Effects of Modified Layered Double Hydroxides on the Thermal Degradation and Combustion Behaviors of Intumescent Flame Retardant Polyethylene Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation Methods
2.3. Characterization
3. Results
3.1. Structure of Modified LDH and Its Nanocomposites
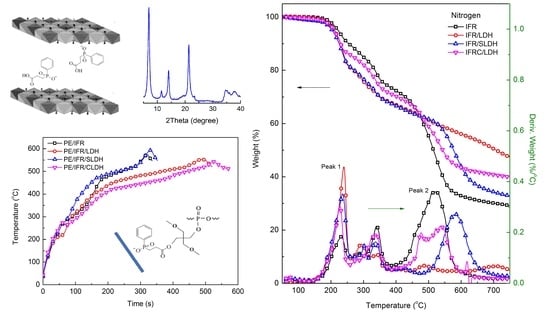
3.2. Thermal Degradation of LDHs, IFR/LDHs, and PE/IFR/LDHs
3.3. Combustion Behaviors of PE/IFR/LDHs
3.4. Char Structure after CONE Testing
3.5. Flame Retardant Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Erfan, R.G.; Fatemeh, K.; Zahra, M.; Ali, S.A.; Fatemeh, M.D.; Masoud, K.; Rasoul, E.N.; Oisik, D.; Atiye, M.; Rhoda, A.M.; et al. The flame retardancy of polyethylene composites: From fundamental concepts to nano-composites. Molecules 2020, 25, 5157. [Google Scholar]
- Tang, T.T.; Liu, X.Y.; Hao, S.X. Mechanism and Preparation Methods of Inorganic Fire-Retardant. Adv. Mater. Res. 2013, 785–786, 757–760. [Google Scholar] [CrossRef]
- Costa, F.R.; Saphiannikova, M.; Wagenknecht, U.; Heinrich, G. Layered Double Hydroxide Based Polymer Nanocomposites. Adv. Polym. Sci. 2007, 101–168. [Google Scholar] [CrossRef]
- Joyeeta, D.; Tuhin, C.; Kinsuk, N. LDH as a multifunctional additive in EVA/TPU blends: Influence on me-chanical, thermal, rheological and flame retardancy properties. Mater. Sci. Eng. B 2018, 236–237, 84–94. [Google Scholar]
- Shen, A.; Wu, H.; Guo, Y.; Yang, X.; He, Z.; Li, Y. Effect of Layered Double Hydroxide on Rheological and Flame-Retardant Properties of Styrene-Butadiene-Styrene–Modified Asphalt. J. Mater. Civ. Eng. 2021, 33, 04020454. [Google Scholar] [CrossRef]
- Lyu, B.; Luo, K.; Gao, D.; Wang, Y.; Ma, J. Modified layered double hydroxide/zanthoxylum bungeanum seed oil composites to improve the flame retardant of leather. Polym. Degrad. Stab. 2020, 183, 109430. [Google Scholar] [CrossRef]
- Han, Y.; Wu, Y.; Shen, M.; Li, T.; Wang, Y.; Zhang, Q.; Wang, Z. Preparation and flame re-tardancy of polystyrene nanocomposites based on layered double hydroxides. Polym. Compos. 2017, 38, 1680–1688. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Q.; Zheng, Y.; Hong, M.; Fu, H. Study on novel flame retarded LDH-TDI-HEA-VTES-acrylate composites and their flame retardant mechanism. React. Funct. Polym. 2019, 147, 104371. [Google Scholar] [CrossRef]
- Lei, Q.; Yanshan, G.; Peng, L.; Dermot, O.; Qiang, W. Synthesis and properties of polypropylene/layered double hy-droxide nanocomposites with different LDHs particle sizes. J. Appl. Polym. Sci. 2018, 135, 46204. [Google Scholar]
- Xu, S.; Li, S.-Y.; Zhang, M.; Zeng, H.-Y.; Du, J.-Z.; Chen, C.-R. Effect of P3O105− intercalated hydrotalcite on the flame retardant properties and the degradation mechanism of a novel polypropylene/hydrotalcite system. Appl. Clay Sci. 2018, 163, 196–203. [Google Scholar] [CrossRef]
- Wang, W.; Kan, Y.; Pan, H.; Pan, Y.; Li, B.; Liew, K.; Hu, Y. Phosphorylated cellulose applied for the exfoliation of LDH: An advanced reinforcement for polyvinyl alcohol. Compos. Part A Appl. Sci. Manuf. 2017, 94, 170–177. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Zhang, Z.; Wang, Q. Preparation of ammonium polyphosphate and dye co-intercalated LDH/polypropylene composites with enhanced flame retardant and UV resistance properties. Chemosphere 2021, 277, 130370. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Lai, X.; Li, H.; Gao, J.; Zeng, X.; Liao, X. Facile fabrication of a novel polyboro-siloxane-decorated layered double hydroxide for remarkably reducing fire hazard of silicone rubber. Compos. Part B Eng. 2019, 175, 107068. [Google Scholar] [CrossRef]
- Wu, K.; Xu, S.; Tian, X.-Y.; Zeng, H.-Y.; Hu, J.; Guo, Y.-H.; Jian, J. Renewable lignin-based surfactant modified layered double hydroxide and its application in polypropylene as flame retardant and smoke suppression. Int. J. Biol. Macromol. 2021, 178, 580–590. [Google Scholar] [CrossRef]
- Xu, S.; Zeng, H.-Y.; Du, J.-Z.; Chen, C.-R.; Wu, K.; Tian, X.-Y.; Pan, Y. The effect of ammonium polyphosphate on the mechanism of phosphorous-containing hydrotalcite synergism of flame retardation of pol-ypropylene. Appl. Clay Sci. 2020, 185, 105348. [Google Scholar] [CrossRef]
- Lyu, B.; Luo, K.; Gao, D.; Wang, Y.; Ma, J. Synergistic effect of layered double hydroxide and montmo-rillonite: Towards super-efficient fireproofing of leather. Appl. Clay Sci. 2021, 212, 106215. [Google Scholar] [CrossRef]
- Ning, H.; Ma, Z.; Zhang, Z.; Zhang, D.; Wang, Y. Core–shell expandable graphite @ layered double hydroxide as a flame retardant for polyvinyl alcohol. J. Therm. Anal. 2021, 1–10. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, Y.; Wang, W.; Gu, X.; Li, H.; Li, J.; Sun, J. Intercalation of phosphotungstic acid into layered double hydroxides by reconstruction method and its application in intumescent flame retardant poly (lactic acid) composites. Polym. Degrad. Stab. 2018, 147, 142–150. [Google Scholar] [CrossRef]
- Wang, L.; He, X.; Lu, H.; Feng, J.; Xie, X.; Su, S.; Wilkie, C.A. Flame retardancy of polypropylene (nano)composites containing LDH and zinc borate. Polym. Adv. Technol. 2011, 22, 1131–1138. [Google Scholar] [CrossRef]
- Kotal, M.; Srivastava, S.K.; Bhowmick, A.K. Thermoplastic polyurethane and nitrile butadiene rubber blends with layered double hydroxide nanocomposites by solution blending. Polym. Int. 2009, 59, 2–10. [Google Scholar] [CrossRef]
- Qiu, L.; Gao, Y.; Zhang, C.; Yan, Q.; O’Hare, D.; Wang, Q. Synthesis of highly efficient flame retardant polypropylene nanocomposites with surfactant intercalated layered double hydroxides. Dalton Trans. 2017, 47, 2965–2975. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, Q.; Wang, J.; Huang, L.; Yan, X.; Zhang, X.; He, Q.; Xing, Z.; Guo, Z. Synthesis of highly efficient flame retardant high-density polyethylene nanocomposites with inorgano-layered double hy-droxides as nanofiller using solvent mixing method. ACS Appl. Mater. Interfaces 2014, 6, 5094–5104. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.R.; Wagenknecht, U.; Heinrich, G. LDPE/Mg–Al layered double hydroxide nanocomposite: Thermal and flammability properties. Polym. Degrad. Stab. 2007, 92, 1813–1823. [Google Scholar] [CrossRef]
- Ye, L.; Wu, Q. Effects of an intercalating agent on the morphology and thermal and flame-retardant properties of low-density polyethylene/layered double hydroxide nanocomposites prepared by melt intercalation. J. Appl. Polym. Sci. 2011, 123, 316–323. [Google Scholar] [CrossRef]
- Khanal, S.; Lu, Y.; Jin, D.; Xu, S. Effects of layered double hydroxides on the thermal and flame retardant properties of intumescent flame retardant high density polyethylene composites. Fire Mater. 2021, 46, 107–116. [Google Scholar] [CrossRef]
- Feng, X.X.; Liu, M.J.; Zhang, J.C. Study on the Preparation and Properties of the Phosphorus-Containing Flame Retardant PET Copolymer. Adv. Mater. Res. 2013, 652–654, 410–413. [Google Scholar] [CrossRef]
- Chen, X.; Xu, D.; Zhang, H.; Feng, X.; Deng, J.; Pan, K. In situ polymerization of flame retardant modification polyamide 6,6 with 2-carboxy ethyl (phenyl) phosphinic acid. J. Appl. Polym. Sci. 2019, 137, 48687. [Google Scholar] [CrossRef]
- You, L.; Hui, Y.; Shi, X.; Peng, Z. Study on the synthesis and characterization of a novel phospho-rus-nitrogen containing intumescent flame retardant. Adv. Mater. Res. 2012, 399–401, 1376–1380. [Google Scholar]
- Ding, P.; Kang, B.; Zhang, J.; Yang, J.; Song, N.; Tang, S.; Shi, L. Phosphorus-containing flame retardant modified layered double hydroxides and their applications on polylactide film with good transparency. J. Colloid Interface Sci. 2014, 440, 46–52. [Google Scholar] [CrossRef]
- Li, B.; Jia, H.; Guan, L.; Bing, B.; Dai, J. A novel intumescent flame-retardant system for flame-retarded LLDPE/EVA composites. J. Appl. Polym. Sci. 2009, 114, 3626–3635. [Google Scholar] [CrossRef]
- Han, Z.D.; Zhang, X.K.; Wang, Y.; Jiang, Z.Q.; Wang, P. Characterization of Layered Double Hydroxide Modified with Sodium Dodecyl Sulfate and its Dispersion in Polyethylene. Key Eng. Mater. 2013, 591, 138–141. [Google Scholar] [CrossRef]
- Camino, G.; Maffezzoli, A.; Braglia, M.; De Lazzaro, M.; Zammarano, M. Effect of hydroxides and hydroxycarbonate structure on fire retardant effectiveness and mechanical properties in ethylene-vinyl acetate copolymer. Polym. Degrad. Stab. 2001, 74, 457–464. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A.; Malucelli, G. Thermal shielding performances of nano-structured intumescentcoatings con-taining organo-modified layered double hydroxides. Prog. Org. Coat. 2015, 78, 504–510. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A.; Malucelli, G.; Camino, G. Testing fire protective properties of intumescent coatings by in-line temperature measurements on a cone calorimeter. Prog. Org. Coat. 2010, 69, 475–480. [Google Scholar] [CrossRef]
- Xu, Z.-Z.; Huang, J.-Q.; Chen, M.-J.; Tan, Y.; Wang, Y.-Z. Flame retardant mechanism of an efficient flame-retardant polymeric synergist with ammonium polyphosphate for polypropylene. Polym. Degrad. Stab. 2013, 98, 2011–2020. [Google Scholar] [CrossRef]
- Xia, Y.; Mao, Z.; Jin, F.; Guan, Y.; Zheng, A. Synthesis of 1-hydroxy ethylidene-1,1-diphosphonic ammo-nium and the promise of this ammonium salt as an intumescent flame retardant in polystyrene. Polym. Degrad. Stab. 2014, 102, 186–194. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Das, A.; Costa, F.R.; Leuteritz, A.; Wang, Y.-Z.; Wagenknecht, U.; Heinrich, G. Synthesis of organo cobalt-aluminum layered double hydroxide via a novel single-step self-assembling method and its use as flame re-tardant nanofiller in PP. Langmuir 2010, 26, 14162–14169. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Wang, C.; Wang, Y.; Wang, Y.; Han, Z. Effects of Modified Layered Double Hydroxides on the Thermal Degradation and Combustion Behaviors of Intumescent Flame Retardant Polyethylene Nanocomposites. Polymers 2022, 14, 1616. https://doi.org/10.3390/polym14081616
Zhang T, Wang C, Wang Y, Wang Y, Han Z. Effects of Modified Layered Double Hydroxides on the Thermal Degradation and Combustion Behaviors of Intumescent Flame Retardant Polyethylene Nanocomposites. Polymers. 2022; 14(8):1616. https://doi.org/10.3390/polym14081616
Chicago/Turabian StyleZhang, Tiefeng, Chunfeng Wang, Yue Wang, Yongliang Wang, and Zhidong Han. 2022. "Effects of Modified Layered Double Hydroxides on the Thermal Degradation and Combustion Behaviors of Intumescent Flame Retardant Polyethylene Nanocomposites" Polymers 14, no. 8: 1616. https://doi.org/10.3390/polym14081616
APA StyleZhang, T., Wang, C., Wang, Y., Wang, Y., & Han, Z. (2022). Effects of Modified Layered Double Hydroxides on the Thermal Degradation and Combustion Behaviors of Intumescent Flame Retardant Polyethylene Nanocomposites. Polymers, 14(8), 1616. https://doi.org/10.3390/polym14081616