp. Epidemiol. 2010, 31, 118–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernando, S.A.; Grat, T.; Gottlieb, T. Healthcare-acquired infections: Prevention strategies. Intern. Med. Rev. 2017, 47, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Larrañeta, E.; Dominguez-Robles, J.; Lamprou, D.A. Additive manufacturing can assist in the fight against COVID-19 and other pandemics and impact on the global supply chain. 3D Print. Addit. Manuf. 2020, 7, 100–103. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, A.K.; Chakraborty, R.; Basu, T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 2014, 25, 12. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lü, X.; Jiang, X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef]
- Ortiz-Benítez, E.A.; Velázquez-Guadarrama, N.; Durán Figueroa, N.V.; Quezada, H.; Olivares-Trejo, J.D.J. Antibacterial mechanism of gold nanoparticles on Streptococcus pneumoniae. Metallomics 2019, 11, 1265. [Google Scholar] [CrossRef]
- Hakkarainen, M.; Albertsson, A.C.; Karlsson, S. Weight losses and molecular weight changes correlated with the evolution of hydroxyacids in simulated in vivo degradation of homo- and copolymers of PLA and PGA. Polym. Degrad. Stab. 1996, 52, 283–291. [Google Scholar] [CrossRef]
- Cimolai, N. MRSA and the environment: Implications for comprehensive control measures. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 481–493. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buruga, K.; Kalathi, J.T. A facile synthesis of halloysite nanotubes based polymer nanocomposites for glass coating application. J. Alloy. Compd. 2018, 735, 1807–1817. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McFarland, A.W., Jr.; Elumalai, A.; Miller, C.C.; Humayun, A.; Mills, D.K. Effectiveness and Applications of a Metal-Coated HNT/Polylactic Acid Antimicrobial Filtration System. Polymers 2022, 14, 1603. https://doi.org/10.3390/polym14081603
McFarland AW Jr., Elumalai A, Miller CC, Humayun A, Mills DK. Effectiveness and Applications of a Metal-Coated HNT/Polylactic Acid Antimicrobial Filtration System. Polymers. 2022; 14(8):1603. https://doi.org/10.3390/polym14081603
Chicago/Turabian StyleMcFarland, Antwine W., Jr., Anusha Elumalai, Christopher C. Miller, Ahmed Humayun, and David K. Mills. 2022. "Effectiveness and Applications of a Metal-Coated HNT/Polylactic Acid Antimicrobial Filtration System" Polymers 14, no. 8: 1603. https://doi.org/10.3390/polym14081603
APA StyleMcFarland, A. W., Jr., Elumalai, A., Miller, C. C., Humayun, A., & Mills, D. K. (2022). Effectiveness and Applications of a Metal-Coated HNT/Polylactic Acid Antimicrobial Filtration System. Polymers, 14(8), 1603. https://doi.org/10.3390/polym14081603